Percutaneous devices for left atrial appendage occlusion: A contemporary review
Homam Moussa Pacha, Yasser Al-khadra, Mohamad Soud, Fahed Darmoch, Abdulghani Moussa Pacha,M Chadi Alraies
Abstract Patient with atrial fibrillation (AF) are at risk of developing stroke with the left atrial appendage (LAA) being the most common site for thrombus formation. If left untreated, AF is associated with 4 to 5 folds increase in the risk of ischemic stroke in all age groups. About 5% to 15% of AF patients have atrial thrombi on transesophageal echocardiography, and 91% of those thrombi are located in the LAA in patient with nonrheumatic AF. Although oral anticoagulants are the gold-standard treatment for stroke prevention in patients with non-valvular AF,some patients are at high risk of bleeding and deemed not candidates for anticoagulation. Therefore, LAA occlusion (LAAO) has emerged as alternative approach for stroke prevention in those patients. Surgical LAAO is associated with high rate of unsuccessful closure and recommended only in patients with AF and undergoing cardiac surgery. Percutaneous LAAO uses transvenous access with trans-septal puncture and was first tested using the PLAATO device.Watchman is the most common and only Food and Drug Administration (FDA)approved device for LAAO. LAAO using Watchman device is non-inferior to warfarin therapy in preventing ischemic stroke/systemic thromboembolism.However, it is associated with lower rates of hemorrhagic stroke, bleeding and death. Amplatzer is another successful LAAO device that has CE mark and is waiting for FDA approval. Optimal antithrombotic therapy post LAAO is still under debate and highly patient-specific. The aim of this paper is to systematically review the current literature to evaluate the efficacy and safety of different LAAO devices.
Key words: Left atrial appendage; Atrial fibrillation; Anticoagulation; Stroke; Mortality
INTRODUCTION
Atrial fibrillation (AF) affects 2.7 to 6.1 million in the United States and 33.5 million worldwide[1-3]. The projected prevalence of AF in the United States is expected to be 12.1 million by 2030[4]. AF-associated stroke is the most feared complication and the leading cause of disability in the United States[5]. If left untreated, AF is associated with 4 to 5 folds increase in the risk of ischemic stroke in all age groups[5,6].Furthermore, AF is associated with increased risk of extracranial thromboembolic events to the aorta; and renal, mesenteric, and peripheral arteries[7]. The proportion of strokes attributed solely to AF increases with age and may reach up to 23.5%[6,8]. Oral anticoagulants (OACs) remain to be the gold standard treatment for stroke prevention, and their role in preventing AF-related strokes is well established[9,10]. Yet,OACs are contraindicated in a subset of patients who are at high risk of bleeding. As a result, left atrial appendage occlusion (LAAO) has emerged as an alternative approach in this group. In the current article, we present the most updated studies describing safety, efficacy and outcome of different LAAO devices.
LITERATURE SEARCH
A systematic literature search was conducted using PubMed, EMBASE, and Cochrane Library to identify relevant articles from 1990 to 2018. The following search terms were used: “atrial fibrillation”, “stroke”, “left atrial appendage”, “occlusion” or“closure”, and “percutaneous” or “surgical.” A total of 78 studies were included for review. Of the included studies on LAAO, 3 studies contained surgical LAAO, two contained Atriclip device, two contained Tiger Paw system, 6 contained Lariat device,4 contained PLAATO device, 19 contained Watchman device, and 12 contained Amplatzer (ACP/Amulet) device.
LEFT ATRIAL APPENDAGE AND THROMBUS FORMATION
Left atrial appendage (LAA) is trabeculated long tubular structure that has narrow junction with the venous component of left atrium. it varies greatly in sizes and shapes and has bent or spiral axis in 70% of patients[11]. Anatomically, LAA is best divided into the ostium, neck, and lobar region[12]. In patients with chronic AF,remodeling of LAA leads to dilation, stretching and reduction in pectinate muscle volume[13].
Approximately, 5% to 15% of AF patients have atrial thrombi on Transesophageal echocardiography (TEE)[14-17], and 91% of those thrombi are located in LAA in patients with nonrheumatic AF[18]. The reason for LAA predilection for thrombus formation in AF is still not well known. One theory suggests that the extent of LAA filling and emptying is influenced more by changes in the left ventricular (which is impaired in AF) than LAA function[19]. Ventricular filling creates intracavitary suction effect which influences the emptying and filling of left atrium and LAA.
