Olfactory proteotyping: towards the enlightenment of the neurodegeneration
Although phylogenetically ancient, the olfactory system has received less attention than other sensorial systems. However,olfactory dysfunction is considered an early prodromal event in neurodegenerative diseases (NDs) (Doty, 2012; Attems et al.,2014), which may vary from severe smell loss (e.g., Alzheimer's and Parkinson's diseases) to relatively moderate loss (e.g., progressive supranuclear palsy) (Doty, 2017). Recently, a cluster of neuropathological and functional discoveries has evidenced the relevant role of the olfactory bulb (OB) during the neurodegenerative process (Attems et al., 2014; Rey et al., 2018). For instance, the double-transgenic APP/PS1 mouse model of Alzheimer's disease (AD)develops early proteomic disturbances accompanied by a specific modulation of the focal adhesion kinase (FAK) and mitogen-activated protein kinase (MAPK) dynamics at the level of the OB,demonstrating that olfactory molecular alterations occur prior to β-amyloid plaque appearance and memory impairments in APP/PS1 transgenic mice (Lachen-Montes et al., 2016). Nevertheless,there are currently specific questions which should be addressed in human neurodegeneration: Does the loss of smell precede the onset of ND-specific neuropathological features? What is the metabolic impairment induced by protein inclusions in the OB? Is it disease-specific or tauopathy/synucleinopathy-dependent? How the neuropathological substrates modulate their constitutive interactome at olfactory level? Are there common olfactory targets across different neurological backgrounds?
In this perspective, we brie fly summarize the current knowledge generated by the application of neuroproteomic strategies for indepth molecular characterization of the OB in AD and Parkinson's disease (PD), and discuss challenges to move the field forward.
Neuroproteomics as a tool to characterize the molecular landscape of the olfactory bulb in proteinopathies:During decades,the molecular complexity of the mammalian olfactory system has impeded its systemic characterization, but shotgun “omic”approaches together with bioinformatics developments are now pushing forward the frontiers of olfaction research at an increasing pace (Zelaya et al., 2015; Lachen-Montes et al., 2017a). Specifically, neuroproteomics is being applied to identify and quantify olfactory-related proteomes in different biological contexts such as olfactory learning, ageing and neurodegeneration (Lachen-Montes et al., 2016). Using protein and peptide fractionation strategies followed by liquid chromatography tandem mass spectrometry(LC-MS/MS), the first proteomic atlas of the human OB was characterized in 2012, identifying 1466 proteins involved in diverse biological functions highlighting nucleotide, RNA binding, hydrolase and phosphatase activities (Fernandez-Irigoyen et al., 2012). Along with recent exhaustive proteomic descriptions performed in human olfactory structures (Lachen-Montes et al., 2016), proteomic work flows are also being used to quantitatively monitor OB molecular derangements in human subjects with different neurological syndromes to discover novel potential therapeutic targets and biomarkers.
Olfactory proteotyping: commonalities and differences across human tauopathies and synucleinopathies:Most of the neuroproteomics workflows focused on the characterization of human AD and PD neurodegeneration, although informative, have ignored the neuropathological progression of the disease across AD/PD related-brain structures. We consider that the elucidation of the progressive proteome-wide alterations that occurs in a stage-dependent manner in early-affected OB region, may help to unveil the biochemical routes affected during the olfactory pathophysiology of AD and PD. Using a discovery pipeline combining neuropathological examination, quantitative proteomics, network biology and biochemical approaches, we have partially characterized the proteostatic imbalance present in the OB from AD and PD subjects (Lachen-Montes et al., 2017b, 2018) (Figure 1). In particular: i) around 20% of the quantified proteome differs between AD and PD phenotypes respect to nondemented controls, ii) differential functional interactors for neuropathological substrates (APP,Tau, and alpha-synuclein) have been detected in the OB, iii) 24 OB proteins are commonly deregulated across AD grading (Braak III, Braak III-IV and Braak V-VI stages), and iv) 65 OB proteins are commonly deregulated across Lewy body pathology (limbic,early-neocortical and neocortical stages) (Lachen-Montes et al.,2017b, 2018).
