Epidemiological, prevention and control updates of yellow fever outbreak in Brazil
Marli do Carmo Cupertino, Rebeca Garcia, Andréia Patrícia Gomes, Sérgio Oliveira de Paula³,Nicholas Mayers,4, Rodrigo Siqueira-Batista ✉
1Medical School, Dinâmica College of the Piranga Valley. Ponte Nova. Minas Gerais/ Brazil
2Laboratory of Epidemiological and Computational Methods in Health, Department of Medicine and Nursing, Federal University of Viçosa. Minas Gerais/ Brazil
3Laboratory of Immunovirology, Department of General Biology, Federal University of Viçosa. Minas Gerais/Brazil
4Department of Veterinary Medicine, Universidade Federal de Viçosa. Viçosa. Minas Gerais/Brazil
Keywords:Infectious disease Epidemiology Arboviruses Yellow fever Aedes aegypti
ABSTRACT Yellow fever is an acute viral disease endemic to tropical countries, like Brazil, where,since the 1940s, has no significant documented outbreaks similar to that observed between 2016/2018 (2 045 confirmed cases and 677 deaths; caused by the sylvatic form). The principal manipulating factors inciting this change were absence of appropriate vaccination campaigns and increased urbanization & population growth in forest areas, with prevalence of the virus in the species inhabiting of these areas. The 2016/2018 outbreaks exhibited incidence in areas with historically low or no yellow fever virus activity, triggering a surge in recorded deaths -mainly in the Southeastern states of Brazil. The Brazilian government aggressively responded,reforming the countries’ prophylactic measures, including vaccine implementation - as of March, 2018, switching from the former double dose regimen of the vaccine, to a single dose protocol, deemed as adequate. Moreover, some states appropriated the fractionated dosage(1/5 of the standard dose), in foresight of potential vaccine shortages. To prevent the uprising of new sylvatic yellow fever cases in Brazil, it’s obligatory the development of effective combative plans, including adaptation of prophylactic measures individually (use of repellents,protective clothing etc.), applicable vaccination campaigns in every endemic region, to raise awareness to locals and visitors alike. Notwithstanding these preventative strategies, the persistence of cases and the recent outbreaks in Brazil, highlight the possible ineffectiveness of combative measures. Based on these considerations, the objective of this review was to raise more awareness of the epidemiological impact of the disease in Brazil.
1. Introduction
Yellow fever (YF) is an acute, non-contagious viral disease that remains endemic and enzootic in the tropical regions of Africa,Central and South America[1]. The incidence in South America is 5 cases per 100 000 inhabitants, while in West Africa it is ten times higher. However, the mortality rate is higher in South America than in West Africa. It is speculated that genetic factors may be associated with this higher mortality rate[2]. Initial symptomology includes fever, arthralgia, myalgia, headache and anorexia, often with jaundice[1]; a small proportion of virus-infected patients developing severe symptoms with approximately half of those dying within the first 7 to 10 days[1,3].
The etiologic agent, an arbovirus (arthropod-borne virus), known as the Yellow Fever Virus (YFV), stems from the family Flaviviridae,genusFlavivirus[1,2,4]. The transmission cycle is dependent to female mosquitoes of the generaAedes,SabethesandHaemagogusinfected with the YFV. These insects playing the role simultaneously as the vectors and reservoirs of the virus[4,5].
The disease is endemic in 47 countries (34 in Africa and 13 in Central and South America)[5,6]. According to the World Health Organization (WHO), there are an estimated 200 000 cases worldwide of YF each year, provoking about 30 000 deaths.Occasionally, travelers from endemic countries may carry the disease to uninfected regions. To avoid the importation of the disease, many countries require proof of YF vaccination prior to issuing a visa,especially if travelers are going to, or have visited, YF endemic areas[7]. In past centuries (17th-19th centuries), the YFV was transported to North America and Europe, causing unprecedented outbreaks that unsettled economies, social development and in some cases, even decimated populations[2,6].
Despite the availability of an effective vaccine, the re-emergence of YF is directly correlated with its continuous dissemination in several countries to date. Together, Brazil, Angola, the Democratic Republic of Congo and Nigeria accumulated thousands of documented cases through 2016-2018, which can be interpreted as a probable indicator of what will occur if no action is taken[7-9].According to the WHO, some aspects have increased the global risk of YF, such as laws in vaccination campaigns, changes in human lifestyles (increased intrusion in the insect vector habitat), along with the increase of vector populations, especially theAedes aegypti(Ae. aegypti)mosquito, which is also a vector of other notable diseases such as Zika, Dengue and Chikungunya[2,10,11]. Fortuitous infection amongst individuals who enter regions in enzootic cycles would be easily controlled if population growth did not lead to the invasion of such forest areas, where the virus is already present in wild animals, highlighting their significant role in the spread of the virus. Another factor involved in the human contamination and outbreaks from YF is the degradation of the wild animal habitats.This obligates these wild organisms, including the insect vectors,to explore new environments, and thus increasing the possibility of viral transmission amongst vulnerable individuals due to lack of vaccination, triggering precedents to large epidemics of the disease.In these conditions, if infected mosquitoes of theAe. aegyptispecies transmit the virus from person to person, it triggers the urban cycle[5,12].
