Considerations for routine coagulation monitoring with rivaroxaban:A case report and review of the literature
Hai-Di Wu, Hong-Yan Cao, Zi-Kai Song, Shuo Yang, Ming-Long Tang, Yang Liu, Ling Qin
Abstract BACKGROUND Rivaroxaban is a non-vitamin K antagonist oral anticoagulant that does not require coagulation monitoring based on current recommendations. Our goal is to explore whether routine coagulation monitoring should not be required for all patients receiving oral rivaroxaban, what relationship between routine coagulation abnormalities and bleeding, and how to deal with the above clinical situations through our case and review of the literature.CASE SUMMARY We report a 67-year-old woman with a history of atrial fibrillation who presented to the hospital with worsening dyspnea and cough. Based on electrocardiogram,venous compression ultrasonography, and computed tomography pulmonary angiography, the diagnosis of atrial fibrillation, deep venous thrombosis, and acute pulmonary embolism was confirmed. Her coagulation assays and renal function were normal on admission; she was not underweight, did not have a history of hemorrhagic disease, and her CHA2DS2-VAS, HAS-BLED, and simplified Pulmonary Embolism Severity Index scores were 3, 0, and 0,respectively. Oral rivaroxaban (15 mg twice daily) was administered. The following day, she presented gastrointestinal and gum bleeding, combined with coagulation abnormalities. Following cessation of rivaroxaban, her bleeding stopped and tests improved over the next 2 d. Rivaroxaban was begun again 3 d after recovery. However, she again presented with gastrointestinal and gum bleeding and the abnormal tests, and the therapy was discontinued. At 30-d follow-up after discharge, she presented normal coagulation tests without bleeding.CONCLUSION Although current guidelines recommend that using non-vitamin K antagonist oral anticoagulants including rivaroxaban do not require coagulation monitoring,a small number of patients may develop routine coagulation test changes and bleeding during rivaroxaban therapy, especially in the elderly. Clinicians should pay attention to these patients and further obtain evidence in practice.
Key words: Rivaroxaban; Routine coagulation monitoring; Anticoagulation; Bleeding;Case report
INTRODUCTION
Rivaroxaban is an Xa factor inhibitor approved for the prevention of stroke in patients with nonvalvular atrial fibrillation (AF)[1]and for the prevention and treatment of venous thromboembolism (VTE)[2]. It has a predictable anticoagulant effect,eliminating the need for routine coagulation monitoring. Compared with vitamin K antagonists (VKAs), it also has a better efficacy/safety ratio, fewer food and drug interactions, a more rapid onset of action, and reduced risk of fatal bleeding.However, many unresolved questions remain about the optimal use of these agents in specific clinical situations involving AF and VTE, whether routine coagulation monitoring should be required for all patients receiving rivaroxaban, and which clinical situations are considered predictive factors associated with coagulation test abnormalities and/or bleeding while prescribing rivaroxaban.
CASE PRESENTATION
Chief complaints
A 67-year-old women presented to the hospital with worsening dyspnea and cough for 4 d.
History of present illness
Her symptoms worsened after mild activities and relieved after rest without taking any medication.
Personal and family history
She had a long-term history of AF.
Physical examination upon admission
On admission, she was fully conscious, with a blood pressure of 110/70 mmHg, an irregular heart rate of 105 bpm on auscultation and oxygen saturation of 92% on room air. She had mild edema in the lower limbs. Her weight was 58 kg and the remainder of her physical examination was normal.
Laboratory examinations
Laboratory tests revealed normal platelets, hemoglobin, electrolytes, liver function markers, renal function markers (serum creatinine and estimated glomerular filtration rate), cardiac troponin-T, and routine coagulation measurements (prothrombin time[PT], international normalized ratio, and activated partial thromboplastin time[aPTT]). However, her N-terminal pro-brain natriuretic peptide was 5364.00 pg/mL(reference, < 450.00 pg/mL), D-dimer level was 5.65 μg/mL (reference, 0.001-0.50 g/mL), and PaO2was 60.5 mmHg (reference, 80-100 mmHg). Her electrocardiogram suggested AF, and her transthoracic echocardiogram revealed a left atrial diameter of 51 mm and an ejection fraction of 48%.