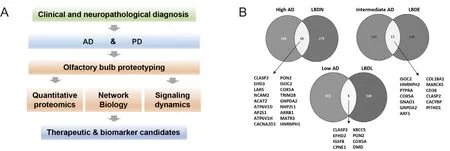
Figure 1 Olfactory proteotyping across Alzheimer's disease (AD) and Parkinson's disease (PD).
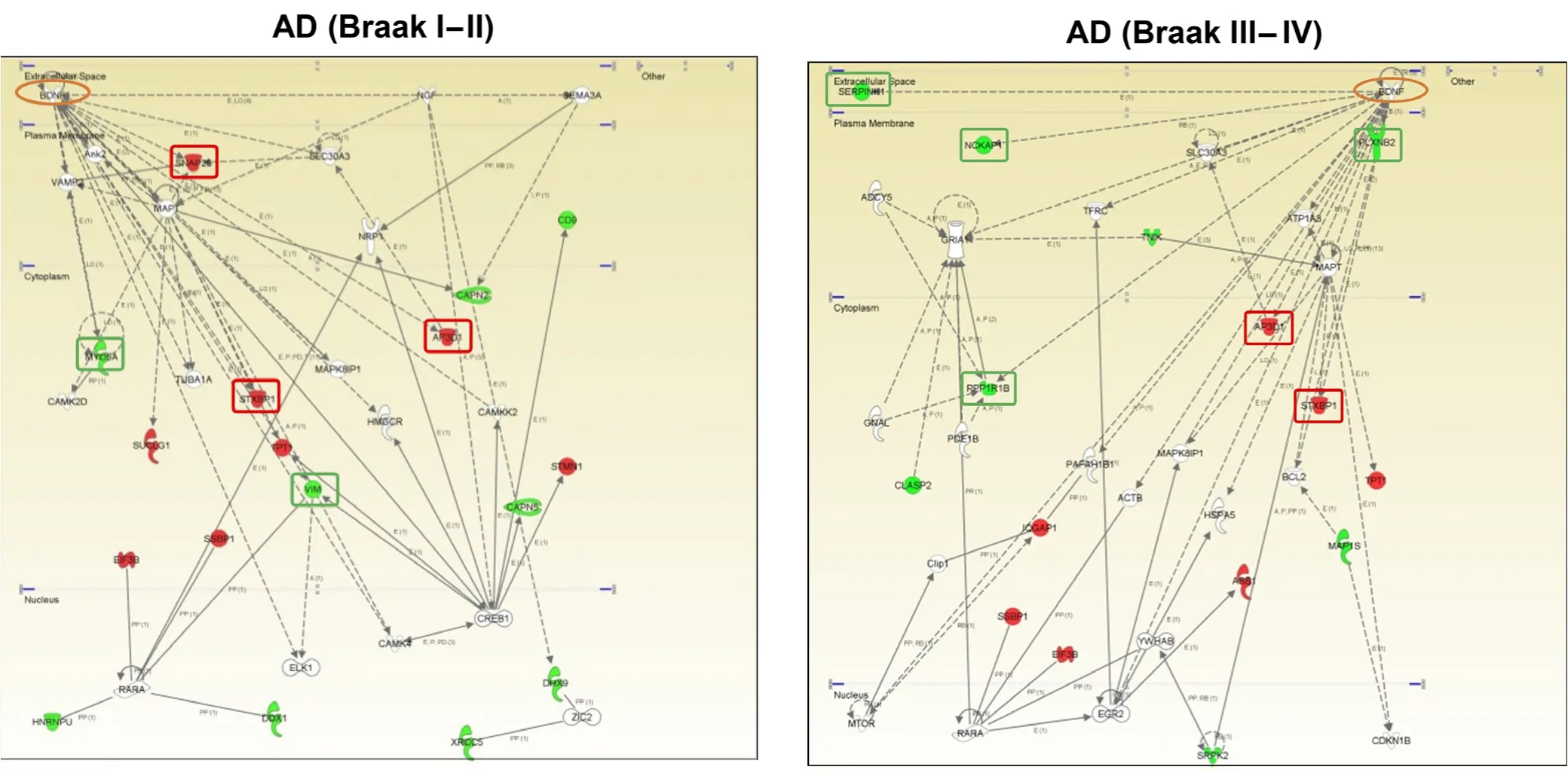
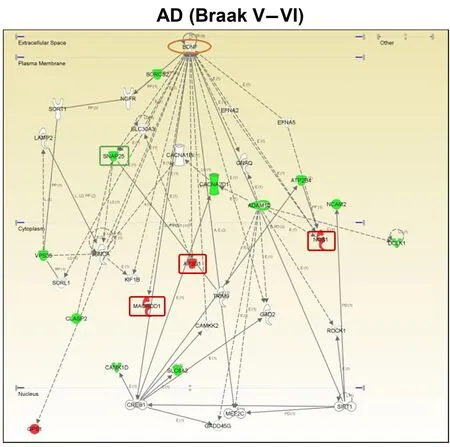
Figure 2 Modulation of the brain-derived neurotrophic factor(BDNF) functional interactome across Alzheimer's disease (AD)stages at the level of olfactory bulb (OB).
Olfactory proteomics as a novel strategy to detect secretable biomarker candidates:Based on the relevance of the smell loss in neurodegeneration, we consider that the application of proteomics in primary olfactory structures is an ideal approach to explore early pathophysiological changes, detecting olfactory proteins that might be tested in biofluids as potential biomarkers. Data-mining of mass-spectrometry-generated datasets has revealed that 30% of the OB proteome is also localized in cerebrospinal fluid. One of these secretable proteins over-expressed in the OB from PD subjects is Glucosamine-6-phosphate isomerase 2 (GNPDA2), an allosteric enzyme that catalyzes the reversible reaction converting D-glucosamine-6-phosphate into D-fructose-6-phosphate and ammonia(Lachen-Montes et al., 2018). Due to ammonia product causes de ficits in excitatory glutamatergic and GABAergic neurotransmission,in flammatory responses, and memory impairments, we focused our attention into the GNPDA2 protein bio fluid profile in patients with PD. Interestingly, GNPDA2 showed an inverse correlation with respect to α-synuclein protein levels detected in cerebrospinal fluid whereas serum levels were decreased in PD population respect to nondemented controls. Although these data may be a consequence of the damaged blood-brain barrier (BBB) previously report in PD,additional experiments are needed to clinically validate the monitoring of the GNPDA2 bio fluid profile across different tauopathies and synucleinopathies, in order to evaluate the specificity and sensitivity of this metabolic protein as a biomarker.
