Inhibition of α5 GABAA receptors has preventive but not therapeutic effects on iso flurane-induced memory impairment in aged rats
Zi-Fang Zhao , Lei Du , Teng Gao, Lin Bao Yuan Luo, Yi-Qing Yin , Yong-An Wang,
1 Department of Anesthesiology, China-Japan Friendship Hospital, Beijing, China
2Department of Radiology, China-Japan Friendship Hospital, Beijing, China
3 Department of Anesthesiology, Beijing Shijitan Hospital Affiliated to Capital Medical University, Beijing, China
4 Institute of Pharmacology and Toxicology, Academy of Military Medical Sciences, Beijing, China
AbstractThe α5 subunit-containing gamma-amino butyric acid type A receptors (α5 GABAARs) are a distinct subpopulation that are specifically distributed in the mammalian hippocampus and also mediate tonic inhibitory currents in hippocampal neurons. These tonic currents can be enhanced by low-dose iso flurane, which is associated with learning and memory impairment. Inverse agonists of α5 GABAARs, such as L-655,708, are able to reverse the short-term memory deficit caused by low-dose iso flurane in young animals. However, whether these negative allosteric modulators have the same effects on aged rats remains unclear. In the present study, we mainly investigated the effects of L-655,708 on low-dose (1.3%) iso flurane-induced learning and memory impairment in elderly rats. Young (3-month-old) and aged(24-month-old) Wistar rats were randomly assigned to receive L-655,708 0.5 hour before or 23.5 hours after 1.3% iso flurane anesthesia.The Morris Water Maze tests demonstrated that L-655,708 injected before or after anesthesia could reverse the memory deficit in young rats. But in aged rats, application of L-655,708 only before anesthesia showed similar effects. Reverse transcription-polymerase chain reaction showed that low-dose iso flurane decreased the mRNA expression of α5 GABAARs in aging hippocampal neurons but increased that in young animals. These findings indicate that L-655,708 prevented but could not reverse 1.3% iso flurane-induced spatial learning and memory impairment in aged Wistar rats. All experimental procedures and protocols were approved by the Experimental Animal Ethics Committee of Academy of Military Medical Science of China (approval No. NBCDSER-IACUC-2015128) in December 2015.
Key Words: iso flurane; postoperative cognitive dysfunction; hippocampus; inverse agonist; α5 GABAA receptors; L-655,708; aged; Morris Water Maze; memory impairment; neural regeneration
Introduction
Memory blockade and amnesic effects are important results of general anesthesia, and the majority of these effects disappear with the rapid elimination of general anesthetics.Unfortunately, learning and memory deficits, referred to as postoperative cognitive dysfunction (POCD) (Cascella and Bimonte, 2017; Gao et al., 2017), may persist for months or can be permanent in patients following surgery and general anesthesia (Newman et al., 2001; Johnson et al., 2002; Gao et al., 2017). Advanced age is a recognized risk factor of POCD(Liu et al., 2018; Monk et al., 2008). Despite improvements in perioperative care, a great number of elderly surgical patients experience POCD, which is associated with poor postoperative outcome and increased mortality (Moller et al., 1998; Gurlit and Möllmann, 2008; Coburn et al., 2010).Accordingly, the exploration of potential preventive or therapeutic methods for POCD in the aged population is of particular significance.
The gamma-amino butyric acid type A receptors (GAB-AARs) are critical inhibitory ion channel receptors in the mammalian central nervous system. Most intravenous and inhaled anesthetics cause memory blockade and amnesia by acting as positive allosteric modulators of GABAARs (Rudolph and Antkowiak, 2004).
α5 Subunit-containing GABAARs (α5 GABAARs) are a distinct subpopulation that are specifically distributed in the extrasynaptic area of hippocampal CA1 pyramidal neurons(Christie and de Blas, 2002; Serwanski et al., 2006), and their activation generates a specific tonic inhibitory current(Caraiscos et al., 2004b; Glykys and Mody, 2006; Chen et al.,2017). Zurek et al. (2014) found that etomidate increased the tonic current generated by α5 GABAARs and cell-surface expression of α5 GABAARs for at least 1 week. This increase in tonic current impaired learning memory. Consistently,Lecker et al. (2013) demonstrated that inverse agonists of α5 GABAARs, L-655,708 and MRK-016, alleviated the overactivity evoked by low-dose inhalational anesthetics. Pretreatment with L-655,708 fully prevented the iso flurane-induced memory deficits in mice aged 2-3 months. Therefore, hippocampal α5 GABAARs may be a potential target for postoperative memory impairment (Saab et al., 2010).