Although often represented as a neglected disease, YF still remains a considerable threat to human health and economy, as exhibited by the outbreaks of 2016-2018 in areas with historically low or no YFV activity. An estimated 400-500 million unvaccinated people live in high-risk areas[12]. Taking into account the re-emergence of YF in different parts of the globe, this article aims to review pivotal aspects of the disease, focusing on the recent outbreaks which have occurred in Brazil between 2016-2018, highlighting the epidemiological and prophylactic characteristics of the disease, and consequently portraying its risk to global health.
2. Etiological aspects
The YFV has a spherical structure, with approximately 40 nm in diameter and a positive-sense single-stranded RNA, behaving like a messenger RNA, which contains about 10 500-11 000 nucleotides in length that codify 3 411 amino acids, and is protected by a protein coating[2].
While there is only one serotype for this virus, there are notable differences between the African and American strains, facilitating the classification of the virus into five genotypes in Africa and two genotypes in the Americas[10,12]. Analyses conducted in 2017 showed that YF genotypesⅠandⅡ follow similar evolutionary and demographic dynamics since the beginning of the 1990s, when a dramatic change in the diversification of genotype Ⅰassociated with growth and dissemination of a new lineage occurred[2,11].
The modern lineage has provoked the largest outbreaks of the disease detected in non-endemic regions of South America since 2000, including the Brazilian outbreak of 2017. Its spread has been accompanied by the accumulation of several amino acid substitutions with non-structural viral proteins[4]. Thus,in situevolutions were identified as key mechanisms that modeled the dynamics of the diseases’ prevalence in the Americas, along with the long-distance propagation through epizootic waves of infection[11-13].
The ecological conditions of Brazil favorably allow for the creation of a sustainable wild cycle for the YFV[14,15]. YF outbreaks require three essential conditions for their maintenance. These are the presence of specific vectors, which have anthropophilic habits;failure of vaccine coverage of the population and contact of the infected vector in a no immunized population. Since immunization and vector control are the two main prophylactic tools[16-18]. The reason for the absence of YF in Asia and the Pacific, despite the presence of the vectors and susceptible human populations, is not yet fully elucidated[15,18-20].
3. Transmission cycles and pathogenesis
The YF transmission cycle is dependent on females of mosquitoes,which act as vectors and reservoirs of the virus. In the Americas,HaemagogusandSabethesconstitute the sylvatic cycle, whereas, and in Africa it isAedesafricanus. In the urban cycle, in both regions,the predominant vector involved isAe. aegypti[21]. These speciesare primatophilic and anthropophilic, and are more abundant in the crowns of the trees - usual monkey habitat[19-21].
YF presents two epidemiologically distinct transmission cycles:sylvatic and urban. From an etiological, clinical, immunological and pathophysiological perspective, it is the same disease in both cycles,however, the mosquito vectors involved are different[15].
3.1. Sylvatic yellow fever (SYF)
In the SYF, non-human primates are the main hosts and amplifiers of the virus. The virus is kept in the wild by transmission between monkeys and wild mosquitoes. At times, in ideal conditions for transmission, a larger number of monkeys become ill and die,accentuating the epizootic factor of the disease. In this cycle, humans act as accidental hosts when entering forest areas and inhabited by mosquito (sylvatic) vectors[21-23].
When the mosquitoes suck the blood of their primate host - during the first three or four days of the fever - the now virus-infected blood is transferred to the stomach of the mosquito. Over the next twelve days, if the concentration is high enough, viruses can infect epithelial cells and reproduce before they are passed into the circulatory system of the mosquito[14]. Thenceforth, the virus will enter the salivary glands, being injected into the victim the next time the mosquito feeds. Inside the primate cell, the virus uses the cellular components to replicate, forming new viral particles which are released from the host’s cell membrane. Duplicated millions of times, the virus fills the body of the host, inducing fever as the most significant etiopathogenic result[10,17]. However, some characteristics of the virus prevent its dissemination, such as, its incapacity to survive outside the body for more than a few hours, cannot infect through air or contact, has a low mutation rate and its fundamental dependency on vector transmission. In human host, after introduction of the virus into the circulation from the mosquito bite, a few hours later the virus reaches the regional lymph nodes, where it silently multiplies in the cells of the reticuloendothelial system. Subsequently, with the release of the viral particles by the cells, viremia occurs, which corresponds clinically to prodromes of the disease and, in particular,fever. Through the bloodstream, the virus reaches and allocates itself in the liver, kidneys, heart, central nervous system, pancreas, spleen and lymphoid structures[21].
Some characteristics of the virus prevent its dissemination: the fact that it cannot survive outside the body for more than a few hours, impedes its infection through air or contact, and as well, its diminished mutation rate. In this way, the virus needs the insect to stay alive outside the host organism[10,17].
The transmissibility period,i.e.the viremia interval or the period in which these hosts can infect the vector mosquitoes, begins 24 to 48 hours before the onset of symptoms and goes up to 3 to 5 days after the onset of symptoms. After being infected, the mosquito is able to transmit the disease throughout its life[18,21].
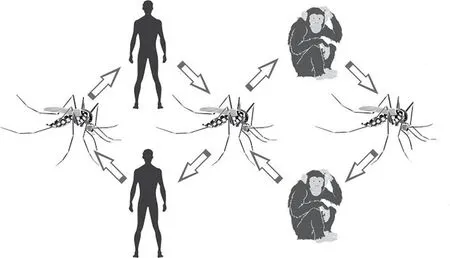
Figure 1. Sylvatic yellow fever cycle in humans and monkeys.