Conclusion and future perspective:Based on the assumption that AD and PD share common underlying mechanisms due to the overlap in clinical presentation and brain neuropathology,cross-disease olfactory proteomic analyses have demonstrated pathology-specific molecular pathways and protein signatures that are common in AD and PD (Lachen-Montes et al., 2017b, 2018).It is evident that data from more neurodegenerative diseases are needed to complete the olfactory proteotyping across taupathies,synucleinopathies and tardopathies. However, the extensive dynamic range in protein concentration and the proteome heterogeneity originated by diverse interconnected neuronal microenvironments, cause difficulties to globally interpret the outputs from olfactory high-throughput molecular maps. In the near future, the acquisition of high-throughput qualitative and quantitative molecular data by technological developments in systems proteomics will allow the precise dissection of unanticipated peptide and protein regulators present in neurodegenerative primary olfactory structures. The application of MALDI imaging (Lachen-Montes et al.,2018) will offer the great advantage to investigate the neuropathological changes taking place directly in olfactory areas, enabling the so-called “molecular histology”. Technological advancements in the field of laser capture microdissection coupled to mass spectrometry will allow the targeted molecular characterization of specific olfactory cell layers present in the OB, providing novel insights regarding the molecular mechanisms that govern the olfactory dysfunction in a cell-type dependent manner. Based on the differences and similarities observed in the activation state of OB survival kinases, future phosphoproteomic studies will allow to precisely decipher novel cell-signaling networks disrupted during olfactory neurodegeneration across tauopathies and synucleinopathies.