However, most existing studies of α5 GABAAR inverse agonists are limited to young animals, and relevant studies have scarcely focused on aging subjects. The aged population is more susceptible than the young to anesthetic-induced cognitive dysfunction and neurological damage (Magnusson et al., 2000; Wang et al., 2000; Culley et al., 2003; Jansson et al., 2004). For example, iso flurane and propofol affected postnatal hippocampal neurogenesis and cell proliferation in an age-dependent manner, and the alterations were significant in aged animals (Erasso et al., 2012, 2013). Therefore, whether the anesthesia-induced changes in hippocampal α5 GABAAR function are similar between young and aged animals and whether inverse agonists of α5 GABAARs have similar effects on postanesthetic memory impairments in aged and young subjects are issues that must be investigated. In the current study, we explored the effects of L-655,708, an inverse agonist of α5 GABAARs, on low-dose isoflurane-induced memory impairments in elderly rats. In addition, we investigated whether hippocampal α5 GABAARs are still a potential target for attenuating POCD in the aged population.
Materials and Methods
Animals
Male specific pathogen-free (SPF) Wistar rats, aged 3 months (average body weight 373.1 g) and 24 months (average body weight 790.5 g) provided by Vital River Lab Animal Technology Co., Ltd., China (animal license No.: SCXK(Jing) 2012-0001) were included in this study. The rats were housed separately in cages under standard laboratory conditions (lights on at 7:00 a.m.; lights off at 7:00 p.m., 22°C, 60%humidity) and were free to water and food.
All experimental procedures and protocols were approved by the Experimental Animal Ethics Committee of Academy of Military Medical Science of China (approval No. NBCDSER-IACUC-2015128) in December 2015. All experimental procedures described here were in accordance with the National Institutes of Health (NIH) guidelines for the Care and Use of Laboratory Animals (NIH Publication No. 85-23,revised 1996).
Grouping
Both young (3-month-old) and aged (24-month-old) rats were randomly assigned to different treatment groups (n= 8) (Figure 1). Rats in the control group inhaled a mixture of oxygen and air (30% oxygen) for 1 hour. Rats in the ISO24group were anesthetized by inhaling 1.3% isoflurane(purchased from Hebei Yipin Pharmaceutical Co., Ltd.,Hebei Province, China) for 1 hour. Rats in the pre-L group received L-655,708 (0.7 mg/kg in 10% dimethyl sulfoxide(DMSO) 2 μL/g) subcutaneously 30 minutes before exposure to 1.3% iso flurane for 1 hour. Rats in the post-L group received L-655,708 subcutaneously 23.5 hours after 1.3%isoflurane exposure. Rats in these four groups were tested in a spatial probe trial at 24 hours after iso flurane exposure.Rats in the ISO72 group were exposed to 1.3% isoflurane for 1 hour and then tested in the spatial probe trial at 72 hours after isoflurane exposure. We also tested the effects of DMSO on cognitive function in a separate experiment in which some rats (aged 3 months, n = 7) received subcutaneous injection of isovolumetric 10% DMSO.
L-655,708
L-655,708 (ethyl (13aS)-7-methoxy-9-oxo-11,12,13,13 atetrahydro-9H-imidazo [1, 5-a]pyrrolo[2,1c][1,4]benzodiazepine-1-carboxylate) used in the present trial was obtained from Sigma-Aldrich Co., St. Louis, MO, USA. For subcutaneous injection, we dissolved crystalline L-655,708 in 10% DMSO.
Anesthesia
Rats in the anesthesia and intervention groups were placed in an anesthetizing apparatus perfused with 30% oxygen [O2]in air with or without 1.3% iso flurane delivered at a rate of 1 L/min. The real-time concentrations of iso flurane, oxygen,and carbon dioxide in the anesthetizing chamber were detected with a commercial Datex-Ohmeda Compact S/5 monitor(Datex-Ohmeda, Inc., Madison, WI, USA), and gas flow was regulated to maintain the desired concentrations. A warming blanket was used to avoid hypothermia during anesthesia. After treatment, the rats were removed from the chamber and allowed to recover in another heated clear chamber for 45 minutes before they were returned to the home cage. There was no mortality during or after the course of animal anesthesia.
Morris water maze test
The experimenters who performed the behavioral tests were blinded to group information. The water maze (Shanghai Xinruan Information Technology Co., Ltd., Shanghai, China) consisted of a circular pool (diameter, 150 cm; depth,50 cm) filled with opaque water (kept at 22°C). Rats were repeatedly trained to swim from the water to a round escape platform (2.0 cm beneath the surface). The pool area was artificially separated into four imaginary quadrants: a, b, c and d, with quadrant c as the target quadrant in the present study. The swimming motions of the animals were automatically recorded by a video computerized tracking system.The acquired data were processed using specific software for the Morris water maze (Shanghai Xinruan Information Technology Co., Ltd., Shanghai, China).
The Morris water maze test consists of place navigation trial and spatial probe trial. The place navigation trial helps animals to acquire specific spatial reference memory and the spatial probe trial tests the retention of acquired spatial memory. During the place navigation trial, each rat underwent four trials per day for 4 consecutive days. During the trial, each rat was gently put into the water from a stationary starting point and given 60 seconds to find the hidden platform. If the rat could not find the target within the designated time, the experimenter would gently guide it to get up the platform and stay there for 30 seconds. The rats that successfully found the target also stayed on the platform for 30 seconds before they were removed. Swim speed, the time spent to find the platform (escape latency), and the distance traveled were recorded. Escape latency is the primary indicator of spatial learning capability; shorter escape latency represents a better spatial learning ability.
