无表面活性剂条件下一锅法制备金属/氧化锌复合材料用于催化二氧化碳加氢制甲醇反应
刘艳芳,胡 兵,尹雅芝,刘国亮,洪昕林
武汉大学化学与分子科学学院,武汉 430072
1 Introduction
Nanocomposites,especially functional metal oxide supported transition metal,have attracted intensive research attention in recent years1–5.The hybrid systems that consist of two or more components usually exhibit special synergic functionalities and novel optoelectronic,magnetic and catalytic properties over their individual counterparts,making them good candidates in a wide range of application areas,such as biology,solar cells,catalysis and optoelectronic devices6–9.Transition metals,especially noble metals,are known to have superior electronic and catalytic properties,which can be further promoted by metal oxide supports in catalysis due to the so-called strong-metalsupport-interaction (SMSI) effect10.More importantly,through controlling particle size and loading content of metals,their chemical properties can be finely tuned and optimized.
Among transition metal/metal oxide nanocomposites,metal/ZnO (M/ZnO) system has been widely used as catalysts for several important chemical processes,such as catalytic conversion of syngas to low-carbon alcohols (Cu/ZnO)11,hydrogenation of CO2(Cu or Pd/ZnO)12,CO oxidation(Au/ZnO)13and ethanol stream reforming (Co or Ni/ZnO)14.In general,M/ZnO nanocomposites can be prepared following two strategies,one being physical routes such as ball-milling15(topdown),the other being chemical synthesis methods (bottom-up).The former allows the mass production of powder samples,but it is very difficult to achieve SMSI,which has been confirmed to be an important factor in heterogeneous catalysis10.Chemical methods could be a better solution to fabricate multi-component catalysts.For example,conventional co-precipitation and wetimpregnation are widely employed for preparation of supported metal catalysts.But they normally require an extra H2reduction step to obtain active metallic phase (M) from oxide precursor(MOx).As the synthesis of individual metal nanoparticles (NPs),especially noble metal NPs,is quite different from that of oxides,especially metal/ZnO,most of metal/oxide nanocomposites involve two or multi-steps synthesis.Therefore,it is of great significance to develop a one-step synthesis procedure.
Since a “hot injection” method was reported by Murray et al.in 199316,many other strategies,such as thermal decomposition17,photodeposition18,in situ redox reactions19,20and thermal reduction21–23,have been also developed for size-control synthesis of metal or oxide NPs.However,most of the reported methods involve expensive or toxic reagents.Moreover,they normally require the use of surfactants,which,albeit effective in controlling the size of nanoparticles,turns out to be a headache for catalytic applications as the surfactants would block the catalytic surface and thus lower the activity24.Therefore,it is quite desirable to develop a simple and versatile route for size-control synthesis of metal/oxide nanocomposites without using any surfactants.
2 Experimental section
Here we present a simple one-pot surfactant-free method to synthesize Pd/ZnO nanocomposites in refluxed ethylene glycol by combining strategies of thermal decomposition and thermal reduction.Typically,a certain amount of Na2PdCl4,0.1 g of NaHCO3and 5 mmol of Zn(OAc)2were mixed with 45 mL of ethylene glycol,followed by a reflux treatment for 30 min (see detailed procedure in ESI).The particle size and loading content of Pd can be easily tailored by changing the dosage of NaHCO3and Na2PdCl4 in the recipe.This new process also proved versatile and can be applied to the fabrication of Au/ZnO,Ag/ZnO,and Cu/ZnO.In addition,we have shown that M/ZnO nanocomposites mixed with Al2O3can be used as catalysts for CO2hydrogenation to methanol.After screening,the Pd/ZnO catalyst with a Pd/Zn ratio of 1 :9 showed the highest methanol yield.