Imaging examinations
Lower limb venous compression ultrasonography showed a deep vein thromboembolism (DVT) involving bilateral intermuscular veins. Furthermore,computed tomography pulmonary angiography confirmed the presence of an embolus in both upper lobes of the pulmonary artery (Figure 1).
FINAL DIAGNOSIS
Atrial fibrillation, deep venous thrombosis, and acute pulmonary embolism.
TREATMENT
To select an appropriate treatment strategy, the CHA2DS2-VAS, HAS-BLED, and simplified Pulmonary Embolism Severity Index (sPESI) scores were calculated; these values were 3, 0, and 0, respectively. Therefore, anticoagulation therapy with rivaroxaban (15 mg twice daily) was initiated. After 1 d, the patient had maroon stools and gum bleeding with routine coagulation test abnormalities. Rivaroxaban was discontinued, and routine coagulation tests were monitored daily (Table 1). Her maroon stools and gum bleeding disappeared, and routine coagulation tests returned to normal 2 d later. The patient received rivaroxaban again 3 d after coagulation tests returned to normal, and routine coagulation tests were performed 2 and 6 h after rivaroxaban administration (Table 1). Routine coagulation tests illustrated abnormalities 6 h after rivaroxaban (Table 1) and her maroon stools and gum bleeding reappeared. No further rivaroxaban treatment was administered.
OUTCOME AND FOLLOW-UP
Following cessation of rivaroxaban, her bleeding stopped and coagulation tests returned to normal. At follow-up 30 d after discharge at home, the patient received aspirin instead of an anticoagulant. Routine coagulation tests were normal and bleeding did not recur.
DISCUSSION
VKAs require regular laboratory monitoring to prevent under-coagulation or overcoagulation. This entails ongoing patient visits to clinics and regular blood collection.Even within the therapeutic range, there is still a significant risk of hemorrhage in patients receiving VKAs[3,4]. In recent years, an improved understanding of coagulation pathways has led to the development of several new parenterally or orally active agents that specifically target single blood coagulation factors.
Rivaroxaban is an orally active, specific, and direct inhibitor of activated factor Xa,with predictable pharmacokinetics and pharmacodynamics across a wide spectrum of patients. Treatment with rivaroxaban does not currently require blood coagulation monitoring[5-7]. It strongly binds plasma proteins (> 90%), peaks in the plasma 2-4 h following oral administration, has a half-life of 5-9 h in healthy young subjects and 11-12 h in elderly subjects, and has a dual mode of elimination (two-thirds are metabolized by the liver and one-third is excreted unaltered by the kidneys)[8].Treatment with rivaroxaban has fewer considerations for food and drug interactions and greater effectiveness and safety than those of VKAs for prevention of stroke in patients with AF and for treatment of VTE[9]. For the majority of patients, rivaroxaban should be considered the first preferred anticoagulation therapy based on the positive results of large trials and current guidelines[1,10,11]. The ROCKET-AF, EINSTEIN-DVT,and EINSTEIN-PE trials also demonstrated that rivaroxaban could be used to prevent ischemic stroke in patients with AF and as a prophylaxis and treatment for DVT and PE[2,12,13].
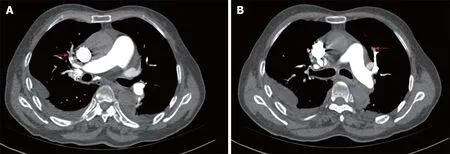
Figure 1 Computed tomography pulmonary angiography images. These reveal left (A) and right (B) superior pulmonary artery embolism.
In real-world practice, however, changes in routine coagulation tests occur in some patients during rivaroxaban treatment. Our patient in particular had a clear diagnosis of AF, DVT, and PE and an indication for anticoagulant therapy. Although we considered factors that might affect the pharmacokinetics and pharmacodynamics of rivaroxaban in our patient, including her age, weight, renal function, liver function,concomitant medications, comorbidities, routine coagulation tests, and overall frailty,and also assessed and stratified CHA2DS2-VAS, HAS-BLED, and sPESI scores prior to initial prescription, the patient experienced two unexpected changes in routine coagulation assays and bleeding events. Therefore, rivaroxaban had to be discontinued. The patient did not use drugs that affected rivaroxiban metabolism and resulted in rivaroxaban accumulation outside and in the hospital.
