土壤组分对磷形态和磷吸附-解吸的影响——基于三峡库区消落带落干期土壤
闫金龙,吴文丽,江 韬,魏世强
土壤组分对磷形态和磷吸附-解吸的影响——基于三峡库区消落带落干期土壤
闫金龙1,2*,吴文丽1,江 韬2,魏世强2
(1.重庆理工大学药学与生物工程学院,重庆 400054;2.西南大学资源环境学院,重庆 4000716)
通过选择性去除土壤组分的方法,探讨了三峡库区消落带落干期3种典型土壤中有机质、铁氧化物组分对磷形态和磷吸附-解吸的影响.结果发现,三峡库区消落带落干期3种典型土壤去除的有机质以易氧化组分为主,去除有机质后,土壤中各种磷形态的含量变化较小.然而,去除游离铁氧化物后,土壤中各种磷形态的含量均发生明显降低.同时,去除有机质、游离铁氧化物组分后并未改变土壤中各种磷形态的相对大小顺序,均为:钙结合磷(Ca-P)>有机磷(OP)>铁/铝结合磷(Fe/Al-P).此外,黄壤(FJ)、紫色潮土(KX)和灰棕紫泥(FL)去除有机质后对磷的吸附能力较原始土壤仅分别降低0.5%、2.3%、6.5%(=0.017<0.05,显著性差异),表明3种土壤中有机质组分对磷吸附的影响较小;而去除游离铁氧化物后对磷的吸附能力分别降低45.6%、51.7%、43.9%(=0.004<0.05,显著性差异),表明土壤中游离铁氧化物组分是决定磷吸附大小的重要因素.另外,3种土壤去除游离铁氧化物后较原始土壤吸附磷的解吸能力明显增加,表明游离铁氧化物组分是控制3种土壤吸附磷的解吸的重要因素.FL土壤去除有机质组分后较原始土壤吸附磷的解吸能力略有降低,而KX和FJ土壤去除有机质组分后较原始土壤吸附磷的解吸能力无明显差异,表明有机质组分对土壤吸附磷的解吸的影响与土壤类型有关.
三峡库区消落带;落干期土壤;磷;有机质;铁氧化物
三峡库区消落带作为典型的陆地和水体过渡地带,是陆地和水体物质和能量的重要交换通道、生态敏感区,其健康和稳定程度直接影响着三峡库区的生态安全.水体富营养化已经成为库区核心的生态环境问题,而磷作为限制性营养元素是控制水体富营养形成的关键因素,消落带土壤同时扮演着磷“汇”和“源”的双重角色,影响着库区水体环境安全[1].因此,深入研究三峡库区消落带土壤磷形态变化及其吸附解吸行为十分重要.
铁氧化物作为土壤中的重要组成组分,具有可变电荷表面,有较大的比表面积,有较高的活性,是决定土壤物理化学性质的重要因素,同时对土壤中磷素的迁移和转化、固定发挥着重要作用[2-3], Zhang等[4]研究发现,土壤对磷的吸附量与无定形铁氧化物、结晶态铁氧化物含量之间呈线性关系.此外,三峡库区消落带因水位涨落将周期性的经历落干和淹水过程,消落带土壤中变价金属氧化物如铁氧化物等随之将周期性经历氧化和还原过程[5].因此,一些学者通过室内模拟淹水-落干过程或野外监测等手段分析了铁氧化物在周期性氧化还原过程中的形态变化及其对磷形态的影响[6-7].上述实验手段能很好地反应消落带土壤铁氧化物与磷形态的关系,但有关铁氧化物与磷形态的关系研究结果也多建立在相关性分析上,未直接给出土壤中铁氧化物对磷形态和磷吸附-解吸的影响.另一方面,有机质作为土壤中的重要组成成分,其对土壤中磷素的地球化学行为也有着重要影响,目前研究多着眼于有机质与磷酸盐在铁氧化物表面的竞争吸附,但也存在一些争议.Borggaard等[8]发现腐殖酸(HA)或富里酸(FA)与磷酸盐共存时对针铁矿、水铁矿吸附磷的影响很小.然而,Antelo等[9]发现土壤腐殖酸存在下,针铁矿对磷酸盐的吸附减少45%.针对三峡库区消落带,仅有部分研究发现土壤的部分磷形态与有机质存在显著相关关系[7,10],未对三峡库区消落带土壤中有机质如何影响磷的环境化学行为做进一步研究.
基于以上,本研究选择三峡库区消落带土壤作为研究对象,以目前国内外较常用的选择性去除土壤组分的方法[11-12],分别去除土壤中有机质、铁氧化物组分,以期探讨土壤有机质、铁氧化物组分对磷形态和磷吸附-解吸的直接影响.
1 材料与方法
1.1 土壤样品和预处理
在三峡库区重庆境内奉节(109°24′25″E, 31°01′08″N)、开县(108°27′21″E,31°11′26″N)和涪陵(107°31′37″E,29°51′30″N)消落带分别采集落干期原始表层土壤(0~10cm)样品(先去除表层沉积物后再采集原始土壤),3种土壤分别为黄壤(FJ)、紫色潮土(KX)和灰棕紫泥(FL).紫色土和黄壤分别是三峡库区第一和第二大类土壤[13].土壤样品运回实验室后自然风干,过60目筛后室温保存.
有机质去除的处理:土壤去除有机质的方法参照文献[14].具体步骤如下:称取10g土壤于100mL离心管中,加入30mL,pH=8.5的6%次氯酸钠(NaClO),于25℃下振荡16h,后在4000r/min下离心10min,去除上清液,重复上述操作3次,后用去离子水洗3次,得到去除有机质的土壤,冷冻干燥后过60目筛,室温下储存待进一步分析.
游离铁氧化物去除的处理:土壤游离铁氧化物的去除采用连二亚硫酸钠-柠檬酸钠-碳酸氢钠法(DCB法)[15].具体步骤如下:称取1g土壤于100mL离心管中,加入20mL 0.3mol/L的柠檬酸钠和2.5mL 1mol/L的碳酸氢钠,置于85℃的恒温水浴锅中,后加入0.5g连二亚硫酸钠并持续搅拌15min后取出,于4000r/min下离心10min,弃去上清液,重复上述操作2次,后用1mol/L的氯化钠溶液洗2次,得到去除游离铁氧化物的土壤,冷冻干燥后过60目筛,室温下储存待进一步分析.