The sylvatic cycle is endemic and epidemic in the tropical regions of Africa and South America. In forest areas or transition areas in Bolivia, Brazil, Colombia, Ecuador and Peru, hundreds of cases occur every year[15]. This cycle type has never been documented in Asia, Australia and the Pacific Islands. As with the urban form,the sylvatic form of the virus is cyclic, and is always preceded by epizootics[21].
The human involvement in the SYF occurs fortuitously, when they invade vector habitats through deforestation, construction(roads) and/or by travelling to high risk areas. Adult men are the most susceptible to be contaminated, given their higher exposure to the risk. There’s a substantial number of unvaccinated individuals, particularly immigrants, who settle in enzootic regions for the exploitation of wood (deforestation) and agricultural projects[10,15,21].
3.2. Urban yellow fever (UYF)
Provoked by the same etiological agent, it induces similar symptomology of those infected by SYF, presenting only an epidemiological differentiation. It had been quite present in the urban spaces of the Americas until health campaigns to combat the vector were launched, which contributed to the eradication of the disease in its urban cycle in the 1940s. Since 1942, there has not been any single case of UYF registered in Brazil, including the last outbreak that occurred in the country in 2016/2017[18].
In the urban cycle, humans are the only host with epidemiological significance and the transmission occurs mainly by theAe. aegyptispecies, which frequents this host’s habitat[14,24]. The risk of UYF transmitted byAe. aegyptimosquitoes has increased in Africa and has the potential to re-emerge in South America, which may lead to a serious public health problem for large demographic centres[5,15].
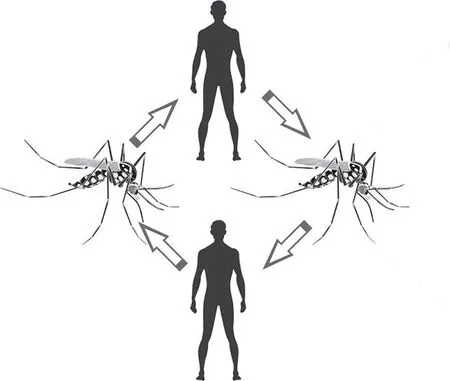
Figure 2. Urban Yellow Fever cycle in the Americas.
There is no participation of any other organisms besides theAe.aegyptiand humans to conclude the cycle[14]. When the mosquito bites an infected individual, it will inoculate the virus, which is now present in its salivary glands, into another individual, who,if not vaccinated, will be contaminated and further contribute to the transmission cycle[25,26]. The vector reproduces in artificial collections of water, easily found in houses, abandoned land,accumulated waste, and others. These mosquitoes are adapted to the urban life, and are not present in semi-sylvatic places or remote areas. They are active throughout the day, but are more prevalent at dawn and the evening. In Africa, transmissions have also been recorded by the speciesAedes vittatusandAedes taylori. Between 1986 and 1991, for instance, there were approximately 20 000 documented cases of UYF in Nigeria, resulting in over 4 000 deaths[18].
In Brazil, the last registered case of UYF occurred in the State of Acre, in 1942. Twelve years later the last documented cases in the Americas were registered in the Venezuela. Since then, no other official cases were diagnosed, with unofficial records of six possible cases in Santa Cruz de La Sierra, Bolivia[10]. However, due to the massive presence of theAe. aegyptispecies in the Brazilian metropoles, even with major health campaigns and public policies,the risk of reintroduction of the virus into the country still remains.Additionally, because YF has many similar non-specific symptoms(mainly fever, myalgia and jaundice) it is the important to include it in differential diagnoses of other diseases, such as malaria,leptospirosis and dengue[1,5].
4. Epidemiological impact
The disease can only spread in species-specific regions, with distinct climatic conditions and virus reservoirs to maintain the cycle[15, 25-27]. As such, even though YF epidemics had occurred in North America and Europe, notably in England, Ireland, France,Italy, Spain and Portugal, the registered cases were of travelers who got contaminated in locations with active transmission of YF, and were later diagnosed in their country of origin[28, 29-31].
In December, 2015 a YF outbreak began in Angola, which then spread to the neighboring Democratic Republic of Congo (DRC)in March, 2016. More than 7 000 suspected cases were reported in the two countries. In Angola, 4 306 suspected cases and 376 deaths were registered, of which 880 cases and 121 deaths were confirmed by laboratory tests as caused by the virus. In DRC, 2 987 suspected cases were reported, of which 81 were confirmed in laboratory and 16 resulted in deaths. The epidemic was overcome with an unprecedented vaccination campaign, which immunized 30 million people in both countries. The outbreak ended December 23rd, 2016 in Angola and February 14th, 2017 in DRC[5,8,9].