IMAGING ASSESSMENT OF LAA
Accurate assessment of anatomic LAA characteristics is crucial prior to LAAO due to substantial variations in LAA anatomy that impact device selection and efficacy. TEE is the most widely used imaging tool for periprocedural LAA assessment. It is used for the detection of thrombi in the LA and LAA as well other cardiac masses and thrombi prior to LAAO[12,20]. Features on TEE associated with increased risk of thrombus formation include: reduced LAA flow velocity, spontaneous left atrial contrast, and aortic atheroma[16]. TEE is very important imaging to support fluoroscopy during device implantation. 3D TEE has shown to be more accurate than 2D TEE in LAA assessment and thrombi detection[21,22]; and therefore, it is recommended for the guidance of LAAO[23]. It is used to guide trans-septal puncture,verify catheter and sheath position in the LAA, aid device delivery and positioning,confirm adequate LAA sealing, and detect complications[12]. Follow-up TEE is also recommended after LAAO to reassess the implanted device, confirm complete LAA closure, and rule out complications. Intracardiac echocardiography (ICE) is comparable imaging to TEE for guiding LAAO and performing the tasks typically provided by TEE during implantation. In one study LAA measurements by ICE during LAAO were significantly correlated to angiography and TEE (Pearson correlation coefficient r = 0.94, P < 0.0001 for both)[24].
Multidetector computed tomography is another imaging modality that is used for the assessment of thrombus formation, LAA anatomy and function, device assessment and detection of complications post procedure[12]. It provides 3D images of the heart by using numerous planes at different points in time during the cardiac cycle and has 100% sensitivity for excluding LAA thrombus[25]. However, its use is limited due to ionizing radiation, lower temporal resolution than TEE and inability to perform during device deployment. angiography has been used for in LAA thrombi detection[26]. However, it is expensive and invasive procedure, and rarely used nowadays due to presence of TEE and other less invasive imaging modalities.
GUIDELINE THERAPY FOR STROKE PREVENTION
The 2014 American Heart Association/American College of Cardiology (AHA/ACC)guidelines for management of AF recommends the use of anticoagulation for prevention of thromboembolism when CHA2DS2-VASCscore is ≥ 2 [class I (A)][16]. The 2016 European Society of Cardiology (ESC) guidelines differentiate between males and females regarding anticoagulation recommendations[27]. While anticoagulation is class I (A) indication for males with a score ≥ 2 and females with a score ≥ 3, it's considered class IIa (B) indication for males with a score of 1 and females with a score of 2. Both American and European guidelines recommend considering surgical excision of LAA in patients who have AF and undergoing cardiac surgery [class IIb(level of evidence is "C" in AHA/ACC and "B" in the ESC guidelines)][16,27]. While AHA/ACC guidelines have no recommendations for LAAO, the ESC guidelines have class IIb (B) recommendation for LAAO in patients with AF and contra-indications for long-term anticoagulation[27]. Similarly, National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand state that LAAO may be considered for stroke prevention in patients with non-valvular AF at moderate to high risk of stroke and with contraindications to OAC (GRADE quality of evidence: Low;GRADE strength of recommendation: Strong)[28].
LAA SURGICAL CLOSURES/EXCISION
Surgical exclusion of LAA is recommended for patients with AF and undergoing concomitant cardiac surgeries. Different surgical methods to isolate LAA include:suture ligation, excision and suture closure, and stapling exclusion with or without excision[29,30]. Surgical isolation of LAA is associated with high rate of unsuccessful closure. For instance, a previous study reported only 40% (55 out of 137) complete LAA closure noted on TEE following surgical closure[30]. Despite that, Friedman et al[31]reported a lower risk of readmission for thromboembolism (4.2% vs 6.2%, HR = 0.67;95%CI: 0.56-0.81) and all-cause mortality (17.3% vs 23.9%, HR = 0.88; 95%CI: 0.79-0.97)among Medicare patients (age > 65) with AF undergoing concomitant cardiac surgery and surgical LAAO, compared with no surgical LAAO. This the largest study to date supporting the role of surgical LAAO during cardiac surgery as a mean of preventing thromboembolism in patients over the age of 65 with AF.