1.2 土壤磷形态测定
基于欧共体提出的SMT磷形态分级方法[16],原始土壤和去除不同组分的土壤均测定了磷的形态.SMT方法中,土壤磷形态被分成以下5个组分:总磷(TP),土壤样品在450℃的马弗炉中灼烧3h后,采用3.5mol/L的HCl在25℃下振荡16h提取得到;无机磷(IP),土壤样品直接采用1.0mol/L的HCl在25 ℃下振荡16h提取得到;有机磷(OP),土壤提取无机磷后的残渣用去离子水洗2次后,烘干并在450℃的马弗炉中灼烧3h,后用1.0mol/L的HCl在25℃下振荡16h提取得到;铁/铝结合磷(Fe/Al-P),土壤样品直接采用1.0mol/L的NaOH在25℃下振荡16h提取得到;钙结合磷(Ca-P),土壤提取Fe/Al-P后的残渣用去离子水洗2次后,用1.0mol/L的NaOH在25 ℃下振荡16h提取得到.
1.3 土壤对磷的吸附-解吸
称取0.5g土壤原样和去除不同组分的土壤样品分别置于100mL离心管中,加入25mL浓度为1,2,5,10,15,20,25mg/L的KH2PO4溶液(以P计),控制体系pH=7±0.1,=0.01mol/L KNO3.转数220r/min下,25℃恒温振荡24h,过0.45 μm滤膜后,测定滤液中磷含量.土壤残渣保留待解吸实验使用.解吸剂为pH=7±0.1,=0.01mol/L KNO3溶液25mL,解吸步骤同吸附过程.所有处理均设置3个平行和空白处理.
1.4 化学分析和数据处理
本研究中,所有化学试剂均为分析纯.土壤原样和去除不同组分的土壤样品均分析以下基本性质:pH值,采用PB-10型pH计(Sartorius, 德国)设置土水比为1:2.5进行测定;BET比表面积测试采用美国麦克ASAP 2020V4.00N2吸附测定;有机质,采用重铬酸钾氧化法测定[17];铁全量(TFe)和游离态铁氧化物含量(Fed)参照张甘霖等的提取方法[18],采用原子吸收光谱法(北京普析通用TAS-990,中国)测定;磷含量,土壤磷形态测定参照鲍士旦的测定方法[17],吸附解吸实验磷含量测定参照文献[19]的测定方法.实验结果数据均为平均值,相关数据处理采用Orgin 8.5和Microsoft Office 2013、SPSS 17进行.
2 结果与讨论
2.1 土壤去除不同组分前后的基本性质
如表1所示,3种供试土壤处理前均为弱碱性,处理后的土壤样品均用去离子水洗涤数次,以降低化学试剂对土壤pH值的影响,3种土壤去除游离铁氧化物和有机质后,供试土壤的pH值均增加.FJ,KX和FL土壤采用NaClO去除有机质的效率分别达到66.8%、61.5%和67.5%,同时对土壤样品中TFe和Fed损失较小,这与文献报道[14]结果一致.另一方面,FJ,KX和FL土壤采用DCB法去除Fed的效率分别达到93.9%、91.9%和87.2%,且TFe分别去除43.9%、43.8%和43.4%.结果表明,3种土壤中游离铁氧化物是土壤中铁的主要成分.此外,土壤比表面积作为一个重要的基本指标,能反应土壤吸附能力的大小,铁氧化物是土壤中比表面积的重要贡献组分[20].当游离铁氧化物去除后,FJ,KX和FL土壤的比表面积分别降低39.6%、40.9%和35.1%.FJ,KX和FL土壤去除有机质后,比表面积分别降低15.2%、19.5%和23.7%,去除有机质的过程中伴随着部分铁氧化物的去除,这可能是造成土壤比表面积降低的原因.
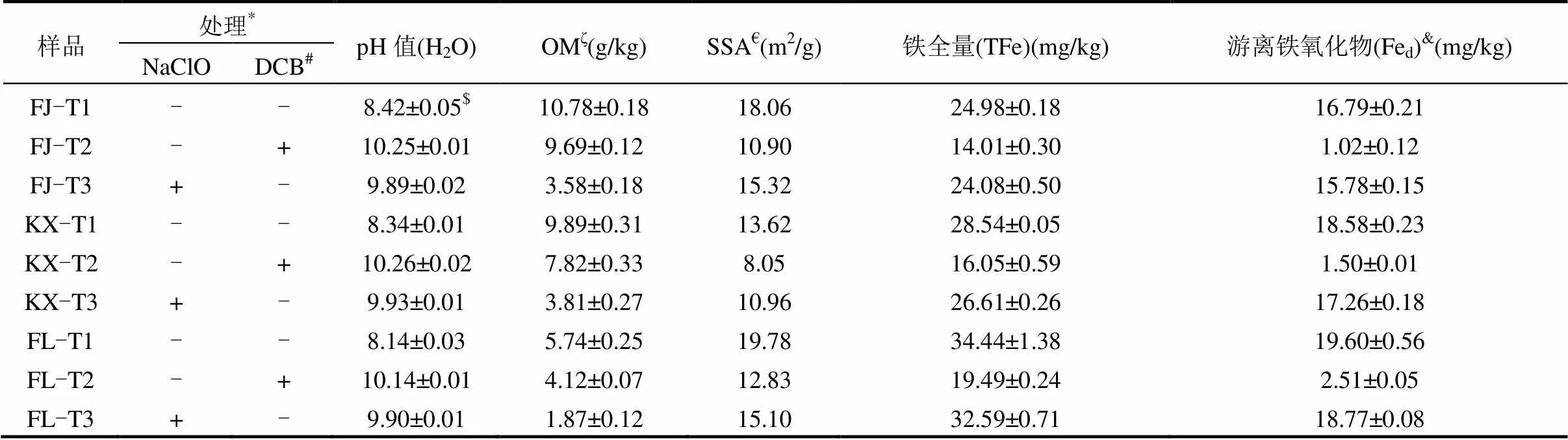
表1 供试土壤去除不同组分前后的基本性质Table 1 Basic characteristics of the soils with and without different treatments
注:T1代表未作任何处理的土壤样品,T2代表去除游离铁氧化物的土壤样品,T3代表去除有机质的土壤样品;* 处理过(+)和没有处理过 (-);# 采用连二亚硫酸钠-柠檬酸钠-碳酸氢钠处理;ζ 有机质;€ 比表面积;& 游离铁氧化物采用DCB提取;数据为3个平行处理的均值±标准偏差.