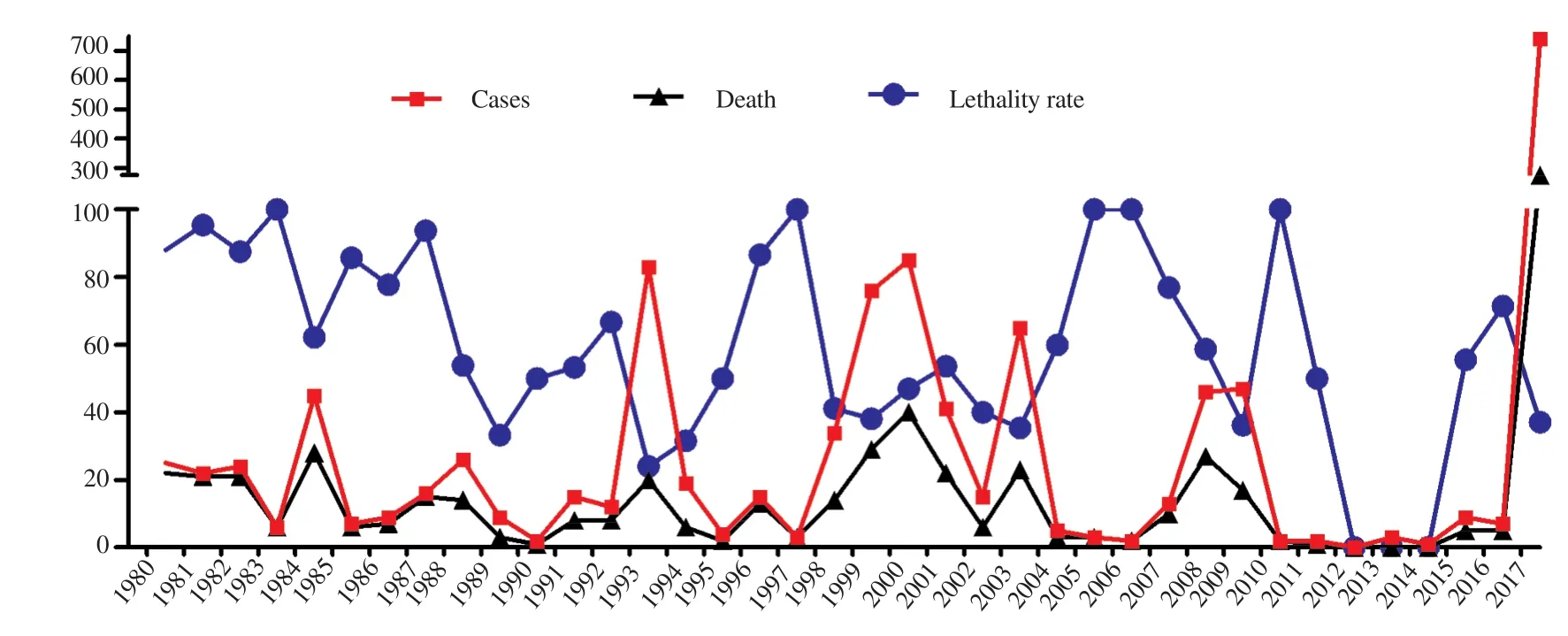
Figure 3. Number of cases, deaths, and lethality rates of sylvatic yellow fever confirmed in Brazil from 1980 to 2017.
In Brazil, SYF is endemic to the Amazon region. Beyond the Amazon region epidemic periods are occasionally recorded, which characterize the re-emergence of the virus in the country[32]. YF has a seasonal pattern of occurrence. In most cases, it occurs between December and May; which marks the rainy season and promotes auspicious environmental conditions for the virus, such as high temperatures and rainfall. Together these conditions (high density of vectors & primary hosts, presence of susceptible individuals, low vaccine coverage and possibly new strains of the virus) may cause the dissemination of YF beyond the limits of the endemic areas.The figures on annual cases in Brazil are variable, with a significant increase of records in the last epidemic (2016-2017) (Figure 3)[27,32-34].As seen in Figure 3, there is a relation between decreases of lethality rates with the increase of number of cases. And the opposite is true: when there were few cases, there was an increase in the lethality rates. This may explain why YF is not a commonly proposed differential diagnosis in patients with clinical symptoms of fever and jaundice at off-peak times, delaying and/or neglecting their diagnoses and treatment. Another hypothesis for the higher rate of deaths is the geographical location of the cases, since they occur mostly in the impoverished regions of countries, where accessibility to good medical care is limited or non-existent[32-34].
Between 1960 - 2015 the number of confirmed cases of YF in Brazil was 1 150, with 407 of those evolving to death. In Central and South America 13 countries are endemic: Argentina, Bolivia,Brazil, Colombia, Ecuador, French Guiana, Panama, Paraguay,Peru, Suriname, Trinidad and Tobago, and Venezuela. During this same period, these countries together have registered 6 873 cases of YF and 2 480 confirmed deaths. Thus, cases in Brazil represented 16.73% of the cases and 16.41% of the deaths in the Americas[27,33-34].Furthermore, in Brazil, 21 of the 27 states and the Federal District were considered as high-risk areas for the transmission of YF. The 2002-2003 outbreak, which occurred prior to the latest outbreak, had 63 cases and 23 deaths confirmed, with a lethality rate of 37%[34,35].A major concern surrounding this outbreak is the reintroduction of the YFV in the urban cycle. There has not been a case of UYF in Brazil since 1942; confirmed cases have only resulted from the sylvatic transmission. The risk of the resurgence of the urban cycle would be materialized simply with anAe. aegyptimosquito bite in someone inadvertently infected by the virus, followed by the inoculation of the saliva with the virus from the mosquito in a healthy individual[36-38].
The 2016/2017 seasonal outbreak is considered to be the largest of its kind observed in the last decades, which, according to the Brazilian Ministry of Health, began December 2016 and ended September 6th, 2017. However, even after the official declaration new cases of YF were observed in the country. In the period between July 1st, 2017 and March 20th, 2018, 920 new cases of the disease were registered, with 300 deaths distributed throughout the Brazilian states[27,34,39,40].