The Atriclip Device System (Atricure, Inc., West Chester, OH, United States) is a surgical LAA exclusion device composed of self-closing, sterile, implantable clip with a reusable deployment tool (Figure 1). It is applied epicardially by either an open surgical or a minimally-invasive technique and placed at the base of the appendage.The clip is made of 2 parallel rigid titanium tubes with elastic nitinol springs covered with a knit-braided polyester sheath (Table 1)[32,33]. The EXCLUDE study (Exclusion of LAA with AtriClip Exclusion Device in Patients Undergoing Concomitant Cardiac Surgery) is a nonrandomized multicenter trial that included 70 patients to evaluate the efficacy of Atriclip device[33]. They enrolled adult patients undergoing elective primary cardiac operations via median sternotomy (coronary artery bypass grafting,valve re- pair or replacement, surgical Maze procedures, or atrial septal defect repair)and have CHADS2> 2. 67 out of 70 patients (95.7%) had successful intraoperative LAA exclusion, and 60 out of 61 patients (98.4%) had successful LAA exclusion seen on computed tomography angiography or TEE imaging after 3 mo[33]. Tiger Paw System (Terumo Cardiovascular Systems, Ann Arbor, MI, United States) is another LAA exclusion device that is used as a concomitant procedure during open cardiac surgical procedures (Figure 1). The device contains implantable fastener of titanium connectors that staples the LAA tissue and is embedded in two rims of silicone that adapts to the LAA morphology and seals the puncture sites (Table 1)[34]. Despite its efficacy in achieving complete LAA closure on prior study[34], a class 1 recall from the market by FDA was made in 2015 due to device malfunction[35].
LARIATE DEVICE CLOSURE SYSTEM
Lariat device (SentreHEART, Inc., Redwood City, California) is LAA closure system that is approved by the United States Food and Drug Administration (FDA) for soft tissue closure, but not LAAO (Figure 1). It is composed of 15-mm compliant occlusion balloon catheter (EndoCATH), 0.025-inch and 0.035-inch magnet-tipped guidewires(FindrWIRZ), and a 12-F suture delivery device (LARIAT) (Table 1). During the procedure, magnet-tipped guidewires are advanced through epicardial and transvenous accesses and connected in the LAA. Then, a suture fashioned as a Lariate or lasso is advanced over the epicardial access guidewire and tightened to occlude LAA base[29,36]. The largest prospective study of Lariate device included patients who:were ≥ 18-year-old; had nonvalvular AF; had CHADS2 ≥ 1; were poor candidate for or failed warfarin therapy; and had a life expectancy of at least 1 year[36]. They reported 95% (81 of 85 patients) complete LAA closure documented on TEE one month after the procedure. 98% of those who underwent TEE (n = 65) had complete LAA closure after 1 year, including cases of incomplete closure at earlier time. Complications in the same study were limited to only two cases of severe pericarditis, two cases of strokes,and one case with pericardial effusion[36]. Another study demonstrated similar efficacy of the Lariate device for stroke prevention[37]. Dar et al[38]demonstrated that LAAO using Lariate device might improve the mechanical function of the left atrium (LA)and reverse LA remodeling based on 2-dimensional speckle tracking echocardiography (a novel method for functional assessment of the LA). However,due to steep learning curve for device deployment (especially epicardial access), LAA leak and lack of direct efficacy comparison with oral anticoagulation, the device was not widely used in the United States[39-42].
PERCUTANEOUS LAA CLOSURE
The most commonly used percutaneous LAAO devices are shown in figure 1 and described in Table 1. Percutaneous LAAO uses transvenous access with trans-septal puncture and was first tested using the Percutaneous LAA Transcatheter Occlusion(PLAATO) device (Appriva Medical Inc., Sunnyvale, CA) in 2001.