2.2 土壤去除不同组分前后的磷形态
由表2可知,土壤不同磷形态的质量回收率较高,与文献报道结果相似[21],表明本研究采用SMT法提取土壤各种磷形态是可行的.由图1和表2可知,供试土壤去除不同组分前后,IP均为土壤中TP的主要成分,3种土壤原样IP占TP的比例为53.0%~65.2%.去除有机质后,3种土壤IP占TP的比例为52.8%~64.5%,而去除游离铁氧化物后,3种土壤IP占TP的比例为52.4%~61.9%.其中,Ca-P又是IP的主要组成部分,3种土壤原样Ca-P占IP的比例为58.4%~74.5%.去除有机质后,3种土壤Ca-P占IP的比例为56.3%~76.1%,而去除游离铁氧化物后,3种土壤Ca-P占IP的比例为74.2%~82.4%.土壤去除不同组分前后磷形态分布的相对大小顺序均为:Ca-P>OP>Fe/Al-P,这与以往研究三峡库区消落带土壤中磷形态分布的结果一致[22].本研究中土壤磷形态分布大小与土壤类型无关,但3种土壤中不同磷形态的绝对含量有差异,不同磷形态均表现出FJ土壤值最大,其次是KX土壤,最小的是FL土壤.此外,去除有机质组分后,3种土壤中不同磷形态含量均略微减少,除FL-T3土壤中OP减少11.7%,其余土壤中各形态磷减少量均低于10.0%.然而,当去除游离铁氧化后,3种土壤中Fe/Al-P含量均降低约75.0%,其余各形态磷含量也有不同程度的降低,可知,铁氧化物在土壤中对其他组分能起到一个基质作用,因此去除游离铁氧化物后,土壤中各种磷形态含量均有不同程度降低.报道称,SMT方法中Fe/Al-P和OP被称为生物可利用磷(BAP)[13].当供试土壤去除有机质后,FJ、KX和FL土壤的BAP含量仅分别降低6.6%、8.3%、10.0%,而当去除游离铁氧化物后,FJ、KX和FL土壤的BAP含量分别降低36.4%、41.1%、60.0%.综上可知,游离铁氧化物是影响三峡库区消落带土壤中磷形态变化的重要因素,而有机质的作用较小.
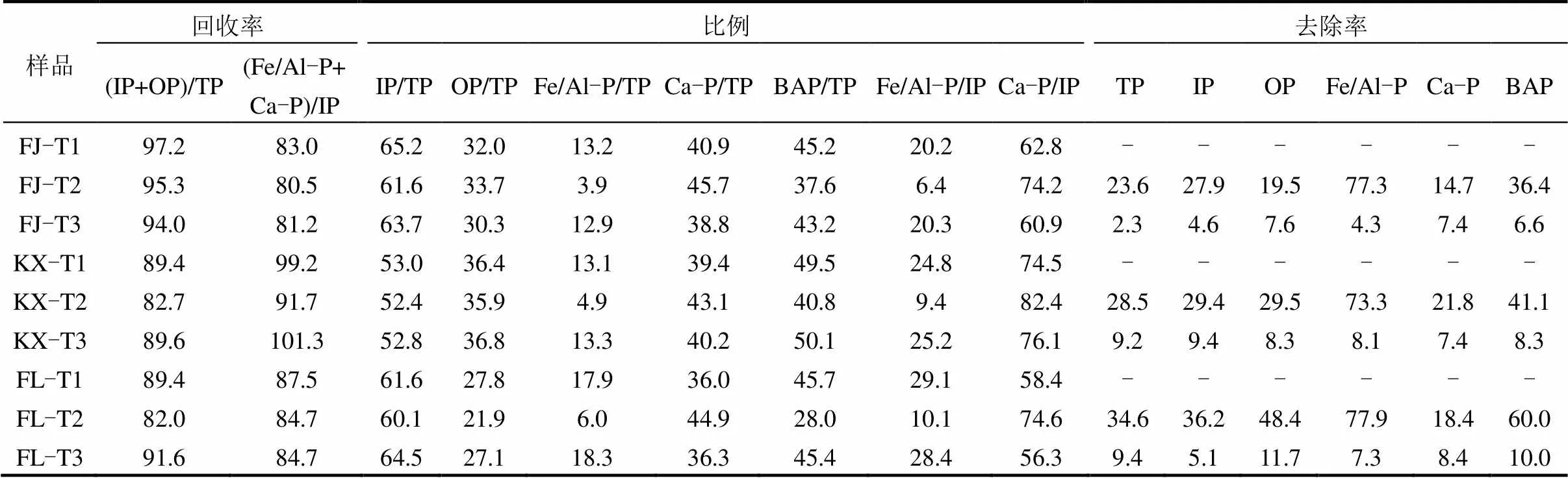
表2 供试土壤去除不同组分前后磷形态去除率,所占比例以及质量回收率(%) Table 2 Recovery of phosphorus fractions and ratio of different phosphorus fractions, removal rate of different phosphorus fractions in the soils with and without treatment(%)
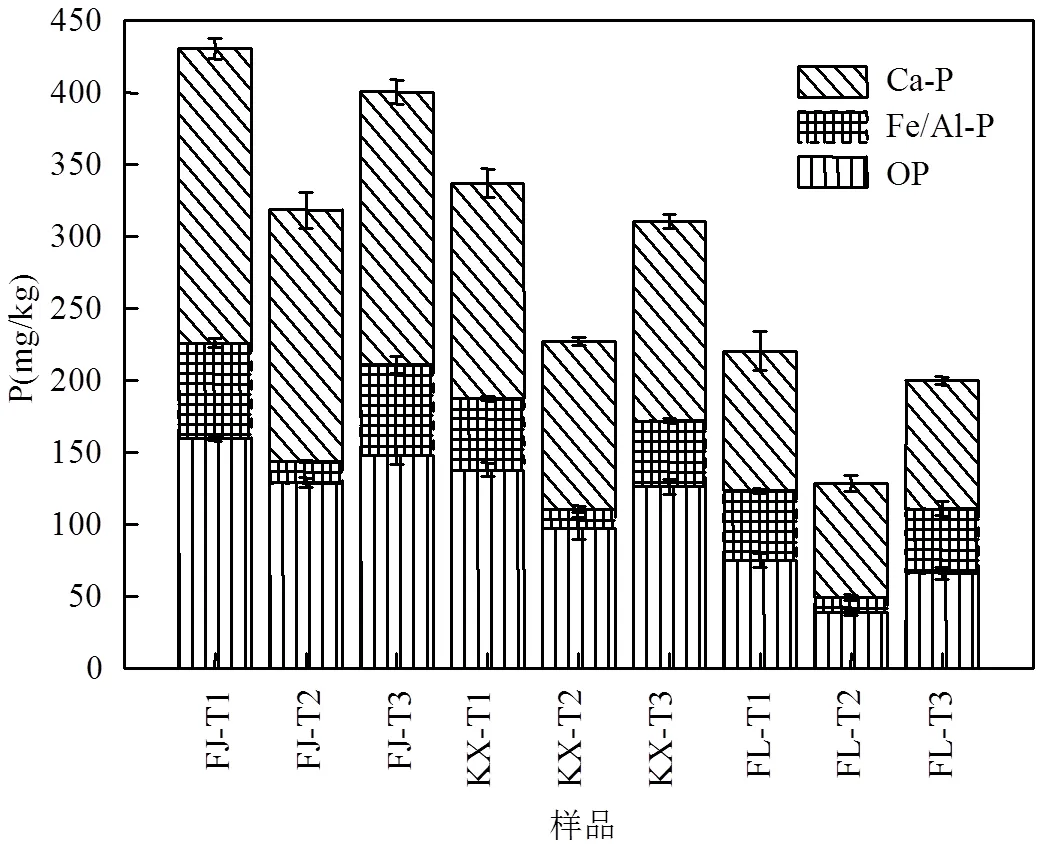
图1 土壤去除不同组分前后的磷形态分布(SMT法提取) Fig.1 Phosphorus fractions extracted by the SMT method in the soils with and without different treatments