In Figure 4, while comparing the 2003 (Figure 4A) and the 2016/2017 (Figure 4B) outbreaks, there was a significant increase in the number of documented cases and deaths, in all affected states.In the 2017 outbreak all the southeastern states of Brazil presented cases and deaths, whereas in the 2003 outbreak only the state of Minas Gerais was affected. The lethality rate was higher in the states with the lowest number of cases, namely Pará, Mato Grosso,Tocantins and Goiás, possibly due to late diagnoses or because they were states with diminished health care support. The average lethality rates in the 2003 and 2017 outbreaks were very similar:33.87% and 33.72%, respectively[34,37,41].

Figure 4. . Comparison between (A) the distribution of yellow fever cases,by Brazilian state, in the 2003 outbreak in Brazil and (B) the cases notified between 01 December 2016 and 28 February 2018 .
Comparing the 2003 and the 2016-2017 outbreak, there was a significant increase in numbers of registered cases and deaths, in all affected states.All states of southeastern Brazil presented cases and deaths in the 2017 outbreak, whereas in the 2003 outbreak only the state of Minas Gerais was affected. The lethality rate was higher in the states with the lowest number of cases - namely Pará, Mato Grosso, Tocantins and Goiás. The average lethality rate in the outbreak of 2003 and 2017 were very similar: 33.87 per cent and 33.72 per cent, respectively[34, 37, 41].
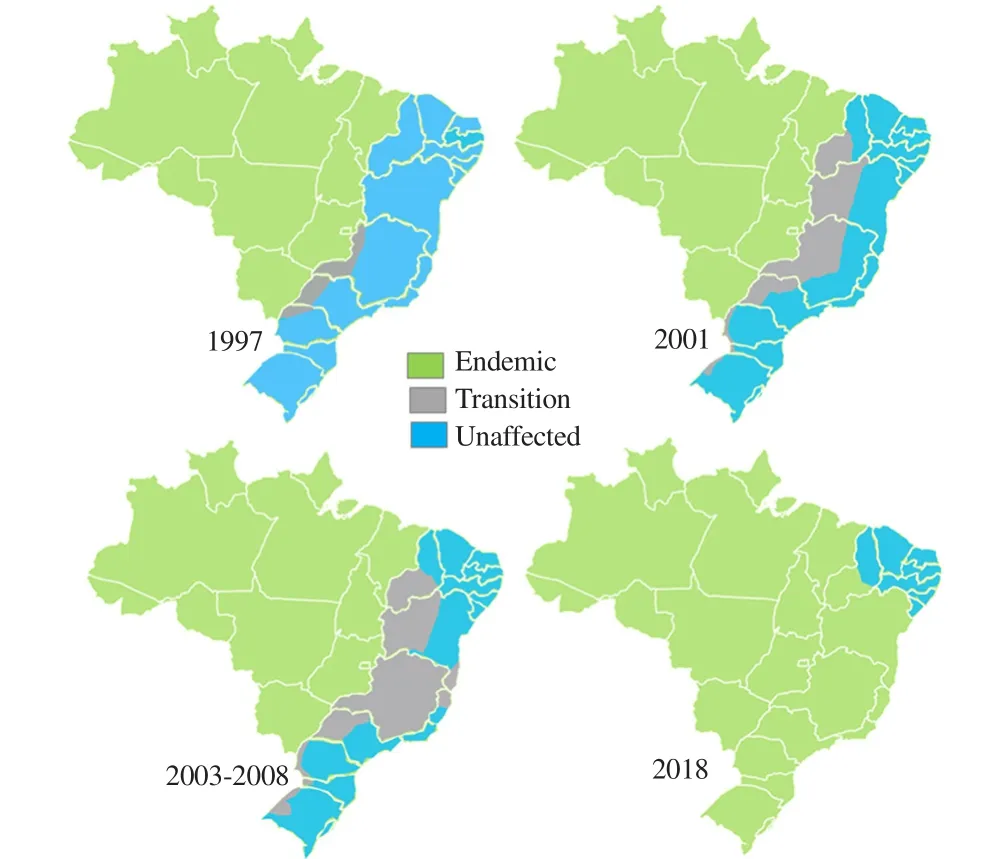
Figure 5. Areas considered endemic (green), of transition (grey), and uninffected (blue) in the years 1997, 2001 and 2003-2008[42].
5. Clinical aspects
The incubation period varies, in general, from 3-6 days. Many patients who contact the virus do not present a conspicuous symptomology. Fever, muscle pain with back pain, headache,and loss of appetite, nausea or vomiting are commonly observed in those patients who develop manifestations. Although in most cases the complaints disappear after 3-4 days, a small percentage of the patients enter in a second, more toxic phase within 24 hours after recovery from those initial symptoms[5,7]. High fever returns and various systems of the body are affected, usually the liver and kidneys. At this stage, it is likely that people develop jaundice, dark urine and abdominal pain with vomiting. Bleeding may occur in the mouth, nose, eyes or stomach. Half of the patients enter the toxic phase within 7-10 days. Those who recover from YF generally have long-lasting immunity against a subsequent infection[43-45].