PLAATO device
This device consists of self-expanding nitinol cage that is covered with polymeric membrane in order to close off blood flow into the LAA (Table 1)[43,44]. It was first tested on 15 patients with non-valvular AF and contraindication to warfarin therapy and are at high risk of thromboembolism based on CHADS2score[43]. Successful occlusion of LAA was observed in all cases and no device related complications were reported. A larger prospective study enrolled patients using similar inclusion criteria to undergo LAAO using PLAATO device[44]. Similarly, they reported high successful device Implantation in 108 out of 111 patients (97.3%) with only 2 patients developed stroke on follow up (2.2% annual risk of stroke). Subsequently, the European PLAATO2 trial reported successful LAAO in 90% (126 out of 140) of patients with reduction of stroke rate from 6.6% (based on CHADS2score) to 2.3% per year[45].Besides, a single center prospective study on 73 cases who had PLAATO device reported death due to device embolization in one patient and implant instability requiring open heart surgery in another one[46]. Interestingly, there was no incidence of stroke for 24 mo of follow-up in the same study. Despite this success, the device was discontinued for unspecified reasons and replaced by Watchman device.

Table 1 Comparison of left atrial appendage occlusion devices
Watchman device
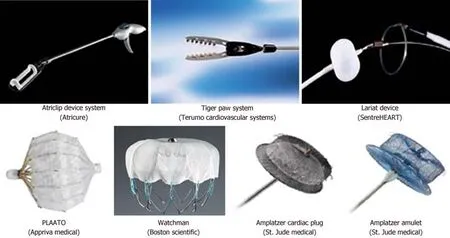
Figure 1 Surgical and percutaneous devices that are used for left atrial appendage occlusion.
The Watchman device (Boston Scientific, Marlborough, MA), is the only FDA-approved percutaneous device for LAAO. The device is composed of self-expanding nitinol frame structure with fixation barbs and a permeable polyester fabric that covers the atrial facing surface of the device (Table 1)[47]. Multiple trials were done to evaluate the safety, efficacy and outcomes of watchmen device.
Pilot study was a non-randomized trial that included 75 patients and was done to assess the feasibility and safety of watchman device[47]. They enrolled adult patients who: had non-valvular AF for 2 years, were eligible for warfarin therapy, and had CHADS2of at least 1. Although this was the first human trial to evaluate the efficacy and safety of Watchman device, the success rate of LAAO was very high and complications were relatively low. 88% of patients had successful device implantation and 93% of them had complete LAAO. Reported complications included; device embolization in 2 patients, device-related thrombus formation in 4 patients, and transient ischemic attack in 2 patients. There was no reported major strokes or procedure-related mortality.
PROTECT AF study (WATCHMAN LAA System for Embolic Protection in Patients with Atrial Fibrillation) was the first randomized trial to compare the efficacy and safety of LAAO using Watchman device with chronic warfarin therapy in patients with non-valvular AF and had CHADS2of 1 or more[48]. Exclusion criteria included contraindications to warfarin, chronic warfarin use, LAA thrombus, a patent foramen ovale with atrial septal aneurysm and right-to-left shunt, mobile aortic atheroma, and symptomatic carotid artery disease. This trial enrolled 707 patients from 59 centers worldwide and assigned them randomly to LAAO with Watchman device (n = 463) or warfarin therapy (n = 244) with INR goal of 2 to 3. Watchman group was treated with warfarin for 45 d after device deployment to allow proper endothelialization.Warfarin was discontinued if TEE showed complete closure or significantly decreased flow around the device. Afterward, patients were given aspirin and clopidogrel for 6 mo followed by lifelong aspirin. At 1065 patient-years (PY) of follow-up (mean follow up 18 mo), Watchman device was non-inferior to warfarin for primary efficacy endpoint of stroke (either ischemic or hemorrhagic), cardiovascular death, or systemic thromboembolism. The Event rates of primary efficacy endpoint were 3% and 4.9%for Watchman and warfarin groups, respectively. Since then, two studies were published with two different follow up period[49,50]. At 2.3 ± 1.1 years (2621 PY),Watchman device continued to be non-inferior to warfarin therapy with 3% and 4.3%event rates of primary efficacy endpoint for Watchman and warfarin groups,respectively[49]. The second trial with 3.8 ± 1.7 years of follow up (2621 PY) showed event rate of 2.3% in the watchman group and 3.8% in the warfarin group (P = 0.0348),leading to 40% risk reduction in primary efficacy endpoint with Watchman device[50].