2.3 土壤去除不同组分前后对磷的吸附
由图2可知,实验浓度范围内,3种土壤去除不同组分前后对磷的吸附量均随着初始磷浓度的增加而增加,未出现明显的吸附平衡.与原始土壤对磷的吸附量相比,3种土壤去除游离铁氧化物后对磷的吸附量明显降低,其降低程度远大于土壤去除有机质组分对磷的吸附量.为进一步分析吸附结果,发现Freundlich等温方程能较好的拟合吸附数据(2>0.982),方程如下:
e=´e1/n(1)
式中:e表示磷吸附量,mg/g;e表示吸附液中磷浓度,mg/L;表示Freundlich吸附系数,反应吸附能力和吸附亲和力的大小,值越大表示吸附能力越强;为常数,与吸附剂表面均一性有关.Freundlich方程拟合参数如表3所示.结果发现,与原始土壤吸附能力相比(基于值比较),FJ、KX、FL土壤去除游离铁氧化物后对磷的吸附能力分别降低67.2%、71.5%、63.6%,一些学者也发现相似的研究结果,土壤中铁、铝氧化物增加有利于土壤对磷的吸附[23].然而,FJ、KX、FL土壤去除有机质后对磷的吸附能力较原始土壤仅分别降低15.6%、21.3%、28.6%.由于土壤有机质的去除过程伴随着部分铁氧化物的损失,导致土壤比表面积降低,因此,对值进行比表面积归一化后得到SSA,通过SPSS对SSA配对两样本检验得到,3种土壤去除有机质或游离铁氧化物后,值分别为0.017和0.004,均小于0.05,表明处理前后的土壤达到显著性差异.FJ、KX、FL土壤去除有机质后对磷的吸附能力(基于SSA值比较)较原始土壤仅分别降低0.5%、2.3%、6.5%,可知,消落带3种土壤去除有机质后造成的磷吸附差异主要是由于土壤比表面积的改变造成,其中有机质所起作用较小,这和一些文献报道结果相似,有机质对土壤吸附磷的能力没有影响或仅起到间接的作用[24-25],甚至有报道称有机质对金属氧化物吸附磷没有影响[8].然而,FJ、KX、FL土壤去除游离铁氧化物后对磷的吸附能力(基于SSA值比较)较原始土壤仍分别降低45.6%、51.7%、43.9%,说明游离铁氧化物在消落带3种土壤中对磷的吸附发挥着重要作用.
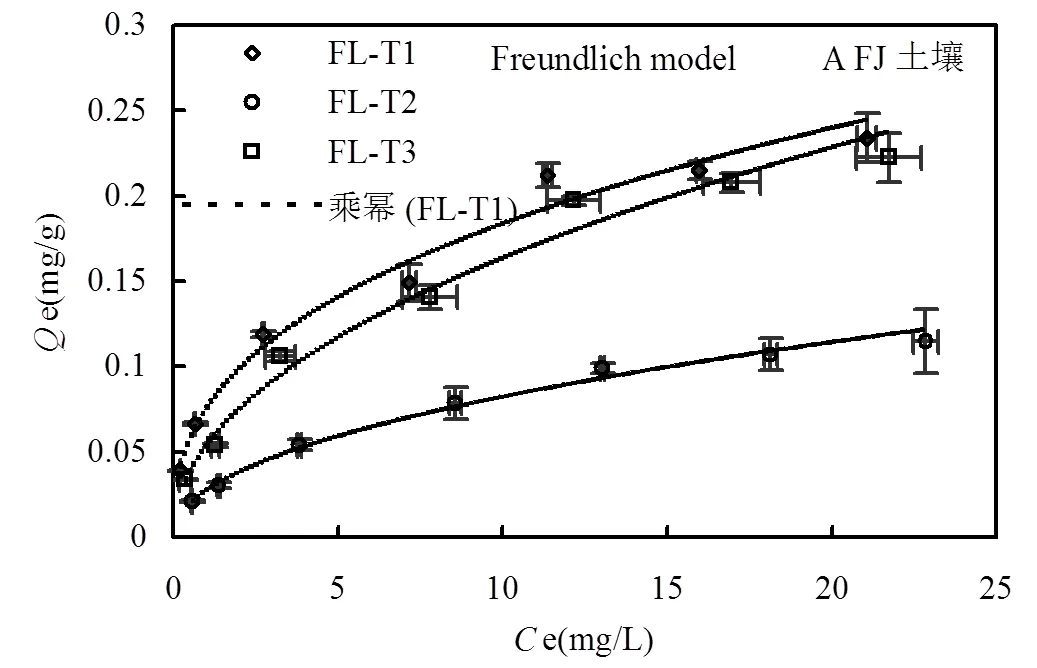
同时,1/越接近于0,说明吸附剂表面异质性越强,且1/值在0.1~0.5之间表明吸附剂有较强的吸附能力[26].由表3可知,Freundlich方程对三峡库区消落带3种土壤去除不同组分前后吸附磷数据拟合得到的1/值大小顺序均为:原始土壤<去除有机质土壤<去除游离铁氧化物土壤,结果同样表明原始土壤表面异质性更强,较去除不同组分的土壤有更强的磷吸附能力.此外,反应吸附能力大小的值与供试土壤基本性质的相关性结果也进一步说明上述结果(图3).值与TFe、SSA呈显著正相关关系,与Fed呈极显著正相关关系,但与有机质未发现有显著的相关关系.综上可知,三峡库区消落带3种土壤中有机质组分对磷吸附的影响较小,而土壤中游离铁氧化物组分是决定磷吸附大小的重要因素.