Depending on the severity of the symptoms, YF can be subdivided into four forms: mild, moderate, severe and malignant[5,43]. The evolution of the disease occurs in two stages. In the first, which lasts from 2 - 3 days, there is fever, moderate to severe headache,generalized myalgia, hyperemia and conjunctival congestion. It is followed by a reduction in signs and symptoms which may vary from hours to days, corresponding to the reduction in the number of viruses in the bloodstream. This reduction occurs once the virus migrates to the liver, spleen, heart and other organs, initiating the second stage. In that second stage there is high fever, significant jaundice and hemorrhages. It is a form characterized by high lethality, which may reach 80% - 90%[43,44].
6. Diagnosis
YF infection should be considered as a differential diagnosis in all cases of febrile syndrome in individuals with contact to endemic regions[7]. The diagnosis is made from a detailed clinical evaluation,with specific and non-specific laboratory tests. The specific tests define the diagnosis of the disease, while the non-specific tests act as a prognosis of the disease[28,46-49].
Non-specific methods include hemogram (leukopenia with neutropenia and lymphocytosis with thrombocytopenia and anemia); erythrocyte sedimentation rate (very low, possibly zero);Aminotransferases (extremely high); bilirubin (elevated); nitrogenous compounds (elevation of urea and creatinine); coagulation disorders(reduction of Factor Ⅷ serum level, increased prothrombin time and activity, prolongations of coagulation and bleeding time); and Urineanalysis (abnormal elements and sediment)[28,49].

Table 1 Immunization scheme in Brazil from March 2017, according to the characteristics of the patients[56,57].
Specific methods include isolation of the virus (intracerebral inoculation in rats or in cell culture and isolation); molecular biology(identification of the genetic material of the virus by the reverse transcription-polymerase chain reaction); immunohistochemistry(histopathological analysis); and antibody screening (serological tests)[47,49-50].
There are diseases with clinical manifestations similar to those of YF that should be differentiated. The main ones are malaria(clinical and geographical similarity of distribution); dengue (it is differentiated from YF by the rarer appearance of jaundice;in the early stages it is clinically indistinguishable from YF)[18];leptospirosis (differentiation occurs through laboratory tests of the leukogram, marked by the presence of leukocytosis with neutrophilia and by the dosage of aminotransferases, which will normally be increased with high erythrocyte sedimentation rate)[47,49]; sepsis viral hepatitis, arenaviruses, filoviruses (Ebola and Marburg),hantavirus, rickettsia diseases (epidemic typhus, endemic typhus,spotted fever and Q fever) and labrea black fever[26,50,51].
7. Treatment
There has not been any successful treatment of YF with interferon gamma, Ribavirin and EICAR drugs; there is no specific antiviral for the virus[3,52]. In mild cases, outpatient care with daily visits is considered, with guidance on the risk of rapid aggravation. In this case it is necessary the prescription of hydration (60 mL/kg/day) and avoiding drugs with hepatotoxic risk. In patients with moderate or severe clinical status, hospitalization with a clinical and laboratory follow-up is necessary. Patients with the malignant form can present high digestive hemorrhage, low level of consciousness or respiratory insufficiency, and require endotracheal intubation and mechanical ventilations. In this condition they may require dialysis, in addition to supplementary medications for homeostasis control[53]. More recently, measures like use of sofosbuvir (new antiviral drug) and liver transplant have been attempted in severe cases, but these procedures need to be investigated thoroughly[54].
8. Prophylaxis and control
At the beginning of the 20th century the fight against YF was focused on vector elimination, reducing outbreaks and using insecticides available at the time. The aquatic phase of the vector cycle was at the epicenter of health campaigns at the time and it seemed to have great population engagement[55].
The history of governmental campaigns during this time showed an effort to eliminate outbreak, and a greater engagement from the population. Measures to combat the disease were solicited,such as adequation of water drainage from the streets, eliminating potential reservoirs, cleaning up plots, in addition to the so-called“Aculex”, devices coupled to the sewers to effectively interrupt the reproduction of theAe. aegypti[55].
When it comes to governmental combative teams, there was the‘Training School for Police Personnel on Outbreaks’, which trained qualified professionals to eliminate the foci of reproduction of the mosquito[55]. Individual protection and the prevention of the reurbanization of the disease are the main aspects to be taken into account in the prophylaxis and control of the YF in Brazil[12].
There is clear epidemiological evidence of the success of the YF vaccine in combating the virus, with a 95% efficacy applying a 0.5 mL subcutaneous dose[14]. It is included in the vaccination calendar of endemic regions in Brazil, being offered by the Brazilian Public Health System, the so-called Sistema Único de Saúde (SUS) (the system was created in 1990 and inspired by the British NHS and is entirely free of any costs, for any person, including foreigners),since 2017, in one dose, which confers sustained life-long protective immunity against YF[56].
The main route of protection against the virus is the vaccine.However, there are alternative functional ways to avoid the bite of a potentially infected mosquito. These may include the use of mosquito nets on beds and windows, the use of clothes that cover large areas of the body, application of repellents with N-diethylmetatoluamide. In addition, there are constant efforts to raise awareness to combat the vector, including policies against leaving stagnant water exposed for the deposition ofAe. aegyptieggs[57,58].The vaccines available in Brazil are the Biomanguinhos/Fiocruz(used in the public health system. Composed of the attenuated live virus which is cultivated in chicken eggs); and Sanofi Pasteur (used in the private health system. Composed of the attenuated live virus which is cultivated in chicken eggs)[57].
