Split-dose or hybrid nonsteroidal anti-inflammatory drugs and Nacetylcysteine therapy for prevention of post-retrograde cholangiopancreatography pancreatitis Laura Pavel, Gheorghe Gh Bălan, Alexandra Nicorescu, Georgiana Emmanuela
Laura Pavel, Gheorghe Gh Bălan, Alexandra Nicorescu, Georgiana Emmanuela Gîlcă-Blanariu, Cătălin Sfarti,Ștefan Chiriac, Smaranda Diaconescu, Vasile Liviu Drug, Gheorghe Bălan, Gabriela Ștefănescu
Abstract BACKGROUND Despite significant technical and training improvements, the incidence of postendoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP) has not significantly dropped. Although many studies have evaluated the efficacy of various agents, e.g. nonsteroidal anti-inflammatory drugs, octreotide,antioxidants, administered via various dosages, routes (oral, intrarectal or parenteral), and schedules (before or after the procedure), the results have been conflicting.AIM To evaluate efficacy of three pharmacologic prophylactic methods for prevention of PEP.METHODS In this prospective, single-center randomized trial, patients who underwent firsttime ERCP for choledocholithiasis were randomly assigned to three groups. The first group received 600 mg N-acetylcysteine 15 min prior to ERCP, and perrectum administration of 50 mg indomethacin both prior to and after completion of the ERCP. The second group was administered only the 50 mg indomethacin per-rectum both prior to and after the ERCP. The third group was administered per-rectum 100 mg indomethacin only after the ERCP, representing the control group given the guideline-recommended regimen. The primary end-point was PEP prevention.RESULTS Among the total 211 patients evaluated during the study, 186 fulfilled the inclusion criteria and completed the protocol. The percentages of patients who developed PEP in each of the three groups were not significantly different (χ2 =2.793, P = 0.247). Among the acute PEP cases, for all groups, 14 patients developed mild pancreatitis (77.77%) and 4 moderate. No severe cases of PEP occurred, and in all PEP cases the resolution was favorable. No adverse events related to the medications (digestive hemorrhage, rectal irritation, or allergies)occurred.CONCLUSION The efficacies of split-dose indomethacin and combined administration (N-acetylcysteine with indomethacin) for preventing PEP were similar to that of the standard regimen.
Key words: Endoscopic retrograde cholangiopancreatography; Prophylaxis; Postendoscopic retrograde cholangiopancreatography pancreatitis; Nonsteroidal antiinflammatory drugs; N-acetylcysteine
INTRODUCTION
Endoscopic retrograde cholangiopancreatography (ERCP) is a complex endoscopic technique. Although ERCP should be a safe procedure for the vast majority of patients, postprocedural complications may arise, among which acute pancreatitis is the most frequently reported. The mean estimated incidence of this complication is 4%-5.5%, representing a wide range of rates reported in the various studies - from as low as 0.4% up to 40%, depending on the presence of various risk factors[1-3].
Despite continuous improvements in the equipment used and the increased training of endoscopy teams, the incidence of this complication has not dropped significantly[2,4-6]. The relatively high incidence and mortality rates (with the latter reaching approximately 0.5%) underlie the substantial research interest in studying the related pathophysiology, risk factors and potential prophylactic measures. This is also reflected by the numerous studies focusing on such objectives that have appeared throughout the literature within the last two decades alone[5-8].
Regarding the risk factors for post-ERCP pancreatitis (PEP), most studies have reported young age, normal serum bilirubin level, personal history of acute PEP,female sex, difficult cannulation of the papilla, injection of contrast agent in the pancreatic duct, and excessive use of the guidewire[1,2,4-6,9-13]. Studies on the prophylaxis of acute PEP have identified pancreatic stent placement as having the highest efficiency in preventing this event in high-risk patients, and consequently this method has been recommended by some guidelines[11-13]. All things considered, pancreatic duct stenting is a difficult, costly maneuver and is not routinely indicated for all patients undergoing ERCP[11].