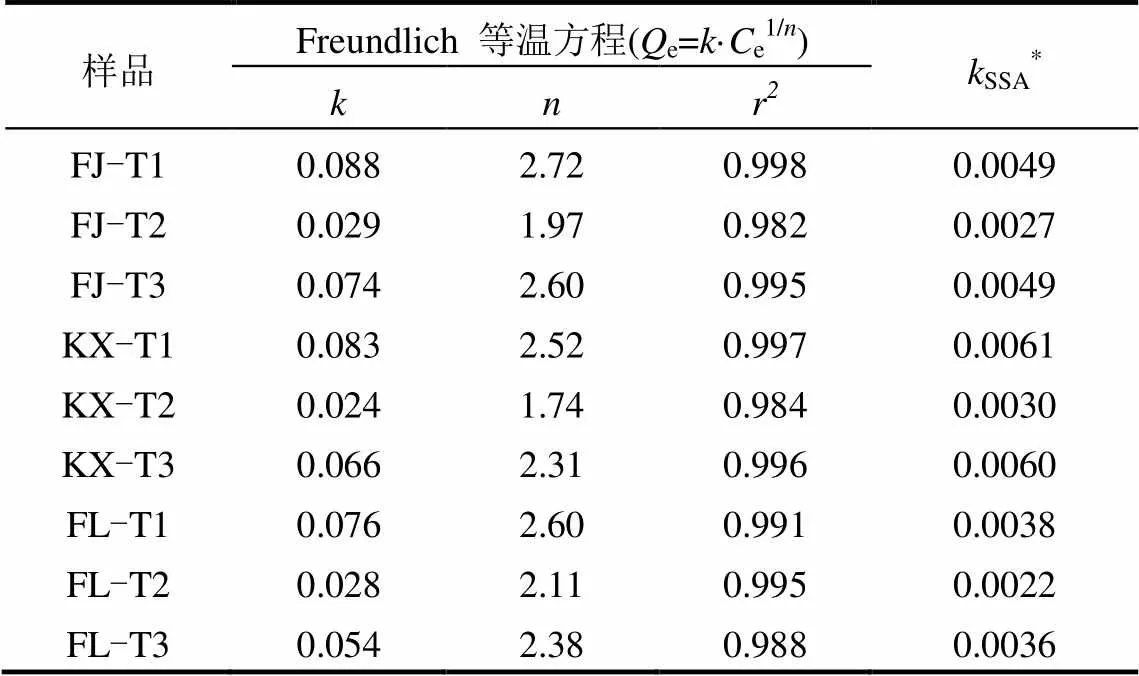
表3 Freundlich模型拟合参数 Table 3 Fitting parameters of Freundlich model
注:SSA=/SSA.
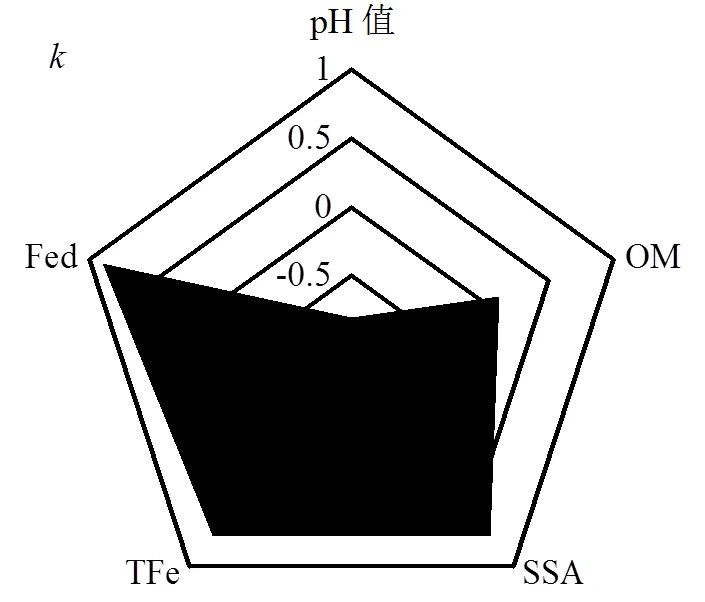
图3 Freundlich方程k值与土壤基本性质的相关性风玫瑰 Fig.3 Wind-rose diagrams of the correlations between k of Freundlich model and different soil constituents
2.4 土壤去除不同组分前后对磷的解吸
土壤磷解吸过程是磷吸附的逆过程,决定磷在土壤中的生物有效性,因此磷解吸过程较吸附过程更为重要[27-28].三峡库区消落带3种土壤去除不同组分前后的磷解吸过程如图4所示,所有供试土壤吸附的磷均出现部分解吸,且磷解吸量均随吸附量的增加而增加,与文献报道的其他研究区域结果一致[29-30].表4显示所有供试土壤的磷解吸量和磷吸附量均存在极显著的正相关关系(> 0.988,<0.01).同时发现线性方程能较好地对磷解吸量和磷吸附量数据进行拟合(2>0.977)(图4,表5),线性方程如下:
=´+(2)
式中:为磷解吸量,mg;为磷吸附量,mg;为斜率,反映解吸能力的大小,其值越大表示解吸能力越强;为截距,反应土壤本底释放磷的能力[23].线性拟合结果发现,消落带3种土壤去除游离铁氧化物后解吸磷的能力在3种处理中均最强(通过比较斜率得出),这可能是土壤中游离铁氧化物的去除导致磷酸盐与土壤的化学作用过程较少造成[31].另一方面,FL土壤去除有机质组分后较原始土壤的磷解吸能力略有降低,说明FL土壤中有机质含量越多有利于磷的解吸,这与一些学者的报道结果相似[32-33].然而,KX和FJ土壤去除有机质组分后较原始土壤的磷解吸能力无明显差异,说明KX和FJ土壤中有机质组分对磷解吸无影响.同时,由表5可知,值均为负数,而值能反应土壤本底释放磷的能力,表明不同处理的消落带3种土壤均有一定的磷吸附能力,3种土壤去除游离态铁氧化物后对磷的吸附能力最弱,3种土壤去除有机质后与原始土壤吸附磷的能力差异不大.综上,三峡库区消落带3种土壤中游离铁氧化物组分是控制磷解吸的重要因素,而土壤中有机质组分对磷解吸的影响与土壤类型有关.