The contraindications of the YF vaccine are children under six months, symptomatic HIV-infected individuals with laboratoryproven severe immunosuppression, individuals with demyelinating neurological disease within 6 weeks after the previous vaccine application, individuals with cancer or who performed organ transplants, individuals with a history of anaphylactic reaction to some component of the vaccine and individuals with previous history of disease in the thymus or organ removal.
8.1. Update of the Brazilian Ministry of Health on doses of vaccine
Until March, 2017, Brazil adopted the double dose regimen of the vaccine: a second dose would be applied 10 years after the first. This indication was based on national and international studies that stated there would be a reduction in long-term immunity in those who had taken only one dose[56,57].
Brazil was the only country to adopt two doses of vaccine, despite WHO guidelines declaring that only one vaccine already conferred immunity. However, given the epidemiological situation in the country over the last 2 years, associated with the fact that production was not meeting the demand, the Ministry of Health adopted the WHO recommendations and started to recommend only one dose of the vaccine as of April 5th, 2017. Therefore, those individuals who had been vaccinated at any time in their life did not require the application of a booster dose and were consequently considered immunized[56-58].
8.2. Chronology of regions with vaccine recommendation
There were changes in which states would have the vaccine recommendation. On March 20th, 2018, a vaccine extension was established for almost the entire Brazilian territory, and will be gradually implemented until April, 2019. This measure includes the Southern and Southeastern regions of the country that were previously areas of temporary recommendation. Some states from Northeast region are now included in the vaccine recommendation,as shown in Figure 6[42,59].
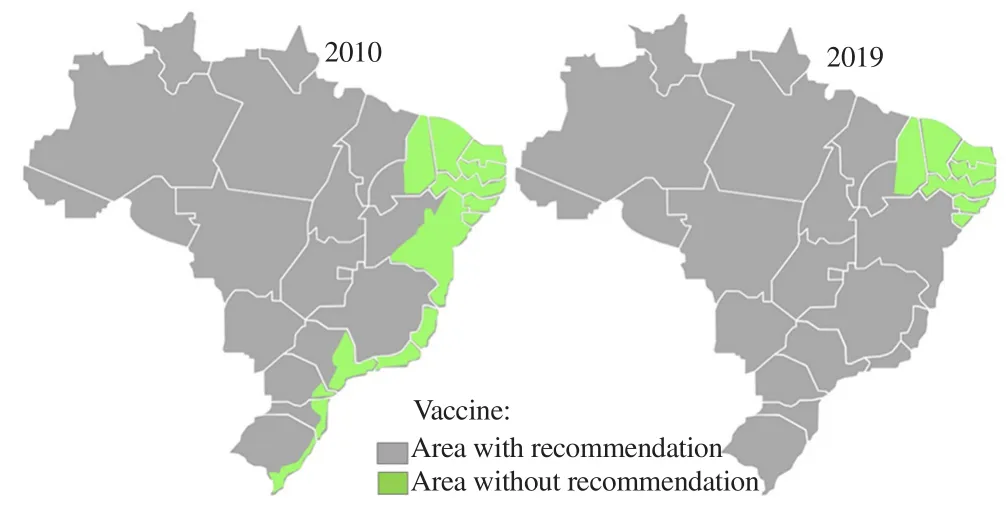
Figure 6. Brazilian area of vaccine recommendation in 2010, and 2019[42, 59].
8.3. Fractional dose of yellow fever vaccine in the outbreak -2018
Because of the potential shortage of the YF vaccines, which was related to the outbreak in Angola and the DRC, the WHO Strategic Advisory Group of Experts analyzed the existing evidence and demonstrated that using one fifth of the standard dose of the vaccine(0.1 mL instead of 0.5 mL) would still provide protection against the disease for at least 12 months and possibly for much longer. SAGE concluded that this available evidence was enough to determine that the fractional dosing of the vaccine could be a safe and effective option for mass vaccination campaigns to control urban outbreaks in acute vaccine shortages. Fractional dosage is considered a short-term measure to preserve stock[60].
This approach is not proposed for routine immunization, as there is not enough data available to show that lower doses would confer the same lifelong protection provided by the full dose vaccination.A fractionated application does not qualify for a YF vaccine certificate in accordance with the International Health Regulations requirements. Travelers must receive the full dose of the vaccine for issuance of the international vaccination certificate[60].
In January, 2018, the Brazilian Ministry of Health, with the approval of the WHO, announced that it would initiate a fractionated vaccination campaign in 76 municipalities in the states of São Paulo,Rio de Janeiro and Bahia to expand immunizations and prevent a new outbreak. The focus was on major cities with high population density, with records of SYF in nearby locations[61]. The only difference between the fractionated vaccine and the previous version,is in the applied volume, which is 0.1 mL, 20% of the standard amount of 0.5 mL. Therefore, a vial that previously immunized one person can now serve up to five people. In studies conducted by Biomanguinhos/Fiocruz, the fractional dose can guarantee antibodies in the body for at least eight years. Moreover, WHO reported no difference in the efficacy of the two vaccines in the study period when comparing immune response[62].
Contraindications of the fractional vaccine: children 9 months to 2 years of age and international travelers; since the fractionated dose is not valid for the issuance of an international YF vaccination certificate. The contraindication is for people with severe egg allergy,pregnant women and people with immunosuppression. Blood donations are not indicated for 4 weeks[61].