Although many studies have evaluated the efficacy of various agents [nonsteroidal anti-inflammatory drugs (NSAIDs), octreotide, antioxidants] administered via various dosages, routes (oral, intrarectal or parenteral), and schedules (before or after the procedure)[14-25], the results have been conflicting. Thus, we established a single-blind prospective randomized trial with the aim of evaluating the efficacy of three pharmacologic prophylactic approaches in the prevention of PEP.
MATERIAL AND METHODS
Patients
The study was performed in a tertiary care center - the Institute of Gastroenterology and Hepatology of the St. Spiridon County Clinical Emergency Hospital in Iai,Romania-between April 2017 and July 2018. The study design was that of a prospective, single-blind randomized trial for comparing and dynamically evaluating the efficacy of three pharmacological combination therapies (indomethacin in various doses with or without an extra dose of N-acetylcysteine (NAC) for prevention of acute pancreatitis in patients with choledocholithiasis and indication for undergoing ERCP.
All patients were evaluated before inclusion in the study. Inclusion criteria consisted of positive diagnosis of choledocholithiasis by magnetic resonance cholangiopancreatography (commonly known as MRCP), age above 18-years-old,willingness to participate in the study, and legal capacity to sign the informed consent form. Exclusion criteria were: Presence of acute or chronic ongoing pancreatitis or other inflammatory diseases at admission; positive history for acute or chronic pancreatitis; jaundice or recurrent upper right quadrant pain; current pregnancy;contraindication for NSAID administration; recent episode of upper digestive bleeding (less than 1 mo); hypersensibility to antioxidants; intraprocedural necessity of a prophylactic pancreatic stent insertion; or inability to perform a proper prospective follow-up.
After initial evaluation, 186 patients who fulfilled the inclusion criteria and agreed to sign the informed consent form were enrolled in the study. These study participants were randomly assigned into one of three groups. The control group (n =98) underwent prophylaxis according to the European Guideline, consisting of intrarectal administration of 100 mg indomethacin after the ERCP. The study group A(n = 32) was administered 600 mg of NAC intravenously 15 min before the ERCP, as well as an intrarectal administration of 50 mg indomethacin both before and after the ERCP. The study group B (n = 56) received 50 mg of indomethacin per-rectum both before and after the ERCP.
Study protocol
After providing written informed consent, each study participant was evaluated to identify their precise indication for ERCP. The evaluation included disease historytaking, clinical examination, and biochemical investigations (i.e., complete blood count, serum and urinary pancreatic enzymes, inflammatory parameters, cholestasis and cytolisis syndrome, renal function evaluation). The positive diagnosis of choledocholithiasis was obtained for all patients through MRCP.
ERCP was performed following the standard protocol, after anesthesiology evaluation and assent, with topical pharyngeal anesthesia consisting of 2% lidocaine and intravenous administration of midazolam, ketamine and propofol. The equipment and materials used for the ERCP included a TJF series 160 duodenoscope(Olympus, Tokyo, Japan), standard sphincterotomes by Olympus and Boston Scientific (Marlborough, MA, United States), Visiglide hydrophilic guidewires by Olympus, extraction balloon catheters by Boston Scientific and Wilson-Cook Medical Inc (Bloomington, IN, United States), and/or Dormia basket extractors by Olympus.Hidrosoluble iopamidol was used as the contrast agent. No pancreatic stents were used with prophylactic intent, per study protocol, as insertion of pancreatic stents was an exclusion criteria-based potential for study bias.
All patients were continuously monitored during the procedure by an anesthetist,and the procedure-related parameters (i.e., number of cannulation attempts, time for cannulation, amount of contrast agent used, duration of procedure, number of involuntary pancreatic guidewire passages) were registered in the database.Moreover, hospitalization time and all procedure- or medication-related adverse events were recorded. No patient received aggressive intravenous hydration. All patients were administered standard local hydration protocol, consisting of 1.5 mL/kg/h of lactated Ringer’s solution during the ERCP and for 8 h after. Several clinical parameters (i.e., abdominal pain, nausea, emesis, fever, change of bowel habit)and biological parameters (i.e., complete blood count, C-reactive protein (CRP), serum amylase, lipase) were recorded at both 6 h and 24 h following the ERCP procedure.