Following the place navigation trial, rats received oxygen-air mixture, iso flurane anesthesia or L-655,708 according to the interventions designated for each group (Figure 1).Then spatial probe trials were conducted 24 hours (rats in the control, ISO24, pre-L, and post-L groups) or 72 hours (rats in the ISO72group) after iso flurane anesthesia or sham to assess the in fluences of different treatments on the retention of acquired spatial memory of rats. In the spatial probe trial, the escape platform was removed, and rats were placed in the opposite quadrant (quadrant a) of the original target quadrant(quadrant c). Each rat was given 60 seconds to swim freely.The time spent for the first arrival at the original platform location (escape latency) and the time spent in quadrant c were acquired and used to evaluate memory retention. Shorter escape latency and/or longer time spent in quadrant c represent encouraging retention of spatial learning memory.
Reverse transcription-polymerase chain reaction (RT-PCR)
RT-PCR was performed to investigate the influence of isoflurane and L-655,708 on the mRNA expression of α5 GABAARs in the hippocampus. Following behavioral tests,rats were sacrificed, and hippocampal tissue was obtained and stored at -80°C immediately. Then the lysed tissue was homogenized by a homogenizer (Qiagen, Inc., Valencia,CA, USA) and then centrifuged. Total RNA was acquired from the supernatant and its concentration was measured using a Nano Drop ND-1000 Spectrophotometer (Thermo Scientific, Wilmington, DE, USA). α5 GABAAR and β-actin primers were purchased from Tianyi Biotech, Beijing, China and sequence information was shown in Table 1. RT-PCR was conducted using the real-time PCR system with SYBR®Premix (TaKaRa, Inc., Dalian, China). α5 GABAAR mRNA levels were standardized to β-actin levels as internal controls.PCR was conducted by 40 cycles at 96 °C for 30 seconds, 55°C for 90 seconds, and 72°C for 60 seconds in a BioRad CFX96™Real-Time System C1000TMThermal Cycler (BioRad Laboratories, Shanghai, China). Threshold cycle (Ct) values were recorded and tested. The Ct values were defined as the amplification cycles when the fluorescent signal in the reaction system significantly exceeded the background fluorescence(threshold). The Ct values were negatively related to the tested mRNA level. Consequently, a lower Ct reflects a higher mRNA level (Dhar et al., 2002; Park et al., 2015).

Table 1 Details of the primers used for RT-PCR
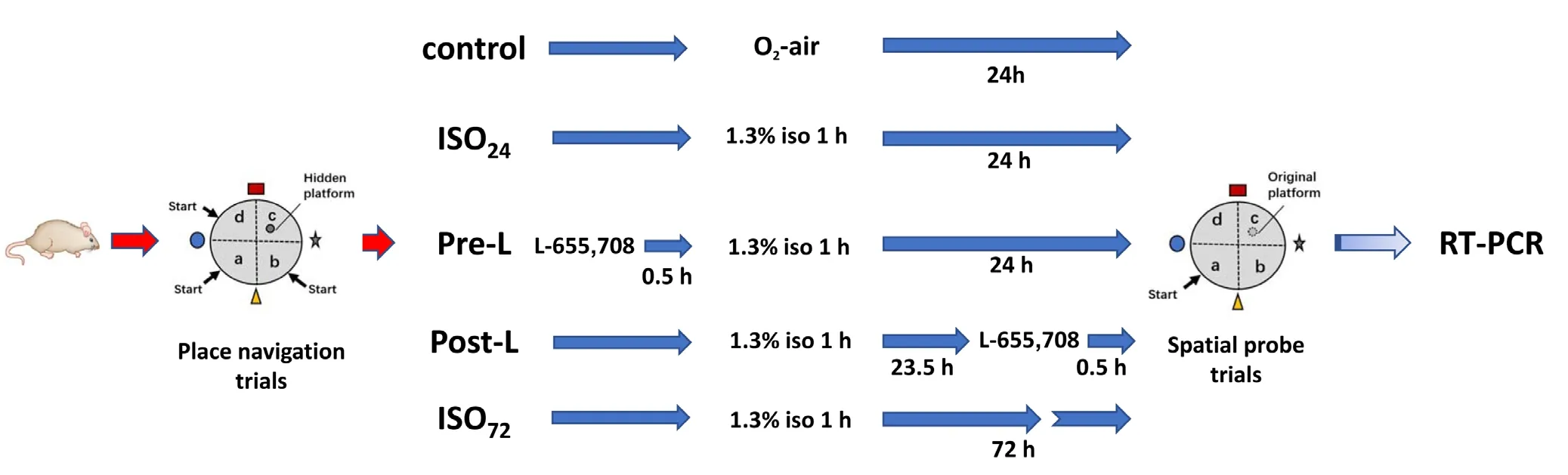
Figure 1 Experimental paradigm timeline.