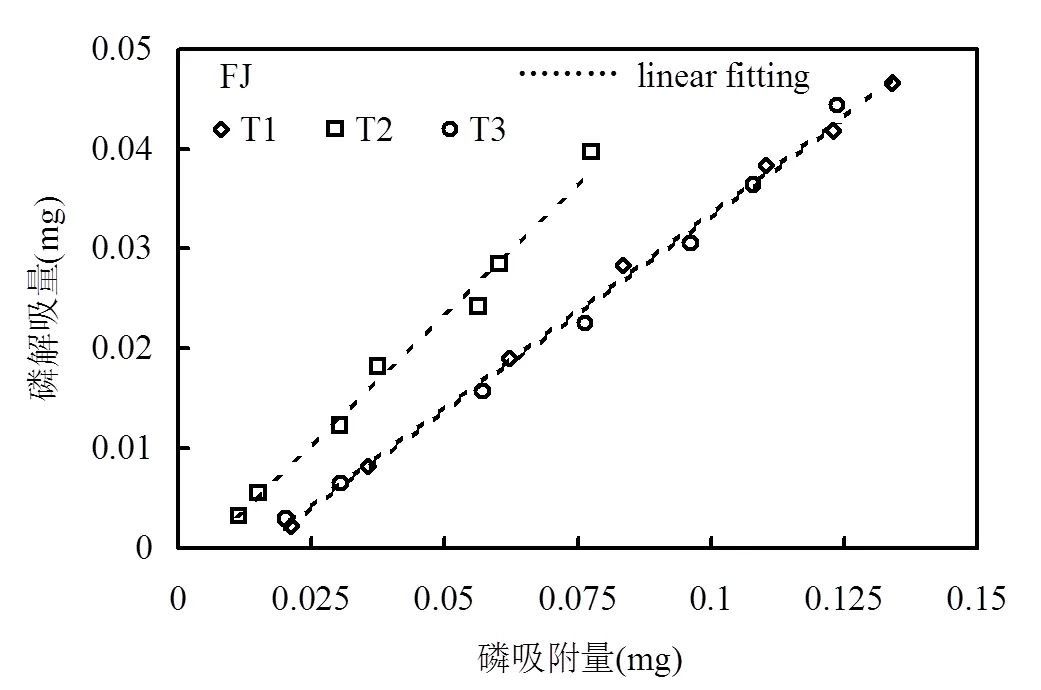
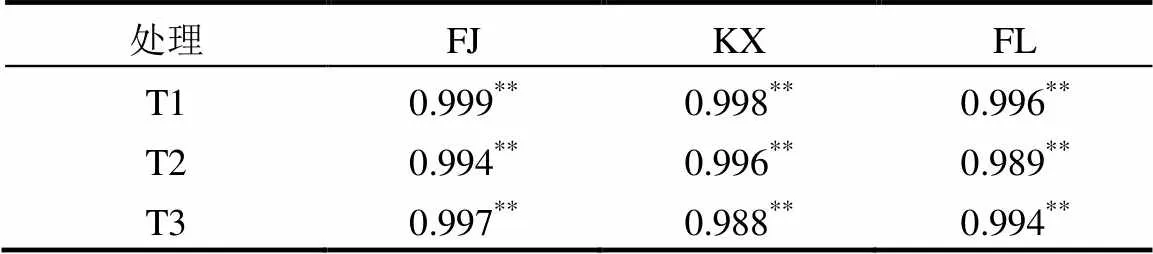
表4 供试土壤的磷解吸量和磷吸附量的相关关系 Table 4 Correlation results of sorbed phosphorus and phosphorus desorption in all original soils and subsamples with different pretreatments
注:**<0.01.
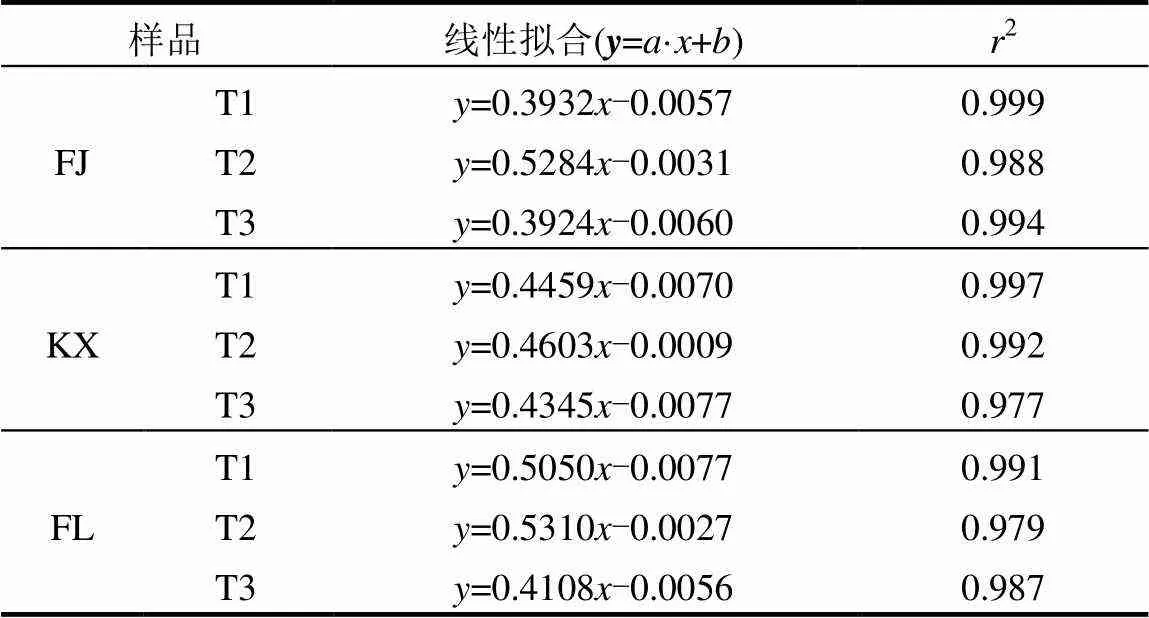
表5 供试土壤磷解吸量和磷吸附量的线性拟合参数结果 Table 5 The linear fitting results between sorbed phosphorus and phosphorus desorption in all original soils and subsamples with different pretreatments
2.5 环境意义
三峡库区消落带由于水位涨落将周期性的经历淹水和落干,消落带土壤每年将有3个月时间处于落干期.因此,部分距离人类活动近的落干土壤常被用于作物耕作,为提高作物的产量常施用有机肥,同时,部分作物秸秆也存在滞留土地的情况,此外,落干期消落带土壤还会生长出各种草类,以上过程都将导致消落带土壤有机质的输入.报道称,有机质(如生物质炭、枯枝落叶、城市堆肥等)的输入将降低土壤对磷的吸附,促使土壤中磷的释放[34-36].然而,本研究结果发现,三峡库区消落带土壤易氧化的有机质对土壤吸附和解吸磷的影响较小.另一方面,消落带土壤去除游离铁氧化物的过程在一定程度上能模拟铁氧化物由落干到淹水的还原溶解过程.结果表明,一旦三峡库区消落带土壤中铁锰等变价金属氧化物发生还原溶解,土壤中各种磷形态都将随着释放,尤其是Fe/Al-P,土壤对磷的吸附能力将大大降低.综上,本研究结果能较好地反应铁氧化物、有机质组分对三峡库区消落带土壤中磷吸附解吸的影响,进一步验证了消落带土壤由落干到淹水过程将导致活性磷的释放,增加库区水环境安全的风险.