3 Results and discussion
As we know,ethylene glycol,serving as a mild reducing agent and solvent,has been widely used in the synthesis of noble metal NPs25.As illustrated in Fig.1,alcohol hydroxyl groups are capable to reduce Pd ions to generate Pd crystals in refluxing conditions.The “thermal reduction” allows the nucleation and growth of Pd particles without using extra reducing agents.To control the size of Pd NPs,NaHCO3,which acts as a size-control agent,is employed in the system by changing the alkaline conditions.Meanwhile,such high temperature would favor a thermal decomposition of Zn(OAc)2 to form ZnO nanocrystals26.When combining the two routes together,one may expect to obtain hybrid Pd/ZnO in one pot.In our surfactant-free system,the new-born species (Pd and ZnO nanoclusters) would stabilize each other from further aggregation by reducing their individual surface energy in the growth process.Importantly,this approach can be extended to fabricate (Au,Cu,and Ag)/ZnO hybrid nanomaterials by simply changing the metal precursor,verifying the effectiveness and feasibility of this novel approach.
Fig.2a shows the X-ray diffraction (XRD) pattern of the Pd/ZnO sample with a Pd/Zn ratio of 1 :6.Clearly,the signals at 31.8°,34.4° and 36.3° can be assigned to (100),(002) and(101) diffraction peaks of wurtzite-ZnO crystals (JCPDS#21-1486),suggesting the existence of ZnO.Meanwhile,the peaks at 40.2°,46.6° and 68.1° correspond to the (111),(200) and (220)planes of a face centered cubic (fcc) lattice of metallic Pd(JCPDS#65-6174).No impurity phase was detected by XRD analysis,indicating that the obtained materials were solely comprised of crystallized Pd and ZnO lattices.Fig.2b shows a transmission electron microscopy (TEM) image of the Pd/ZnO sample.It reveals pseudo sphere-like ZnO crystals,decorated with small-sized Pd particles,as distinguished from different contrasts.The average size of ZnO NPs is around 30 nm,while that of Pd is estimated to be about 8 nm.The high-resolution TEM image in Fig.2c shows a d-spacing value of 0.23 nm,which can be assigned to the characteristic (111) lattice plane of metallic Pd27.
For comparison,pure ZnO sample was synthesized under the same conditions without adding Pd precursor.The TEM image(Fig.2d) shows some big spherical aggregates that consist of irregular ZnO NPs.The size of these aggregates ranges from 30 nm to 100 nm.It seems that Pd plays an important role in preventing aggregation of ZnO nanocrystals.This could be explained by the decrease of the surface energy of ZnO NPs after the decoration of new-born Pd nanoclusters on the surface of ZnO.
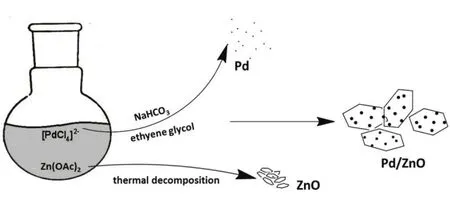
Fig.1 Schematic illustration of hybrid Pd/ZnO nanoparticle fabrication.
To further confirm the formation of Pd/ZnO hybrid material,XPS analysis was performed.Fig.3a displays a wide-range XPS spectrum of the Pd/ZnO sample,confirming the coexistence of Zn,Pd,O and C.More specifically,Fig.3b shows the highresolution spectra of Zn 2p.The binding energy at 1021.5 and 1044.6 eV can be assigned to Zn(II) 2p3/2and 2p1/2in the form of ZnO,respectively.The deconvolution of O 1s using curve fitting in Fig.2c clearly shows two main peaks,one being lattice oxygen (O2-) from ZnO,the other being adsorbed oxygen on the surface of the composite28.The Pd 3d spectrum (Fig.3d)consists of two main peaks at binding energies of 339.9 and 334.6 eV,corresponding to Pd 3d3/2and 3d5/2of metallic Pd species29.Interestingly,the Pd 3d region can be divided into two peak groups,and the signals of higher binding energies (341.2 and 335.8 eV) come from partially oxidized Pd (δ+),which accounts for around 21% of total surface Pd content.Many studies on oxide-supported metal clusters show that surface defects of the supports could serve as anchoring sites for metal clusters30.When Pd are immobilized onto the surface of ZnO,Pd-O-Zn interfaces would form.This part of Pd normally shows positive charge via electron transfer from Pd to ZnO,indicating strong interactions between Pd and ZnO (SMSI)31.