PREVAIL study (Evaluation of the Watchman LAA Closure Device in Patients With Atrial Fibrillation vs Long Term Warfarin Therapy) was another randomized trial that assessed the safety and efficacy of Watchman device in patients non-valvular AF[51]. Investigators included a higher risk patients than PROTECT AF (CHADS2 score of 1 plus any of the following higher-risk characteristics: female age ≥ 75 years,baseline ejection fraction ≥ 30% but < 35%, age 65 to 74 years and either diabetes or coronary disease, and age ≥ 65 years with congestive heart failure). Patients were assigned randomly to receive LAAO using Watchman (n = 269) or warfarin therapy (n= 138) in 2:l ratio. Warfarin and antiplatelet therapy post device implantion was in a similar fashion to PROTECT AF trial. Although non-inferiority criteria was not achieved for overall efficacy endpoint (stroke, systemic embolization or cardiovascualr death), the rate of second efficacy endpoint (stroke or systemic embolization) was 2.5% in the Watchman group and 2% in the warfarin group at 18 mo follow-up, achieving criteria for non-inferiority. Compared to PROTECT AF study, procedural success increased from 90.9% to 95.1% (P = 0.04), while all 7-d procedure-related complications (composite of cardiac perforation, pericardial effusion with tamponade, ischemic stroke, device embolization, and other vascular complications) decreased from 8.7% to 4.2% in PREVAIL (P = 0.004).
PROTECT AF and PREVAIL results were pooled for patient level meta-analysis and with combined follow-up of 5 years (4343 PY)[52]. The primary efficacy endpoint(stroke, systemic embolization or cardiovascualr death) was similar between LAAO and warfarin groups (2.8 vs 3.4 events/100 PY; P = 0.27). In subgroup analysis of the same meta-analysis, the rate of all stroke or systemic embolism was similar between both groups (1.7 vs 1.8 events/100 PY; P = 0.87). However, there was statistically significant decrease in the rates of hemorrhagic stroke (0.17% vs 0.87%, P = 0.002),disabling / fatal stroke (0.37% vs 0.94%, P = 0.027), cardiovascular/unexplained death(1.3% vs 2.2%, P = 0.027), all-cause death (3.6% vs 4.9%, P = 0.035), and post-procedure bleeding (1.7% vs 3.6%, P = 0.0003) in LAAO arm when compared with warfarin arm.This meta-analysis underscores the mortality reduction and stroke prevention,patrticularly hemorrhagic stroke, associated with LAAO using Wathcman device.
Continued Access to PROTECT AF (CAP)[53]and Continued Access to PREVAIL(CAP2)[54]Registries were designed to treat patients with similar baseline characteristics and according to same protocols after PROTECT AF and PREVAIL trials enrollment had been completed. Procedural performance and associated medications were identical in each registry. However, registries did not mandate 1-year neurological assessment. A Meta-analysis of 2406 patients from the PROTECT AF and PREVAIL trials and their respective registries (CAP and CAP2) with 5,931 PY of follow-up (mean of 2.69 years) reported: similar rate of all-cause stroke between both arms (1.75 vs 1.87 events/100 PY, P = 0.94): higher rate of ischemic stroke in Watchman group (1.6 vs 0.9 events/100 PY, P = 0.05); and lower rates of hemorrhagic stroke, cardiovascular death (1.1 vs 2.3 events/100 PY, P = 0.006), and non-procedural bleeding (6.0% vs 11.3%, P = 0.02) in Watchman group[54]. Although the rate of allcause stroke was similar between both arms, the reduction in hemorrhagic stroke with Watchman device was balanced by a relative increase in ischemic stroke rates. This may relate to possible technical failures of the device: failure to completely obliterate LAA flow, anatomical remodeling of the LAA ostium over time resulting in more leaks, or the development of thrombus on the device[54]. Compared with the pooled results of PROTECT AF and PREVAIL trials mentioned above, the difference in ischemic stroke rate was not observed between LAAO and warfarin groups at longer and combined follow-up of 5 years[52].
EWOLUTION study (Registry on Watchman Outcomes in Real-Life Utilization) is a multicenter, prospective, non-randomized cohort that aimed to collect peri-procedural and long-term outcome data for patients implanted with Watchman device for LAAO[55]. This world-wide registry enrolled 1025 patients at 47 centers from the United States, Europe, Middle east and Russia who are more than 18-year-old and require LAAO based on ESC guidelines[55-57]. The device was successfully implanted in 98.5% and complete LAAO was achieved in 99.3% noted on TEE[56,57]. the rates of procedure-related serious adverse events (defined as; perforation, tamponade,embolism, neurological events, thrombosis, and bleeding) were 2.8% at 7 d and 3.6%at 30 d with bleeding being the most common adverse event[57]. This is lower than the 7-d procedure-related serious adverse events observed in PROTECT AF (8.7%) and PREVAIL (4.2%) trials. At 1 year follow up; mortality was 9.8%, device-related thrombus was seen in 3.7% of patients, and 1.1% of patients suffered from ischemic stroke, leading to 84% risk reduction of stroke. There was no hemorrhagic stroke observed during follow-up[56].