Statistical analysis
All measurements were presented as the mean ± SD and analyzed using SPSS 14.0 software (SPSS Inc., Chicago, IL,USA). The escape latency and swimming speed during the place navigation trials were analyzed by repeated measures one-way analysis of variance. Using two-way analysis of variance, age and treatment interactions in the spatial probe trials and RT-PCR were analyzed. Spatial probe trials and RT-PCR results were statistically compared between experimental and control groups using one-way analysis of variance followed by Tukey's post hoc test. A level of P < 0.05 was considered statistically significant.
Results
Age and treatment interactions
Two-way analysis of variance and RT-PCR assay (escape latency and time c were evaluated separately) revealed significant differences in spatial probe trial between two age groups (P < 0.001 for all) and among different treatment groups (P < 0.001 for all), but there was no significant difference in age and treatment interactions (P > 0.05 for all).One-way analysis of variance with Turkey's post hoc test was conducted for young cohort or aged cohort separately.
Rat performance in the place navigation trials
In the place navigation trial, both young and aged rats finished the learning task successfully, which was evidenced by gradually decreased escape latencies across the 4 days of training (Figure 2; P < 0.05). Consistent with the results of a previous study (Saab et al., 2010), DMSO injection had no impact on the acquisition or storage of spatial memory as demonstrated by the similar performance in the place navigation trial between DMSO-injected and control rats.
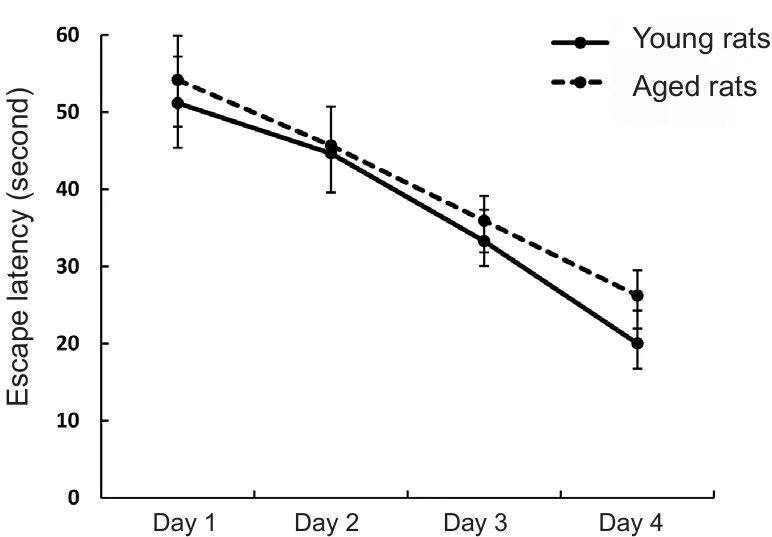
Figure 2 Performance of young (3 months old) and aged (24 months old) rats in the place navigation trials.
Effects of iso flurane and L-655,708 on spatial memory performance of rats in the spatial probe trial
Twenty-four hours after anesthesia, young rats showed significantly prolonged escape latency and decreased time c than control rats (Figure 3A and B; P < 0.01). However, at 72 hours after anesthesia, there was no significant difference in escape latency or time c between control and ISO72groups(Figure 3A and B). These data indicate that rats exposed to isoflurane exhibited impaired spatial memory at 24 hours after anesthesia but not at 72 hours. The escape latency and time c in the pre-L and post-L groups were not significantly different from those in the control group (Figure 3C and D),but the escape latency was shorter and time c was longer in these groups when compared to the ISO24group (Figure 3C and D). These data indicate that subcutaneous administration of L-655,708 has preventive and therapeutic effects on isoflurane-induced spatial memory deficits. Escape latency was prolonged and time c was shortened in aged rats than in control rats, suggesting that spatial memory impairment occurred 24 hours after exposure to isoflurane (Figure 4A and B; P < 0.001). Rats in the ISO72group showed increased escape latency and decreased time c compared to control animals (Figure 4A and B; P < 0.01), indicating that the memory impairment of aged rats persisted at 72 hours after anesthesia. Escape latency was shorter and time c was longer in the pre-L group than in the ISO24group (P < 0.05) and there were no significant differences in escape latency and time c between pre-L and control groups (Figure 4C and D;P > 0.05). These suggest that subcutaneous pretreatment with L-655,708 prevented iso flurane-induced memory deficits.
In contrast, administration of L-655,708 at 23.5 hours after iso flurane anesthesia did not affect the escape latency or time c during the search for the escape platform. In the post-L group,rats showed a significantly longer escape latency and shorter time c than control rats (Figure 4C and D; P < 0.01 and P <0.001), indicating that administration of L-655,708 at 23.5 hours after anesthesia had no effects on impaired memory.