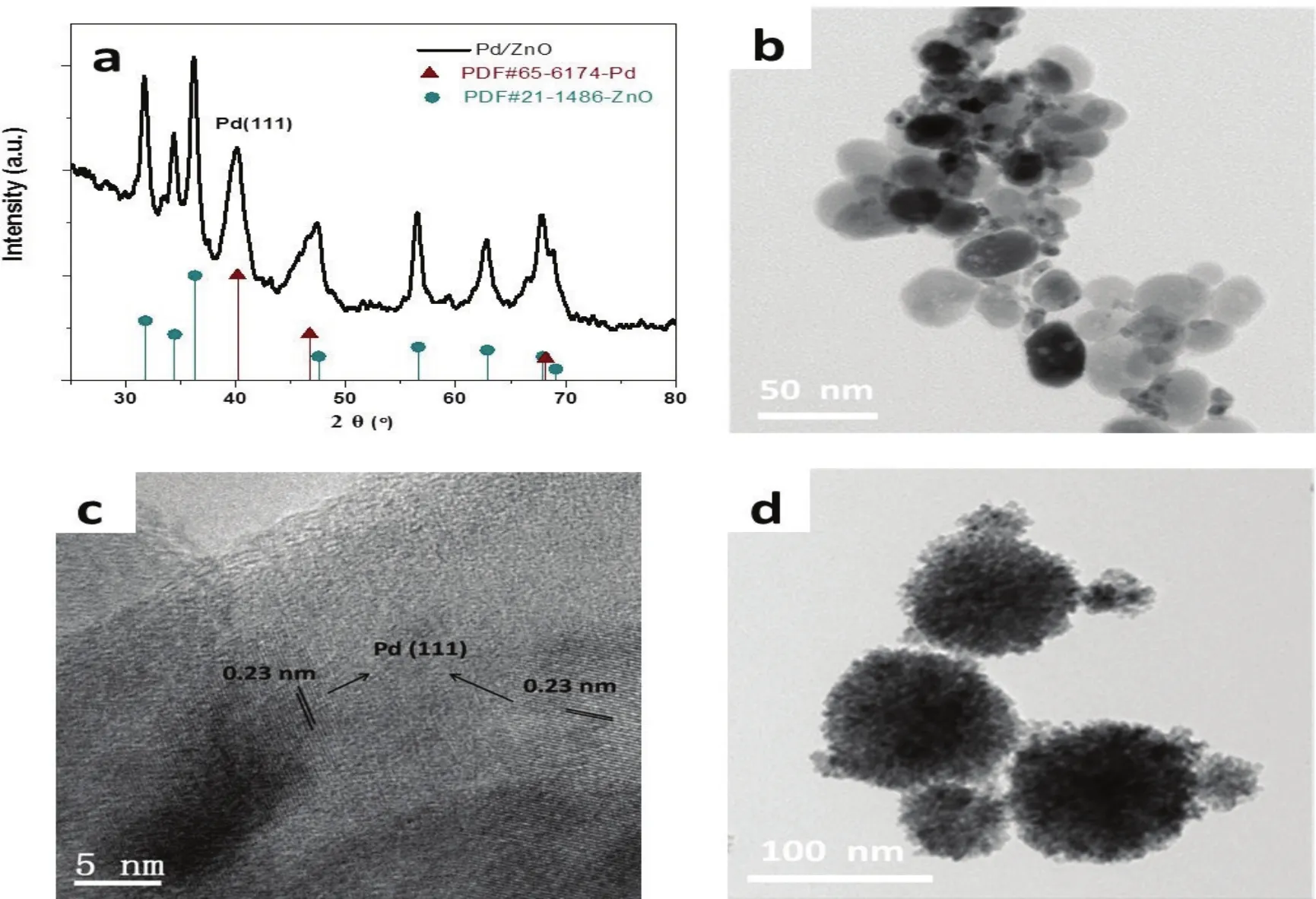
Fig.2 (a) XRD pattern,(b) TEM and (c) high resolution TEM images of the Pd/ZnO sample prepared at a Pd/Zn ratio of 1 :6,and(d) TEM image of pure ZnO sample.