Due to the olfactory neuroepithelium is the only portion of the central nervous system that is exposed to the external environment,intranasal therapies based on enzyme replacement or specific drug delivery are emerging as an alternative route to overpass the BBB and deliver potential therapeutic agents to the brain (Agrawal et al., 2018). Said that, we consider olfactory proteomics as a novel source of pathological olfactory substrates to develop unanticipated preclinical studies in order to potentially treat or cure the neurodegenerative process in its early phases.
This work was funded by grants from the Spanish Ministry of Economy and Competitiveness (MINECO) (No. SAF2014-59340-R), Department of Economic Development from Government of Navarra(No. PC023-PC024, PC025, PC081-82 and PI059) and Obra Social la Caixa to ES. The Proteomics Unit of Navarrabiomed is a member of Proteored, PRB3-ISCIII, and was supported by grant PT17/0019 to JFI, of the PE I+D+i 2013-2016, funded by ISCIII and ERDF.
This project is part of the HUPO Brain Proteome Project and these results are lined up with the Spanish Initiative on the Human Proteome Project (SpHPP). Part of this work was presented in the 27thHUPO Brain Proteome Project Workshop (May 9th-10th, 2017, Ruhr-University Bochum, Germany) and the XII European Proteomics Association(EUPA) Congress (June 16th-20th, Santiago de Compostela, Spain).
Joaquín Fernández-Irigoyen, Enrique Santamaría*
Proteored-Institute of Health Carlos III (ISCIII), Clinical Neuroproteomics Unit, Navarrabiomed, Navarra Health Department, Public University of Navarra, Navarra Institute for Health Research (IdiSNA), Pamplona, Spain
*Correspondence to:Enrique Santamaría, PhD,esantamma@navarra.es.
克什米尔地区一直是印度和巴基斯坦的争议地区,其归属权之争由来已久,导致政局一直不稳定。20世纪中期的两次印巴战争使得矿区无法勘探和开掘,本计划于1990年实施的新矿区开发项目也因政治动乱而没能进行。同时由于地理环境也比较特殊——平均海拔超过4000米,人类生存条件恶劣,常年处于低温严寒状态,适宜开采的时间每年仅2-3个月。加之山上基本设施匮乏,大型开采机械又无法运到山上,这些因素都造成克什米尔蓝宝石开采成本巨大。以至于克什米尔矢车菊、皇家蓝,很多人也只闻其名不见其物!
orcid:0000-0001-8046-8102 (Enrique Santamaría)
Received:October 31, 2018
Accepted:November 29, 2018
doi: 10.4103/1673-5374.249220
Copyright license agreement:The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Dolores E. Lopez, University of Salamanca, Spain;Cristina Maccallini, University G. d'Annunzio, Italy.
Additional files:
Additional file 1: Ingenuity pathway analysis legend.
Additional file 2: Open peer review reports 1 and 2.
- 中国神经再生研究(英文版)的其它文章
- Busting the myth: more good than harm in transgenic cells
- Comparative study of microarray and experimental data on Schwann cells in peripheral nerve degeneration and regeneration: big data analysis
- Lessons from glaucoma: rethinking the fluid-brain barriers in common neurodegenerative disorders
- Characteristics and advantages of adenoassociated virus vector-mediated gene therapy for neurodegenerative diseases
- Gene expression changes in dorsal root ganglia following peripheral nerve injury: roles in in flammation, cell death and nociception
- Nicotinamide adenine dinucleotide phosphate oxidase activation and neuronal death after ischemic stroke