Effects of iso flurane and L-655,708 on rat hippocampal α5 GABAAR expression as assessed by RT-PCR
In young rats, α5 GABAAR expression in the hippocampus was significantly increased 24 hours after anesthesia compared to that in the control rats but returned to control level at 72 hours after anesthesia (Figure 5A; P < 0.001 and P >0.05). There was no significant difference in α5 GABAAR expression between control and pre-L groups (Figure 5B;P > 0.05), indicating that treatment with L-655,708 at 0.5 hours prior to isoflurane exposure prevented the change in hippocampal α5 GABAAR expression. Furthermore, in young rats, α5 GABAAR expression in the post-L group was not significantly different from that in the ISO24group (Figure 5B; P > 0.05). In the aged groups, α5 GABAAR mRNA expression in the hippocampus was significantly lower at 24 hours after 1.3% isoflurane anesthesia than that in the control group, and this trend persisted at least 72 hours after anesthesia (Figure 5A; P < 0.05).
Similarly, there was no significant difference in hippocampal α5 GABAAR mRNA expression between aged control and aged pre-L groups (Figure 5B; P > 0.05). These observations indicate that prophylactic administration of L-655,708 was also able to prevent the alterations in α5 GABAAR expression in the aged hippocampus. No significant difference in hippocampal α5 GABAAR mRNA expression was observed between aged ISO24and post-L groups (Figure 5B; P > 0.05).
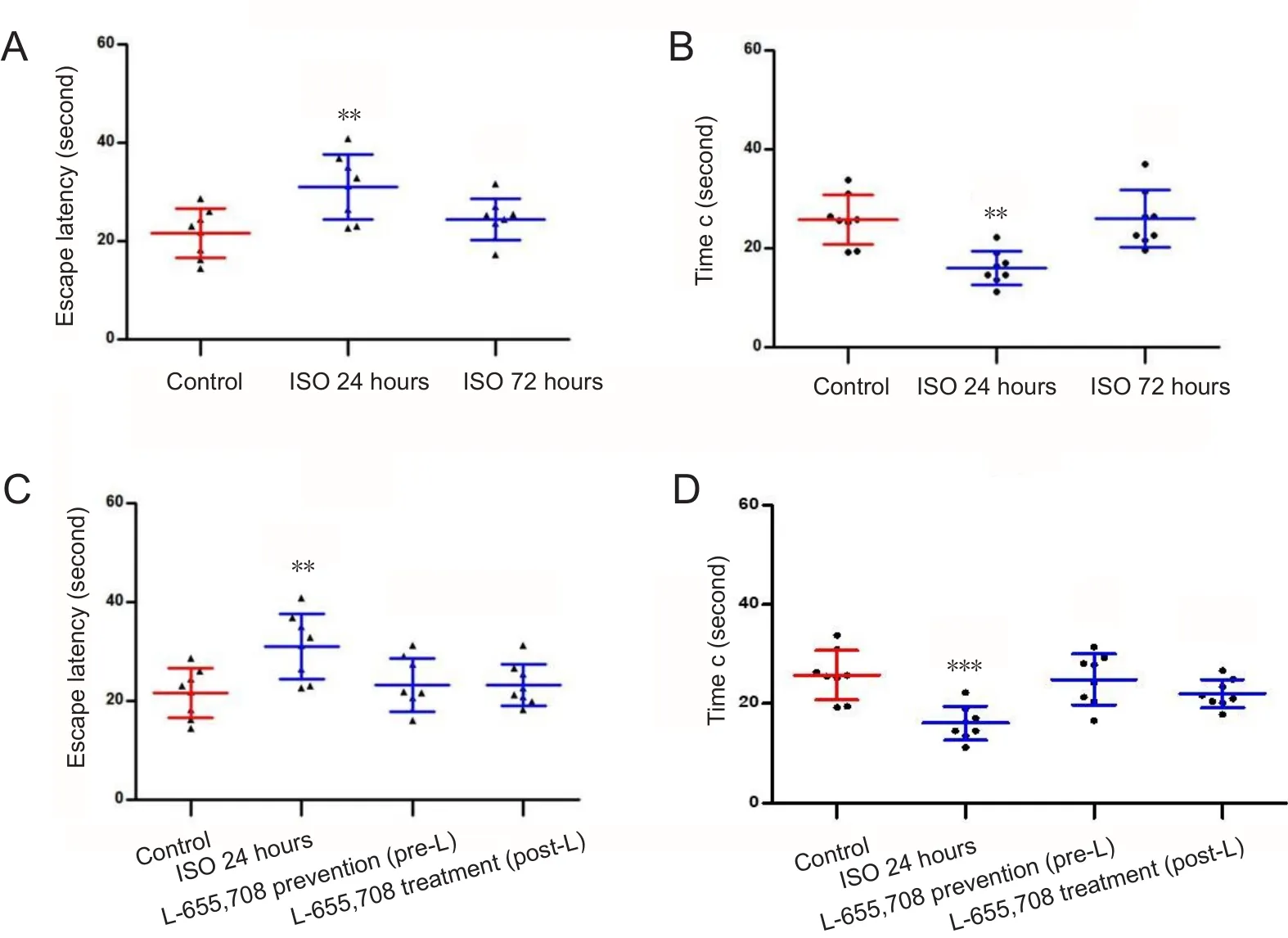
(A, B) Effects of iso flurane (ISO)on the escape latency (A) and time c (B) of young rats in the spatial probe trials. (C, D) Effects of L-655,708 on the escape latency (C) and time c (D) of young rats in the spatial probe trials. (D) Effects of L-655,708 on the time c of young rats in the spatial probe trials. **P < 0.01,***P < 0.001, vs. control group.Data are expressed as the mean± SD (8 rats in each group, oneway analysis of variance with Turkey's post hoc test). Escape latency: The time spent before the first arrival at the original platform location; time c: the time spent in quadrant c.