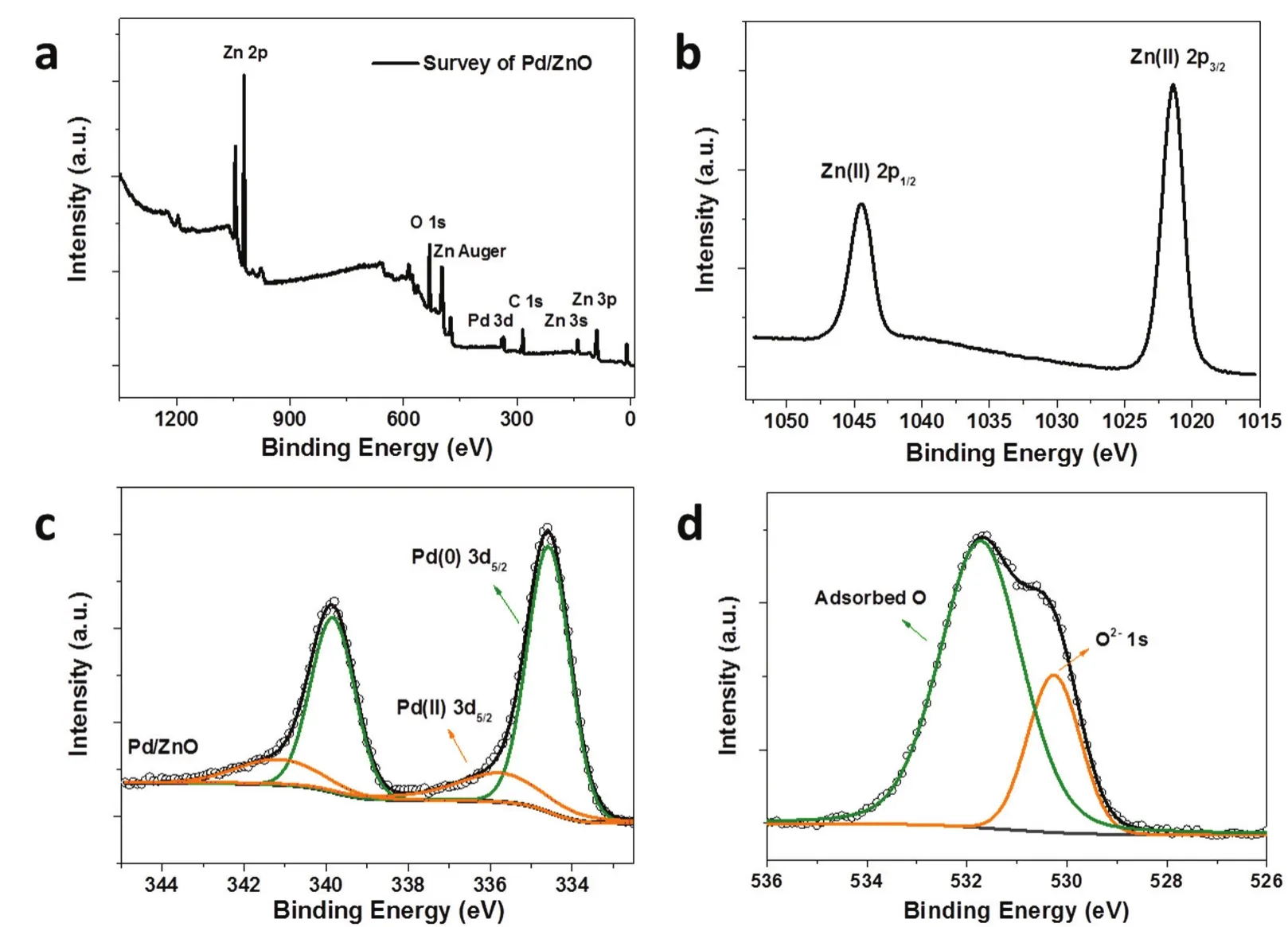
Fig.3 XPS analysis of the Pd/ZnO nanocomposite.(a) The wide-range XPS spectrum;high resolution spectra of (b) Zn 2p,(c) Pd 3d,(d) O 1s.