The ASAP study (ASA Plavix Feasibility Study with Watchman LAA Closure Technology Trial to assess) was a European multicenter, prospective, non-randomized study of Watchman device in patients with non-valvular AF who had CHADS2score≥1 and were not eligible for OACs[58]. After the device implantation, participants were given thienopyridine antiplatelet agent (clopidogrel or ticlopidine) for 6 mo and aspirin indefinitely. Out of 150 patients, 142 (94.7%) had successful implantation and 13 (8.7%) developed device-related adverse event. During mean follow up of 14.4 ±8.6 mo, 4 patients developed stokes (2.3% per year) and 3 of them were ischemic (1.7%per year). There was 77% risk reduction in stroke compared to expected stroke risk based on CHADS2score (7.3% per year). Till this moment, there is no published randomized data on the safety and efficacy of LAAO in patients with contraindications to anticoagulation. The ASAP TOO study (The Assessment of the Watchman Device in Patients Unsuitable for Oral Anticoagulation) is ongoing multicenter prospective randomized trial plan is to enroll up to 888 patients with nonvalvular AF who are not candidate for OAC and have CHA2DS2-VASC≥ 2[59]. The study will randomize patients to Watchman vs control. Control patients will be prescribed single antiplatelet therapy, or no therapy based on physician discretion.
Amplatzer cardiac plug and amulet
Amplatzer cardiac plug (ACP) (AGA, St. Jude Medical, Minneapolis, MN, United States) is another LAAO device that consists of a lobe and disc made of nitinol mesh and polyester patch, connected by central waist. Amulet®is a second-generation device of the Amplatzer with several incremental design improvements. It is larger in size and has higher number of stabilizing wires, which allows successful closure of more LAA anatomies (Table 1). Comparative studies have shown similar results with ACP and Amulet AMPLATZER devices in terms of safety, implantation success and appropriate LAAO[60,61]. Multiple retrospective and prospective studies for ACP and Amulet reported successful device implantation in 95% to 100% patients, with major periprocedural adverse events (death, stroke/TIA, device embolization, MI/perforation/tamponade/effusion, and major bleeding) ranging from 3.2% to 8%[62-67].An FDA approval trial is currently ongoing, with the aim of collecting randomized controlled data from the Amulet and Watchman devices from 1,600 patients worldwide. PRAGUE 17 is another ongoing prospective, multicenter, randomized trial That plan to enroll 396 patients with non-valvular AF and assign them to LAAO using Amulet or Watchman vs non-vitamin K oral anticoagulants (NOACs). The aim at 24 mo of follow-up is to determine whether LAAO is non-inferior to NOACs in terms of primary efficacy endpoint and peri-procedural complications[68].
COMPARISON OF MULTIPLE LAA OCCLUSION DEVICES
A meta-analysis on 2779 patients who had percutaneous LAAO with multiple devices[PLAATO (18%), Watchman (57%), and ACP (24%)] showed successful implantation in in 2611 patients (94%). The adjusted pooled incidence of stroke was 1.2 per 100 PY(95%CI: 0.9-1.6/100) and the combined efficacy outcome (stroke, systemic embolism,or cardiovascular death) rate was 2.7 per 100 PY (95%CI: 1.9-3.4/100). For combined adverse events, the random effect pooled rate was 6.5% (95%CI: 4.9%-8.2%)[69]. One single-center retrospective study in Italy compared the use ACP vs Watchman in 156 patients (ACP in 99 and watchman in 66 patients) and demonstrated procedural success in 99.4%. During follow-up, only 1 patient suffered from transient ischemic attack and 2 from cardiac death. Furthermore, the data showed excellent safety and efficacy with similar clinical outcomes in both devices[70]. Another multicenter retrospective registry for LAAO using various devices showed an overall success of 92.5%. The combined adverse event rate was 3.5%, leading to annual relative risk reduction for ischemic stroke, thromboembolic events, and major bleeding of 90.1%,87.2%, and 92.9%, respectively[71]. RELEXAO (Registry on Real-Life Experience With LAA Occlusion) registry is a French retrospective cohort of patients with AF who were treated with LAAO[72]. In the study cohort from RELEXAO, Fauchier et al[72]reported no differences in death, ischemic stroke, major bleeding, or device related thrombus between Watchman and Amplatzer devices. Those studies underscore the high success rate in placing various LAAO devices, and their safety and efficacy in preventing strokes and adverse events.