3 结论
3.1 三峡库区消落带FJ、KX和FL土壤去除的有机质以易氧化的组分为主,去除有机质组分后,土壤中各种磷形态的含量变化较小,有机质含量与各种磷形态之间无显著相关关系.然而,3种土壤去除游离铁氧化物后,土壤中各种磷形态的含量均发生明显的降低.3种土壤去除有机质、游离铁氧化物组分后并未改变土壤中各种磷形态的相对大小顺序,均为:Ca-P>OP>Fe/Al-P.
3.2 FJ、KX、FL3种土壤去除有机质组分后对磷吸附的影响较小,而土壤去除游离铁氧化物组分后对磷的吸附能力明显降低,表明游离铁氧化物是决定土壤吸附磷的重要因素.
3.3 FJ、KX、FL3种土壤去除游离铁氧化物后较原始土壤吸附磷的解吸能力明显增加,表明游离铁氧化物组分是控制3种土壤吸附磷的解吸的重要因素.
3.4 FL土壤去除有机质组分后较原始土壤吸附磷的解吸能力略有降低,而KX和FJ土壤去除有机质组分后较原始土壤吸附磷的解吸能力无明显差异,表明有机质组分对土壤吸附磷的解吸的影响与土壤类型有关.
[1] Jin X, He Y, Kirumba G, et al. Phosphorus fractions and phosphate sorption-release characteristics of the sediment in the Yangtze River estuary reservoir [J]. Ecological Engineering, 2013,55:62-66.
[2] Wang X, Li W, Harrington R, et al. Effect of ferrihydrite crystallite size on phosphate adsorption reactivity [J]. Environmental Science and Technology, 2013,47(18):10322-10331.
[3] Yan J, Jiang T, Yao Y, et al. Preliminary investigation of phosphorus adsorption onto two types of iron oxide-organic matter complexes [J]. Journal of Environmental Sciences, 2016,42(4):152-162.
[4] Zhang Y, Lin X, Werner W. The effect of soil flooding on the transformation of Fe oxides and the adsorption/desorption behavior of phosphate [J]. Journal of Plant Nutrition and Soil Science, 2003,166(1): 68-75.
[5] 李璐璐.三峡库区消落带土壤及沉积物中磷素分布与赋存特征研究 [D]. 重庆:西南大学, 2014.Li lulu. Characteristics of phosphorus distribution and occurrence in soils and sediments of the water-level fluctuation zone in the Three Gorges Reservoir Areas [D]. Chongqing:Southwest University, 2014.
[6] 朱 强.三峡库区消落带土壤磷吸附特性及淹水-落干周期下的变迁 [D]. 武汉:华中农业大学, 2012.Zhu Qiang. Phosphorus adsorption and transition by flooding-drain cycle in tidal zone soils of the Three Gorges Reservoir [D]. Wuhan: Central China Agricultural University, 2012.
[7] 郭 念.三峡库区消落带典型土壤铁还原行为对磷释放的影响 [D]. 重庆:西南大学, 2014.Guo Nian. Effect of iron reduction on phosphorus release in the typical soil during the flooding in water-level fluctuating zone of Three Gorges Reservoir region [D]. Chongqing: Southwest University, 2014.
[8] Borggaard O K, Raben-Lange B, Gimsing A L, et al. Influence of humic substances on phosphate adsorption by aluminium and iron oxides [J]. Geoderma, 2005,127(3):270-279.
[9] Antelo J, Fiol S, Pérez C, et al. Analysis of phosphate adsorption onto ferrihydrite using the CD-MUSIC model [J]. Journal of colloid and interface science, 2010,347(1):112-119.
[10] 曹 琳.三峡库区消落带水-沉积物界面磷干湿交替分布特征及转化机理研究 [D]. 重庆:重庆大学, 2011.Cao Lin. The distribution characteristics and transformation mechanism of phosphorus research on water/sediments wet-dry alternation in water level fluctuating zone of Three Gorges Reservoir area [D]. Chongqing:Chongqing University, 2011.
[11] Ololade I A, Oladoja N A, Alomaja F, et al. Influence of organic carbon and metal oxide phases on sorption of 2, 4, 6-trichlorobenzoic acid under oxic and anoxic conditions [J]. Environmental Monitoring and Assessment, 2015,187(1):4170-4185.
[12] Kasozi G N, Nkedi-Kizza P, Li Y, et al. Sorption of atrazine and ametryn by carbonatic and non-carbonatic soils of varied origin [J]. Environmental Pollution, 2012,169(15):12-19.
[13] 郭松松.三峡库区消落带磷赋存形态及吸附/释放规律研究 [D]. 重庆:重庆大学, 2012.Guo Songsong. Phosphorus fractions and phosphate sorption-release characteristics of the surface soil in water-level-fluctuating zone of Three Gorges Reservoir [D]. Chongqing:Chongqing University, 2012.
[14] Mikutta R, Kleber M, Kaiser K, et al. Review: Organic matter removal from soils using hydrogen peroxide, sodium hypochlorite, and disodium peroxodisulfate [J]. Soil Science Society of America Journal, 2005,69(1):120-135.
[15] Li Z, Huang B, Huang J, et al. Influence of removal of organic matter and iron and manganese oxides on cadmium adsorption by red paddy soil aggregates [J]. RSC Advances, 2015,5(110):90588-90595.
[16] Ruban V, López-Sánchez J F, Pardo P, et al. Harmonized protocol and certified reference material for the determination of extractable contents of phosphorus in freshwater sediments-a synthesis of recent works [J]. Fresenius Journal of Analytical Chemistry, 2001,370(2/3): 224-228.
[17] 鲍士旦.土壤农化分析(第三版) [M]. 北京:中国农业出版社, 2000: 30-35;70-97.Bao Shidan. Soil agrochemical analysis (Third Edition) [M]. Beijing: China Agriculture Press, 2000:30-35;70-97.