A series of Pd/Zn molar ratios were investigated using this simple synthesis method.The final products were analyzed using ICP-MS to check the actual elemental compositions,as listed in Table S1 (in Supporting Information).The detected atomic ratios of Pd/Zn are well consistent with the theoretical recipe value,evidencing a total conversion of initial Pd and Zn species to their corresponding products.It is quite advantageous to achieve a precise control of multi-component ratios by using this simple method.Fig.S1 shows XRD patterns of a series of Pd/ZnO composites with the Pd/Zn ratio varying from 1 :2 to 1 :48.As expected,when the ratio is reduced,the peak intensity ratio of Pd (111)/ZnO (002) decreases continually.At the same time,the widening of Pd (111) reveals a decreasing trend of the Pd size.According to the Scherrer formula,the particle size calculated on a basis of the Pd (111) peak goes up from 6.14 nm at Pd/Zn molar ratio = 1 :12 to 10.12 nm at Pd/Zn molar ratio = 1 :2.Fig.S2 shows TEM images of the Pd/ZnO samples.Despite different Pd loading,all composites show similar morphology,with small Pd dots on sphere-like ZnO crystals.All ZnO particles have similar size of around 30 nm,while the size of Pd particles varies with the Pd/Zn molar ratios,as shown in Fig.4a.When the Pd loading goes up,the corresponding Pd particle size also increases,in good agreement with aforementioned XRD analysis.This phenomenon may be attributed to relatively insufficient surface defects of ZnO to anchor extra Pd species,thus losing control of the growth of Pd particles.
The amount of NaHCO3is another key factor to control the size of Pd NPs.By keeping the molar ratio of Pd/Zn at 1 :6,we investigated the effect of the added amount of NaHCO3on Pd size.Fig.S3 shows the XRD patterns of fresh samples with the dosage of NaHCO3varying from 0,0.1,0.4,0.6,1.0 to 1.5 g.Accordingly,the Pd (111) peak (at 40.24°) became widened and weakened,revealing a decrease of Pd size.The average sizes are calculated and shown in Fig.4b.Clearly,it decreases gradually from 8.0 to 4.4 nm with increased amount of NaHCO3.This can be further confirmed by TEM observation,as seen in Fig.S4.It has been accepted that alkaline conditions would accelerate the initial nucleation of noble metals,thus favoring a smaller particle size32.Therefore,NaHCO3 can serve as a size-control tool for Pd NPs.