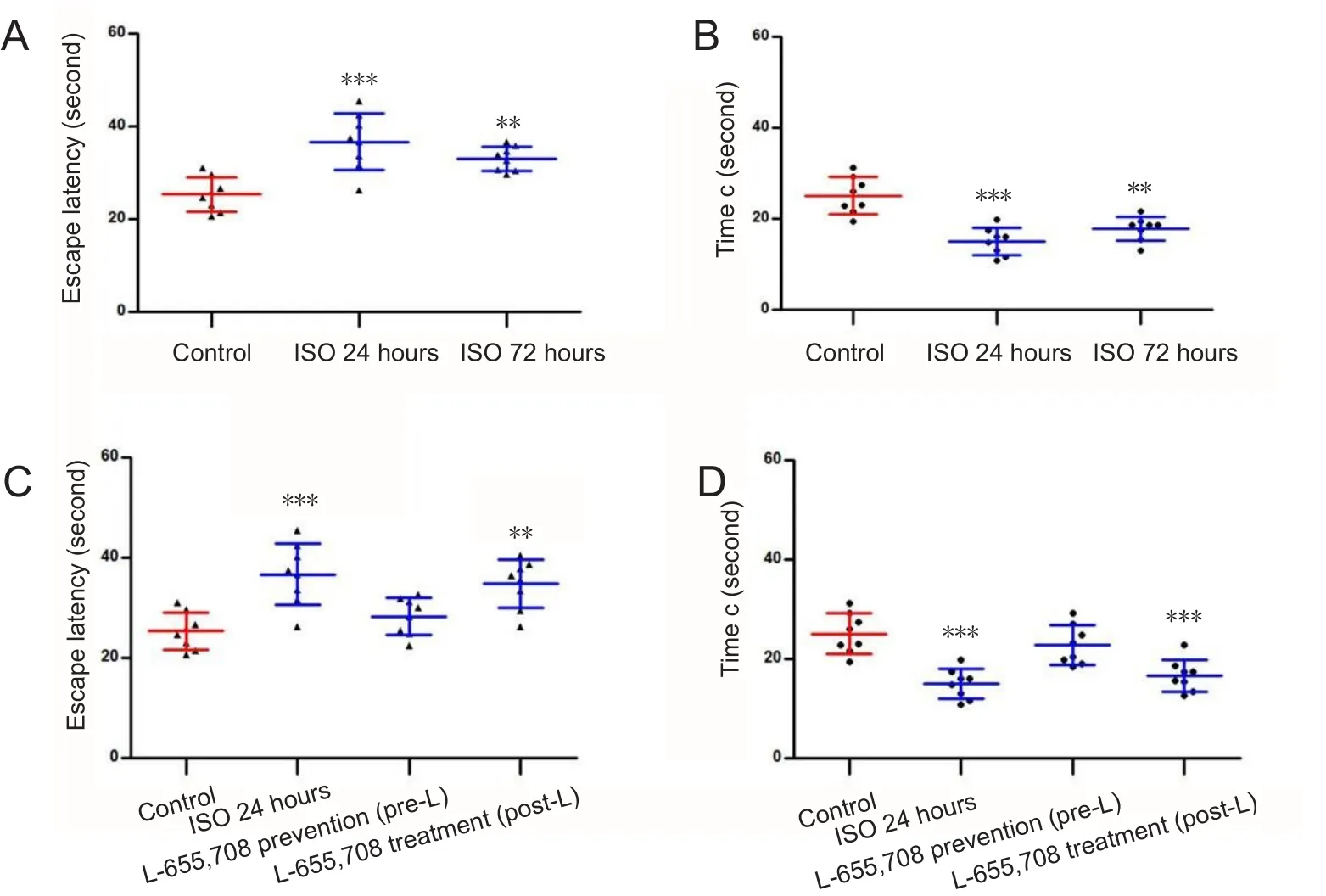
Figure 4 Performance of aged(24 months old) rats in the Morris maze test.

Figure 5 Hippocampal α5 GABAAR mRNA expression determined by RT-PCR.
Discussion
Results from this study showed that 1.3% iso flurane exposure was associated with post-anesthetic spatial reference memory loss and that memory impairment persisted longer in aged rats. Administration of an inverse agonist of α5 GABAARs, L-655,708, before or after anesthesia was able to reverse the memory loss in young rats, while in aged groups,only application before exposure had an effect.