[18] 张甘霖,龚子同.土壤调查实验室分析方法 [M]. 北京:科学出版社, 2012:126-128;156. Zhang Ganlin, Gong Zitong. Soil survey laboratory analysis method [M]. Beijing:Science Press, 2012:126-128;156.
[19] 水和废水监测分析方法 [M]. 4版.北京:中国环境科学出版社, 2002:243-246. Water and wastewater monitoring and analysis methods (Fourth Edition) [M]. Beijing:China Environmental Science Press, 2002:243- 246.
[20] Kaiser K, Guggenberger G. The role of DOM sorption to mineral surfaces in the preservation of organic matter in soils [J]. Organic Geochemistry, 2000,31(7/8):711-725.
[21] Katsaounos C Z, Giokas D L, Leonardos I D, et al. Speciation of phosphorus fractionation in river sediments by explanatory data analysis [J]. Water Research, 2007,41(2):406-418.
[22] Zhang B, Fang F, Guo J, et al. Phosphorus fractions and phosphate sorption-release characteristics relevant to the soil composition of water-level-fluctuating zone of Three Gorges Reservoir [J]. Ecological Engineering, 2012,40:153-159.
[23] Tang X, Wu M, Li Q, et al. Impacts of water level regulation on sediment physic-chemical properties and phosphorus adsorption- desorption behaviors [J]. Ecological Engineering, 2014,70:450-458.
[24] Kang J, Hesterberg D, Osmond D L. Soil organic matter effects on phosphorus sorption: a path analysis [J]. Soil Science Society of America Journal, 2009,73(2):360-366.
[25] Afif E, Barrón V, Torrent J, et al. Organic matter delays but does not prevent phosphate sorption by cerrado soils from Brazil [J]. Soil Science, 1995,159(3):207-211.
[26] Lu L, Gao M, Gu Z, et al. A comparative study and evaluation of sulfamethoxazole adsorption onto organo-montmorillonites [J]. Journal of Environmental Sciences, 2014,26(12):2535-2545.
[27] Wang L, Tao L. Effects of exogenous rare earth elements on phosphorus adsorption and desorption in different types of soils [J]. Chemosphere, 2014,103(5):148-155.
[28] Sharpley A N, Mcdowell R W, Kleinman P J A. Phosphorus loss from land to water: integrating agricultural and environmental management [J]. Plant and Soil, 2001,237(2):287-307.
[29] Zou P, Fu J, Cao Z. Chronosequence of paddy soils and phosphorus sorption–desorption properties [J]. Journal of Soils and Sediments, 2011,11(2):249-259.
[30] Xia Y, Lou Y S, Yang C G, et al. Characteristics of phosphate adsorption and desorption in paddy soils [J]. Scientia Agricultura Sinica, 2002,35(11):1369-1374.
[31] Lai D Y F, Lam K C. Phosphorus sorption by sediments in a subtropical constructed wetland receiving stormwater runoff [J]. Ecological Engineering, 2009,35(5):735-743.
[32] McDowell R, Condron L. Influence of soil constituents on soil phosphorus sorption and desorption [J]. Communications in Soil Science and Plant Analysis, 2001,32(15/16):2531-2547.
[33] 王艳玲.吉林玉米带黑土磷素形态及吸附-解吸特性研究 [D]. 吉林:吉林农业大学, 2004.Wang Yanling. Study on the forms of phosphorus and characteristics of adsorption-desorption of the corn belt Phaeozem in Jilin [D]. Jinling:Jinling Agricultural University, 2004.
[34] Hosseinpur A R, Kiani S, Halvaei M. Impact of municipal compost on soil phosphorus availability and mineral phosphorus fractions in some calcareous soils [J]. Environmental Earth Sciences, 2012,67(1):91-96.
[35] Schreeg L A, Mack M C, Turner B L. Leaf litter inputs decrease phosphate sorption in a strongly weathered tropical soil over two time scales [J]. Biogeochemistry, 2012,113(1-3):507-524.
[36] Xu G, Sun J N, Shao H B, et al. Biochar had effects on phosphorus sorption and desorption in three soils with differing acidity [J]. Ecological Engineering, 2014,62:54-60.
Effect of organic matter and iron oxides on phosphorus forms and adsorption-desorption on dry-period soils in the water- level-fluctuating zone of the Three Gorges Reservoir.
YAN Jin-long1,2*, WU Wen-li1, JIANG Tao2, WEI Shi-qiang2
(1.College of Pharmacy and Biological Engineering, Chongqing University of Technology, Chongqing 400054, China;2.College of Resources and Environment, Southwest University, Chongqing 400716, China)., 2019,39(3):1124~1131
Selective removal of organic matter or iron oxide from three typical dry-period soils were explored to investigate its direct effect on P fractions and adsorption-desorption behavior in the water-level-fluctuating zone (WLFZ) of the Three Gorges Reservoir (TGR). The data showed that kinds of P fractions in three dry-period soils were not significant decreased with removal of readily oxidizable organic matter. However, kinds of P fractions were significantly decreased with removal of free iron oxides in the three soils. Notably, different P fractions were both in the order as follows: Ca-P > OP > Fe/Al-P, before and after removal of organic matter or free iron oxides in the three soils. Moreover, after removal of organic matter, the adsorption capacity of yellow soil (FJ), purple alluvial soil (KX), grey brown purple soil (FL) for P was only decreased by 0.5%, 2.3%, 6.5%(=0.017<0.05, significant difference), respectively, which indicated that P adsorbed on the three soils were little influenced by organic matter. In addition, after removal of free iron oxides, the adsorption capacity of FJ, KX, FL soil for P was significantly decreased by 45.6%, 51.7%, 43.9%(=0.004<0.05, significant difference), respectively, which revealed that P adsorbed on the three soils were dominated by free iron oxides. More importantly, the desorption capacity of three soils for P was increased after removal of free iron oxides, which presented that free iron oxides were also the predominant factor to control desorption behavior of freshly sorbed P. Then, the desorption capacity of FL for P was little decreased after removal of organic mater, and there were no distinction for it before and after removal of organic matter in KX and FJ soils, which showed that the desorption capacity of three soils for P were influenced by organic matter related to soil category.
water-level-fluctuating zone (WLFZ) of the Three Gorges Reservoir (TGR);dry-period soil;phosphorus;organic matter;iron oxide
X142
A
1000-6923(2019)03-1124-08
闫金龙(1989-),男,四川渠县人,博士,讲师,主要研究方向为环境污染化学.发表论文20余篇.
2018-08-13
国家自然科学基金资助项目(41171198,41403079);重庆理工大学科研启动基金(0110170340)
* 责任作者, 讲师, yanjinlong6439@126.com