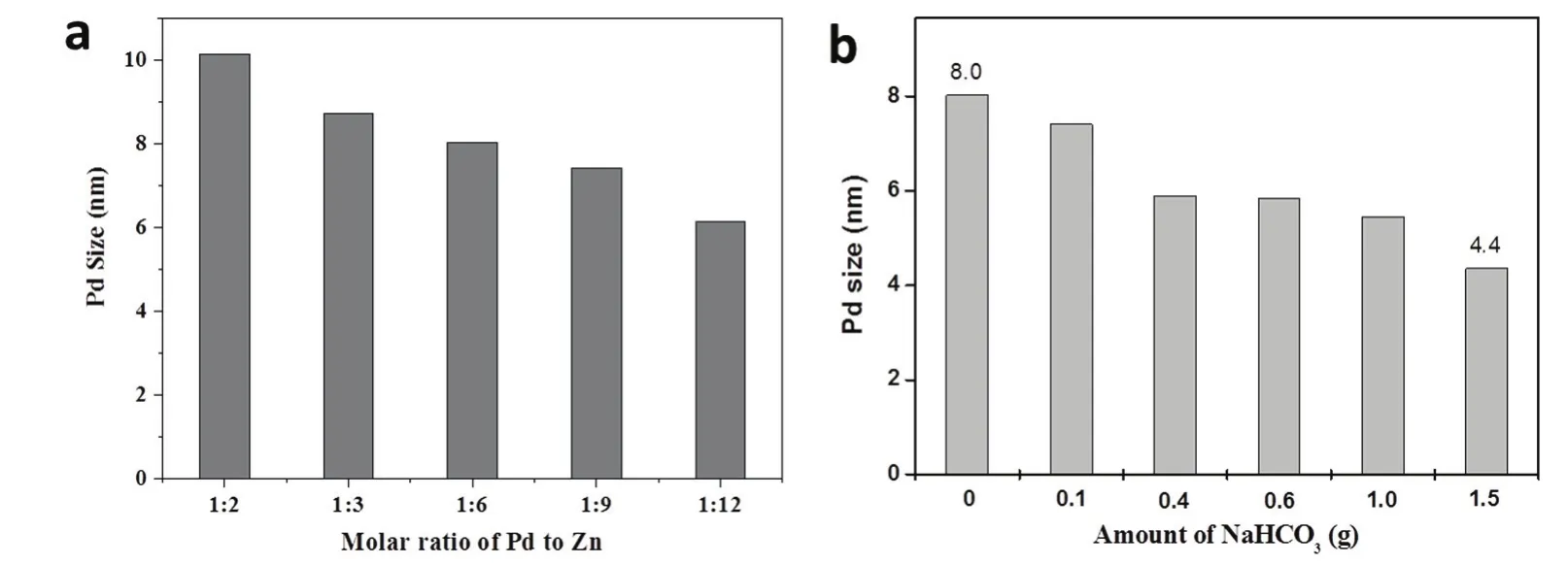
Fig.4 Histogram of Pd particle size versus (a) the mole ratio of Pd to Zn and (b) the amount of NaHCO3.
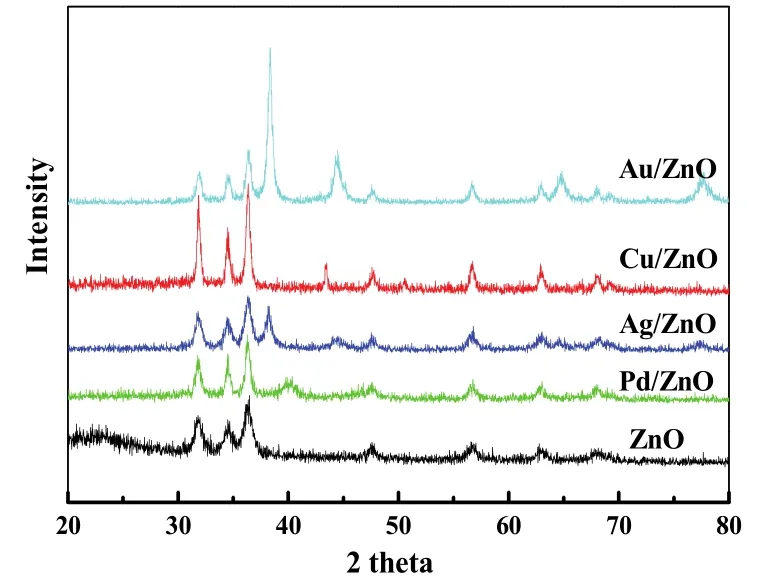
Fig.5 XRD patterns of ZnO and M/ZnO (M = Pd,Au,Ag,and Cu) synthesized at a M/Zn molar ratio of 1 :6.