8.4. Prevention of new sylvatic yellow fever cases in Brazil
For the development of effective combat plans, a State Reporting Program is essential to identify epizootic outbreaks and to record places where human contamination has occurred. In Brazil, the updates are made through epidemiological notes published by the Ministry of Health, which organizes the data by time and place,broadcasting it on official government websites, and administering the correct distribution of funds to control the disease. Wildfires and deforestation are actions that can increase the area of risk, being the modifiers of the primates’ natural habitat. Human settlement in places infested withHaemagogusandSabethesvectors is another determinant factor, which can increase the risk of transmission and regulation of the cycle[53,63].
It is essential in these cases that those who enter sylvatic cycle regions are immunized against YF and adopt individual protection measures such as the use of repellents and clothing covering the bodily surfaces. Thus, avoiding possible infection and redevelopment of the virus if they are stung byAe. aegyptiin urban areas while still infected[10]. Brazil offers the vaccine for free in the health centers by SUS for all citizens, and the National Health Surveillance Agency issues the International Certificate of Vaccination or Prophylaxis for those traveling to countries that require immunization[64].
8.5. Prevention of yellow fever reurbanization in Brazil
The logistics involved in the prevention of UYF are based mainly on measures to combat the vector. The insect control is directly related to its resistance to insecticides in places where this control method is used. One known mechanism of resistance is the biodegradation by detoxifying enzymes. However, there have been genomic changes included in this mechanism that have rarely been identified, which hinders individual resistance genotyping[26,28,58].
Two important metabolic resistance markers are: variations in the copy number and polymorphisms of the detoxification enzymes. Analysis of the copies identified 41 gene amplifications associated with resistance,and polymorphism analysis detected more than 30 000 variants. These 2 variables differ in continents, which shows that insecticide resistance is expressed in different ways around the world, indicating the need for different control standards, sufficient for the variations in each region[65].
Behavioral measures include disruption of theAe. aegyptilife cycle through improvements in water distribution and collection systems, garbage collection, health education campaigns to eliminate potential outbreaks of mosquito egg deposition, and the larvicide use in places where such elimination is not possible. In addition, health surveillance at border and immigration locations (such as ports and airports) and appropriate guidance for travelers in infected areas is necessary[10,28].
The third updated edition of the WHO’s International Health Regulations (1969), requires notification of all YF cases. Member States should notify WHO and border countries if there are imported,transferred or autochthonous cases of YF in non-endemic regions and/or newly found or reactivated cases in non-human vertebrates.Regulation also has specifications for water, air and ground transportation vehicles that originate in regions with active virus transmission[18,26,28].
Notwithstanding all the implemented forms to combat the vector and prophylaxis, in the 21st century, especially the vaccine made available by SUS, the persistence of cases and the recent outbreak in Brazil, demonstrates that combative measures are not always fully effective[18,66].
9. Final considerations
In Brazil, since the 1940s, there were no documented outbreaks of magnitude similar to what occurred in the period between 2016 -2018.From December, 2016 to August, 2018, there were 2 045 confirmed cases and 677 deaths by YF in Brazil[66,67]. In addition, there were reports of the exportation of the virus, mainly by unvaccinated visitors.So, strategies to ensure vaccination of travelers from endemic regions needed to be monitored and encouraged in order to reduce the risk of international spread[7,66].
One of the striking features of the last outbreak in Brazil (2016-2018)was the spatial expansion throughout geographical regions considered free - since the 1960s - of the YFV[28,58]. Although no cases of UYF were reported, there was an imminent threat of urban transmission,due to the existence of favorable conditions for viral transmission,the main one being the presence ofAe. aegyptimosquitoes[66].The Brazilian government responded aggressively to control the outbreak by implementing vaccination in the affected areas, totaling more than 57 million doses used. The outlook until 2019 is for immunization throughout almost the entire Brazilian territory,requiring supplementary dosages for more than 77.5 million people.Such preventive measures should be accompanied by constant epidemiological surveillance to minimize the possibility of further outbreaks[66,67].
The precise detection and notification of YF cases is essential to reduce the spread of YF outbreaks. Difficulties in ensuring precise diagnostic test can delay the confirmation of new cases. This is an issue that is related to the limited overall supply of vaccines. In some cases, use of fractionated doses is required, as in Brazil, 2017.The investment in strengthening vaccine production and diagnostic laboratories to promote early detection and to ensure effective case containment measures is necessary to guarantee adequate prophylaxis in risk zones.
Acknowledgments
This study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-National Council for Scientific and Technological Development) and the Research Program of the Faculdade Dinâmica do Vale do Piranga (PROAPP/FADIP). We thank Paula Carolina Andrade Mariano and Ademir Nunes Ribeiro Júnior for their contributions, and technical support,creating the cycles and designing the images.
 Asian Pacific Journal of Tropical Medicine2019年2期
Asian Pacific Journal of Tropical Medicine2019年2期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Observational study to assess pregnant women's knowledge and behaviour related to toxoplasmosis in Essaouira province, Morocco
- Insecticide susceptibility status and resistance mechanism of Anopheles cracens Sallum and Peyton and Anopheles maculatus Theobald (Family:Culicidae) from knowlesi malaria endemic areas in Peninsular Malaysia
- Survival rate and the determinants of progression from HIV to AIDS and from AIDS to the death in Iran: 1987 to 2016
- Occurrence of Chlamydia spp. in wild birds in Thailand
- Modeling and predicting dengue fever cases in key regions of the Philippines using remote sensing data