First, our review of the literature illustrated that rivaroxaban prolongs PT in a concentration-dependent manner[14]. The impact of rivaroxaban on PT is less sensitive,not specific, varies markedly with different thromboplastin reagents, and is influenced by a variety of other factors, including hepatic impairment and vitamin K deficiency[15]. A normal PT does not rule out clinically relevant concentrations of rivaroxaban and may provide some quantitative information about the risk of bleeding[16]. At present, neither randomized control trials nor clinical trials involve coagulation monitoring for rivaroxaban. Based on previous case reports describing hemorrhaging caused by rivaroxaban, few patients with bleeding who were treated at a normal dosage had prolonged PT[17-19]. Prolongation of both PT and aPTT was reported in only two patients, who had both consumed large oral doses of rivaroxaban (1960 mg and 1400 mg) as a method of suicide[20,21].
Second, aPTT is not suitable for meaningful evaluation of rivaroxaban efficacy due to the nonlinear relationship of aPTT level with rivaroxaban concentration,insufficient sensitivity, and significant variability between reagents. Excessive rivaroxaban may cause PT prolongation, but it has no effect on aPTT. In case reports of bleeding caused by rivaroxaban, none had changes in aPTT[22-24].
Finally, studies have shown that anti-Xa chromogenic assays with rivaroxaban calibrators and controls can accurately measure the anticoagulant effect of rivaroxaban over a wide range of therapeutic levels and plasma concentrations. Low and high plasma levels can be measured with acceptable inter-laboratory precision. In these assays, the absence of anti-Xa activity excludes clinically relevant drug levels[25].These specific assays accurately quantify plasma levels of the anticoagulant[5,26-28], but are not routinely available at most clinical centers. Excessive plasma concentrations,such as those upon intentional overdose, potentially expose patients to an increased risk of bleeding. Although data on the relationship between plasma levels and clinical outcomes are beginning to emerge, there is no current evidence that routine monitoring or dose titration will improve outcomes, and no studies have investigated whether measuring drug levels and adjusting dosage based on laboratory coagulation parameters reduce the risk for bleeding or thromboembolic complications.
As the use of rivaroxaban increases, clinicians have begun to pay attention to changes in routine coagulation tests and their relationship with bleeding in patients.Some studies suggest that coagulation monitoring may be useful in elderly patients,those who are underweight, or those with renal dysfunction, suspected overdose or bioaccumulation, bleeding, higher HAS-BLED scores, planned invasive procedures and surgery, or those patients with multiple diseases for which multiple drugs are being taken[18,19,29].
The combination of AF and VTE (PE and DVT) is not only common in patients whoreceive anticoagulation therapy, but is also associated with higher morbidity and mortality[30]. Before initiating anticoagulant therapy to a patient with AF and/or VTE,a clinician should decide whether anticoagulation is indicated by risk/benefit analysis and approved by regulatory authorities and specified guidelines. In addition, patientrelated clinical factors and patient preferences should be considered[31].