We also synthesized other M/ZnO nanocomposites including Au/ZnO,Ag/ZnO and Cu/ZnO following the same strategy.XRD patterns (Fig.5) confirm the formation of corresponding metal NPs supported on ZnO.Precisely,all metals show their typical (111) diffraction peaks at a scanning region from 38° to 45°.The intensity and width of diffraction peaks vary for different samples,indicative of apparent size difference.Among the three M/ZnO systems,Au NPs show the largest size with around 30 nm,Ag the second with around 15 nm,Cu the smallest with only 9 nm,as calculated from Scherrer formula.The size difference may be explained by different reduction potentials for selected metal precursors,thus affecting the subsequent growth process.Fig.S5 shows TEM images of the three samples and pure ZnO.Clearly,we can see the incorporation of Au,Ag and Cu species can also stabilize ZnO NPs from aggregation,similar to the aforementioned Pd/ZnO system.
Cu/ZnO/Al2O3 composite is a well-known catalyst towards the catalytic hydrogenation of CO2to produce methanol33.Other metal/ZnO composites are also catalytically active for this reaction,such as Pd/ZnO34.We tested our one-pot synthesized M/ZnO nanomaterials mixed with Al2O3 (33.3% (w)) as catalysts for the hydrogenation of CO2(see Table 1).For the Cu/ZnO and Ag/ZnO catalyst,the methanol selectivity is very low,with a value of 23% and 26% respectively.The Au/ZnO catalyst favors the highest methanol selectivity (82%),but lowest CO2conversion,with only 6.6%.When the Pd/ZnO is used,the CO2 conversion reaches 20.7% while the methanol selectivity is still kept at a high value (71%),giving the highest methanol yield (14.7%).The excellent catalytic performance may be explained by the following two factors:one is that Pd is a good catalyst for the dissociation of H2to give active H atoms35,the other being that SMSI between Pd and ZnO would favour the formation of surface oxygen vacancies on ZnO36.
Reaction temperatures were then investigated over the Pd/ZnO (1 :6) system.When the temperature is reduced from 260 to 240 °C,the CO2conversion slightly decreases from 20.7% to 19.0% while the methanol selectivity goes up marginally from 71% to 77%,maintaining a nearly identical methanol yield (14.6%).When the temperature further drops to 220 °C,the CO2conversion decreases to only 9.8% although a higher methanol selectivity (84%) is achieved.The final methanol yield drops significantly from 14.6% to 8.2%.A lower temperature would be favourable for the selectivity of methanol because this route is exothermic whilst the CO2-to-CO route is endothermic33.However,the total CO2conversion rate would drop quickly with the decrease of temperature.Therefore,it is of great significance to choose a proper temperature for a desirable methanol yield.
The loading of Pd in Pd/ZnO catalysts was further studied at a reaction temperature of 240 °C,as summarized in Table 1.When Pd loading is increased,the methanol yield increases first and then decreases,with a maximum of 21.0% at a Pd/Zn ratio of 1 :9.Similarly,the CO2conversion also reached a peak(30.49%) for this sample,along with an acceptable methanol selectivity (68.7%).In general,the activity of Pd/ZnO is closely related to the exposed Pd surface sites which determine the amount of dissociated H species.In our case,the Pd particle size shows an increased trend with increased loading content,which would in turn decrease the effective Pd surface sites.Therefore,the activity of Pd/ZnO catalysts from our synthesis method would be very sensitive to the loading content of Pd.

Table1 Catalytic performance of M/ZnO/Al2O3 catalysts (M = Ag,Cu,Au,and Pd).
4 Conclusions
In summary,we demonstrate a facile one-pot surfactant-free synthesis of M/ZnO (M = Pd,Au,Ag,and Cu) nanocomposites in ethylene glycol under the refluxing condition.In this strategy,Pd and ZnO can stabilize each other from further aggregation.Pd loading can be precisely tailored by changing recipe Pd/Zn ratios.NaHCO3can serve as a size-control tool for Pd particles by adjusting alkaline conditions.It is also found that the Pd/ZnO sample prepared by this simple method shows strong interactions between Pd and ZnO,which promotes a high methanol yield at a Pd/Zn ratio of 1 :9.This facile method would open up a new route for one-pot synthesis of M/ZnO nanocomposites with clean surface for catalysis.
Supporting Information:available free of charge via the internet at http://www.whxb.pku.edu.cn.