ANTITHROMBOTIC THERAPY AFTER DEVICE IMPLANTATION
Optimal anticoagulation/antiplatelet protocol post LAAO is highly patient-specific and recommended for a limited period post LAAO to prevent device associated thrombus[72]. Different anticoagulation strategies have been described in multiple studies including: warfarin, NOACs, DAPT, single antiplatelet (SAPT), or no therapy at all (Table 2). The anticoagulation protocol described In PROTECT AF and PREVAIL trials consists of warfarin for 45 d followed by aspirin and clopidogrel for 6 mo, then aspirin indefinitely[48-51]. In EWOLUTION registry for example, anticoagulation regimens post LAAO were variable and included: warfarin in 16%, NOAC in 11%,DAPT in 60%, single antiplatelet (SAPT) in 7%, and no therapy in 6%[56]. A study on post LAAO anticoagulation in patients from EWOLUTION registry demonstrated that NOAC and DAPT were similar to warfarin in terms of device thrombus, stroke or bleeding risks[73]. Compared with EWOLUTION registry, antithrombotic regimen post LAAO In RELEXAO registry was different and included: OACs 28.8%, SAPT in 36.2%, DAPT in 23.2%, OACs plus DAPT in 4.3%, and no therapy in 7.5%. In ASAP study, patients were given DAPT for 6 mo followed by aspirin indefinitely as they were ineligible for OACs[58]. A Questionnaire sent by European Heart Rhythm Association Electrophysiology to the participating centers to assess the indications and anticoagulation regimen post LAAO, showed that DAPT for 6 wk to 6 mo followed by aspirin monotherapy as the most common regimen[74]. Interestingly, 41%of centers would prescribe no therapy and less than 10% followed PROTECT AF and PREVAIL protocol. The European Heart Rhythm Association/European Association of Percutaneous Cardiovascular Interventions (EHRA/EAPCI) expert consensus statement recommends treatment with DAPT for 1 to 6 mo followed by aspirin indefinitely in patients with high bleeding risk[75].
COMPLICATIONS
Complications related to LAAO are either acute or delayed and most of them can be detect by peri-procedural imaging. Table 3 summarizes LAAO related complications,their incidence and treatment options.
CONCLUSION
LAAO is a reasonable alternative approach that is used for preventing embolic events in patients with AF who are deemed not eligible for anticoagulation. While AHA/ACC guidelines have no recommendations for LAAO, the ESC guidelines have class IIb (B) recommendation for LAAO in patients with AF and contra-indications for long-term anticoagulation. Similarly, Australian guidelines recommend considering LAAO in patients with non-valvular AF at moderate to high risk of stroke and with contraindications to OAC. Watchman is the only FDA approved device for LAAO and indicated to reduce the risk of thromboembolism from the LAA in patients with nonvalvular AF who: are at increased risk for stroke and systemic embolism based on CHADS2 or CHA2DS2-VASc scores; are deemed by their physicians to be suitable for warfarin; and have an appropriate rationale to seek a non-pharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device compared to warfarin. Amplatzer is another successful LAAO device that has CE mark and is waiting for FDA approval. Optimal antithrombotic regimen post LAAO is highly patient-specific and recommended to prevent device associated thrombus. Due to wide variety of shapes, sizes, indications, and implantation techniques in different LAAO devices, there is a need for further research to identify the best type of LAAO device that suites each patient profile. We believe that the development of established clinical guidelines and expert consensus supporting the use of LAAO in the foreseeable future will ultimately improve patient outcomes.

Table 2 Antithrombotic therapy regimens following left atrial appendage occlusion

Table 3 Complications related to left atrial appendage occlusion