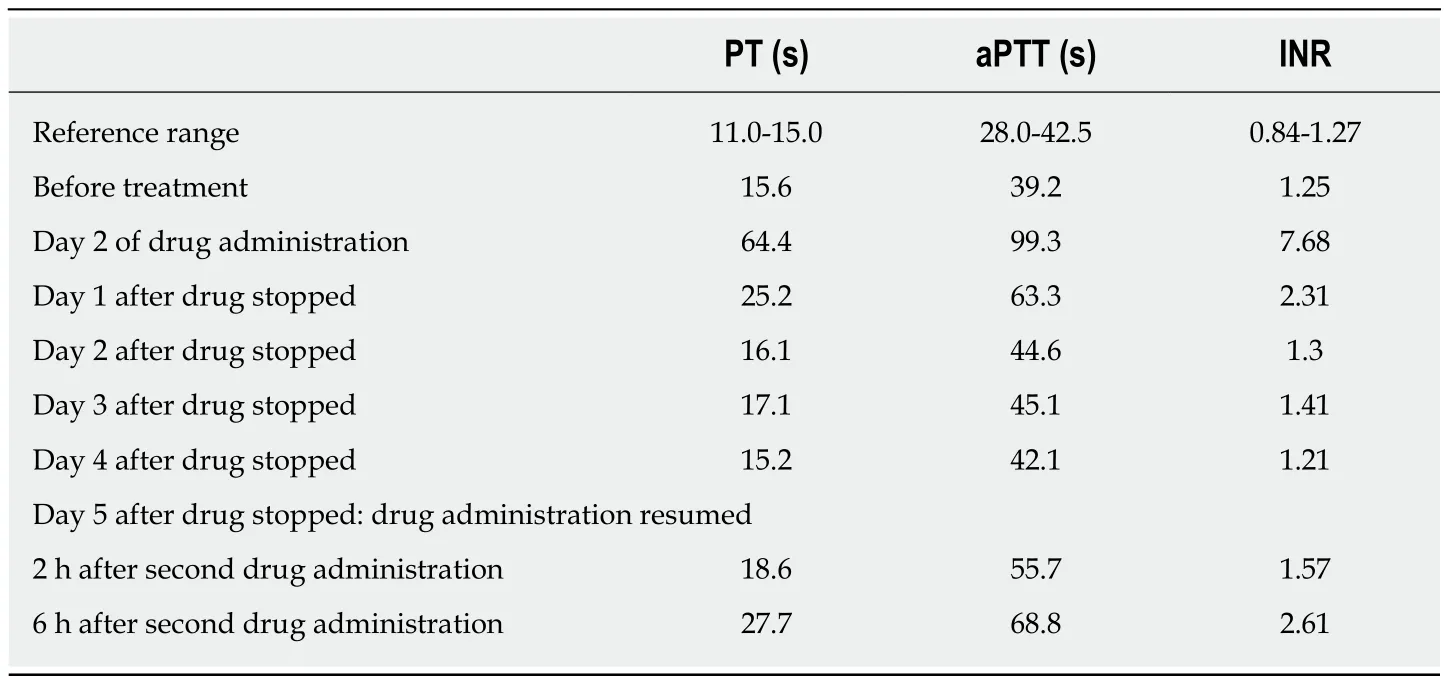
Table 1 Serial coagulation studies
It is clear from the case described here that differences between individuals exist in real-world situations, especially in elderly adults. Our patient was an elderly woman who had a relatively high risk of routine coagulation changes and bleeding, and she presented rarely both PT and aPTT prolongation at a normal dose of rivaroxaban.However, anti-Xa chromogenic assays were not performed and the quantities and functions of clotting factors were not detected.
Summarily, we suggest that clinicians should now be particularly aware of patients with an increased risk of bleeding and consider monitoring the coagulation status of these individuals during rivaroxaban therapy, because which patients and situations are required to detect routine coagulation is unclear. Conditional centers should also consider using the accurate anti-Xa chromogenic assays to improve detection of drug concentrations and evaluation of bleeding factors.
CONCLUSION
Although current guidelines recommend that using non-vitamin K antagonist oral anticoagulants including rivaroxaban do not require coagulation monitoring, we found that in clinical practice, a small number of patients may develop routine coagulation changes with bleeding during rivaroxaban therapy. The results of literature review and our case showed that routine coagulation assays may be required in special populations including elderly patients, particularly low-weight females or those with renal insufficiency. Clinicians should pay attention to these patients and further obtain evidence in practice
ACKNOWLEDGEMENTS
We thank all participants for their support and participation.
 World Journal of Clinical Cases2019年3期
World Journal of Clinical Cases2019年3期
- World Journal of Clinical Cases的其它文章
- Biventricular pacing for treating heart failure in children: A case report and review of the literature
- Gerstmann-Sträussler-Scheinker disease: A case report
- Castleman disease presenting with jaundice: A case report and review of literature
- Papillary cystadenoma of the parotid gland: A case report
- Ghost cell odontogenic carcinoma of the jaws: Report of two cases and a literature review
- Intravenous leiomyomatosis with different surgical approaches:Three case reports