GABAARs are often increased by general anesthetics at high concentrations, but α5 GABAAR is specifically enhanced by low-dose inhaled anesthetic (iso flurane) (Caraiscos, 2004a). Saab et al. (2010) found that 1.3% iso flurane-induced memory impairment in young mice was reversed by L-655,708. Therefore, we kept the concentration of isoflurane to be 1.3%, though the minimal alveolar concentrations of iso flurane were different in mice (1.34 ± 0.10%) and rats(1.46 ± 0.06%) (Mazze et al., 1985). In this study, impaired memory retention was both observed 24 hours and 72 hours after anesthesia in aged rats. Aged subjects have been demonstrated to be more vulnerable to anesthetics-induced cognitive deficits than young subjects, and clinical studies have also verified that advanced age is a risk factor for POCD (Culley et al., 2003, 2004; Callaway et al., 2015). Furthermore, we found that subcutaneous injection of L-655,708 0.5 hours before or 23.5 hours after anesthesia enhanced the cognitive performance of young rats exposed to iso flurane anesthesia, indicating that a preventive or therapeutic dose of L-655,708 was able to reverse the low-dose iso flurane-induced memory deficits, which is in accordance with the results from another study (Saab et al., 2010). In aged rats,prophylactic administration of L-655,708 before anesthesia improved cognitive performance. However, administration after iso flurane exposure was unable to reverse the post-anesthetic memory impairment, indicating that L-655,708 has preventive but not therapeutic effects on cognitive memory dysfunction caused by low-dose iso flurane in aged rats.
α5 GABAARs are of particular interest due to its specific distribution in the hippocampus (Martin et al., 2009; Olsen and Sieghart, 2009). α5 GABAARs are highly sensitive to GABA and show a slow desensitization rate (Soh and Lynch,2015), allowing them to mediate the tonic inhibition regulated by the low-concentration ambient GABA that exists outside of hippocampal neuronal synapses (Caraiscos et al.,2004b; Glykys and Mody, 2006). Alterations in the activity and density of hippocampal α5 GABAARs partially contribute to the amnesic effects of general anesthetics (Cheng et al., 2006; Martin et al., 2009). Zurek et al. (2014) found that isoflurane increased the activity and cell-surface expression of hippocampal α5 GABAARs, thereby impairing cognitive performance. Moreover, transgenic α5 GABAAR knockout mice show resistance to the memory-blocking effects of inhaled isoflurane and sevoflurane (Zurek et al.,2012). α5 GABAAR activity and tonic inhibitory current in hippocampal pyramidal neurons have been shown to be preferentially potentiated by low-concentration isoflurane and etomidate and thus produce memory-blocking effects(Caraiscos, 2004a). Consistently, pharmacological inhibition of α5 GABAARs has been found to improve cognition.Negatively modulating the activity of α5 GABAARs by pretreatment with α5IA in human subjects specifically reversed the decline in the capability to recall a word list after alcohol drinking (Nutt et al., 2007; Atack, 2010). Furthermore,the anesthetic-induced potentiation of α5 GABAARs was significantly attenuated by inverse agonists in a concentration-dependent manner (Lecker et al., 2013). In a series of newly published studies (Pálvölgyi et al., 2016; Etherington et al., 2017; Gacsályi et al., 2017), a potent competitive antagonist of α5 GABAARs, S44819, enhanced hippocampal synaptic plasticity in vitro and improved cognitive function.
L-655,708 possesses 107-, 61-, and 54-fold greater selectivity for the α5 subunit than α1, α2 or α3 subunit-containing GABAARs, respectively (Quirk et al., 1996). A previous study suggested that L-655,708 preferentially suppressed the hippocampal α5 GABAA receptor-mediated tonic currents(Casula et al., 2001). Interestingly, upon exploration of the effects of L-655,708 in aged rats, we found that these effects differed from the effects of L-655,708 on iso flurane-induced memory deficiency in young rats. To investigate this issue,we performed RT-PCR and determined that, compared to a sham exposure, exposure to 1.3% iso flurane increased α5 GABAAR mRNA expression in the young hippocampus but decreased the expression in the aged hippocampus 24 hours after anesthesia. The increased expression of α5 GABAAR in the young rats returned to baseline 72 hours after anesthesia. However, in aged rats, reduced expression of hippocampal α5 GABAARs was detected at both 24 hours and 72 hours after anesthesia. Importantly, α5 GABAAR mRNA expression was at baseline level in both the young and aged rats that received subcutaneous L-655,708 0.5 hours before anesthesia. The activity and expression of hippocampal α5 GABAARs are integrally linked to normal spatial reference memory. Therefore, the opposite alterations in hippocampal α5 GABAAR expression observed in young and aged rats could both result in spatial memory impairment. Iso flurane anesthesia induced a decrease of α5 GABAAR expression in aged hippocampus. Consequently, treatment with the inverse agonists was not able to reverse the imbalance between the excitatory receptor system and inhibitory receptor system that was evoked by anesthesia. For this reason, L-655,708 was unable to reverse the memory impairment in the aged group that received L-655,708 after anesthesia exposure.Koh et al. (2013) found that positive allosteric modulators of α5 GABAARs, compound 6 and compound 44, improved cognition in aged rats with memory impairment. Therefore,whether the administration of α5 GABAAR agonists is able to reverse the isoflurane-induced memory impairment in aged subjects should be explored in a future study.
A question raised here is why hippocampal α5 GABAAR expression was differentially affected in young and aged rats.We speculate that neuroin flammation may explain the alterations in hippocampal α5 GABAARs in young rats. Volatile anesthetics can result in noninfectious neuroin flammation and the consequent excessive production of proin flammatory cytokines, including interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (Cibelli et al., 2010; Wu et al., 2012; Kong et al., 2013; Zhang et al., 2013). The overexpression of these proinflammatory cytokines is involved in POCD. For example, IL-1β activates p38 MAPK, which is associated with the phosphorylation of radixin (an actin-binding protein that regulates the surface expression of α5 GABAARs) (Srinivasan et al., 2004; Koss et al., 2006; Loebrich et al., 2006).Wang et al. (2012) found that IL-1β increased the surface expression of hippocampal α5 GABAARs and tonic currents in young mice, which were associated with impairments in contextual fear memory. Therefore, the alterations in the expression of α5 GABAARs that occurred in young rats might be results of an acute in flammatory reaction induced by isoflurane anesthesia. However, aged subjects are more susceptible to POCD and neurological damage than young subjects (Magnusson et al., 2000; Wang et al., 2000; Culley et al., 2003; Jansson et al., 2004). The hippocampus is especially vulnerable to aging, consequently leading to cognitive disorders including memory impairments (McLay et al.,1997). Isoflurane might cause hippocampal neuron injury or apoptosis and a subsequent decline in α5 GABAAR expression, thereby disturbing the balance of the excitatory and inhibitory receptor systems involved in spatial memory(Zhang et al., 2012; Kong et al., 2013; Wang et al., 2016). The up-regulation of proin flammatory cytokines in fluences the synaptic plasticity of the hippocampus and triggers neurodegeneration. Therefore, we speculate that the reduction in hippocampal α5 GABAAR expression in aged rats might be the result of hippocampal damage caused by isoflurane exposure. This abnormality in α5 GABAAR expression persisted at least 72 hours after anesthesia. Interestingly, our behavioral investigation showed that a preventive dose of L-655,708 before anesthesia avoided the deterioration in cognitive memory both in young and aged rats. We propose that prophylactic injection of L-655,708 at the dose applied in our study may protect the hippocampus from neuroinflammation and proin flammatory cytokine-induced injury.Therefore, investigations into the mechanism behind this effect are of interest.
The present study has several limitations. First, the group size of rats is small for the cognitive behavior tests, although similar animal number has been used in some other studies (Koh et al., 2013; Lin et al., 2012). Second, simple RTPCR data is insufficient to well elucidate the actual change of hippocampal α5 GABAAR. Therefore, it is of necessity to profoundly explore relevant mechanism using larger group size and more experimental data (western blotting, immunohistochemical or electrophysiological data). Moreover,the mechanism by which iso flurane in fluences the function of hippocampal neurons and the regulation of α5 GABAAR expression remains to be determined. We speculate that the isoflurane-induced abnormal release of proinflammatory cytokines is involved in the regulation of hippocampal α5 GABAAR expression. However, the associations between the in flammatory reaction, pathological lesion, hippocampal α5 GABAAR expression and memory impairment that occur after iso flurane exposure remain to be verified.
In conclusion, we found in the present study that administration of the inverse α5 GABAAR agonist before but not after anesthesia was able to reverse the low-dose (1.3%)isoflurane-induced spatial reference memory impairments in aged Wistar rats, which was different from that observed in young rats. In addition, the differential effects between young and aged rats might result from the converse alterations in the expression of hippocampal α5 GABAARs caused by iso flurane anesthesia.
Author contributions:Study design: YQY and ZFZ; experiment implementation: ZFZ, LD, TG, LB, and YL; data analysis: YQY, ZFZ, and LD; paper writing: ZFZ and LD; paper review and editing: YQY and YAW. All authors approved the final version of this paper.
Conflicts of interest:The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support:None declared.
Institutional review board statement:All experimental procedures and protocols were approved by the Experimental Animal Ethics Committee of Academy of Military Medical Science of China (approval number: NBCDSER-IACUC-2015128) in December 2015. All experimental procedures described here were in accordance with the National Institutes of Health (NIH)guidelines for the Care and Use of Laboratory Animals.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Busting the myth: more good than harm in transgenic cells
- Comparative study of microarray and experimental data on Schwann cells in peripheral nerve degeneration and regeneration: big data analysis
- Lessons from glaucoma: rethinking the fluid-brain barriers in common neurodegenerative disorders
- Characteristics and advantages of adenoassociated virus vector-mediated gene therapy for neurodegenerative diseases
- Gene expression changes in dorsal root ganglia following peripheral nerve injury: roles in in flammation, cell death and nociception
- Nicotinamide adenine dinucleotide phosphate oxidase activation and neuronal death after ischemic stroke