Diagnosis of PEP was established according to the criteria described by Cotton et al[26], namely by patient report of new-onset abdominal pain after the ERCP or worsening of pre-existent abdominal pain, and at least 3-fold increase of either serum lipase or amylase levels above the upper limit of normal (ULN). The normal ranges of serum lipase and amylase reported by the local laboratory for adults were levels below 60 U/L and 100 U/L respectively. An asymptomatic increase of either serum lipase or amylase above the ULN was not interpreted as PEP.
Patients were withdrawn from the study protocol monitoring at 24 h after undergoing the ERCP if no significant events were reported. For patients who experienced adverse events, withdrawal occurred after resolution and the patients were re-evaluated at 30 d after discharge. The data collected in all monitoring stages were recorded in an electronic database, being presented as raw values, percentages,and mean ± standard deviation. For intergroup comparisons, the Student’s t-test was used for continuous data and the χ2or Fisher’s exact tests were used for qualitative variables when appropriate. Results were considered significant when the P value was < 0.05. All statistical analyses were conducted using SPSS software (IBM Corp.Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.).
The study protocol and the patient consent form were approved by the Ethics Committee of Grigore T. Popa University of Medicine and Pharmacy and St Spiridon Clinical Emergency Hospital, Iasi, Romania.
RESULTS
Study groups description
A total of 211 patients were evaluated for study participation, of which 186 fulfilled the inclusion criteria, represented by 102 women (54.8%) and 84 males (45.2%). The patients were randomized to one of the three groups as described above. The median age of all included patients was 64.32 ± 14.91 years, without statistically significant differences between the three groups: Control group: 64.47 ± 15.403 years vs Study group A: 63.56 ± 14.099 years vs Study group B: 64.50 ± 15.684 years (ANOVA test, F =0.025, P = 0.97). Also, there was a similar number of male and female patients in the three groups (t-test, t = 1.056, P = 0.294, non-significant). The structure of each group with consideration to demographic, clinical and laboratory values at baseline is shown in Table 1.
Evaluation of the known risk factors involved in PEP (sex, age, bilirubin, amount of contrast medium/agent used, number of cannulations and history of acute PEP)showed no statistically significant differences between the three groups for any of the factors (Table 2). Multifactor comparative analysis of the demographic and risk factors for acute pancreatitis showed no statistically significant differences between the three groups of patients regarding the factors that might influence the features studied below (i.e., PEP occurrence, specific laboratory results, or responsiveness to treatment). Thus, the selected study groups are similar and represent an appropriate foundation of data for minimally-biased evaluation, promoting our ability to investigate valid comparisons between the study arms.
Comparative analysis of the evolution of clinical features and laboratory results at 6 h and 24 h post-ERCP in the three study groups
Efficacy of the three studied regimens was evaluated by comparing the clinical evolution and the laboratory results for the three study groups. PEP was diagnosed based on findings from clinical exam and laboratory tests (i.e., pancreatic enzymes and inflammatory syndrome markers, such as white blood cell count and CRP,measured at 6 h and 24 h post-ERCP. For the evaluation of statistical significance, the Pearson's chi-squared test was used.
Of the total 186 patients included in our study, 18 developed clinically significant acute pancreatitis after the ERCP procedure. The percentages among the three study groups were similar, with 8 patients from the control group (8.16%), 4 from Study group A (12.5%) and 6 from Study group B (10.71%) affected; the differences showedno statistical significance (χ2= 2.793, P = 0.247). Of note, in the majority of cases (n =16) the debut was precocious, under 6 h post-ERCP, and only in 2 cases was the debut late (both those cases belonged to Study group B) (Figure 1, Tables 3 and 4). Among the acute PEP cases, 14 were mild (77.77%) and 4 were moderate. No severe cases occurred, and in all cases the outcome was favorable. No adverse events related to the study medications, including digestive hemorrhage, rectal irritation and allergies,occurred.
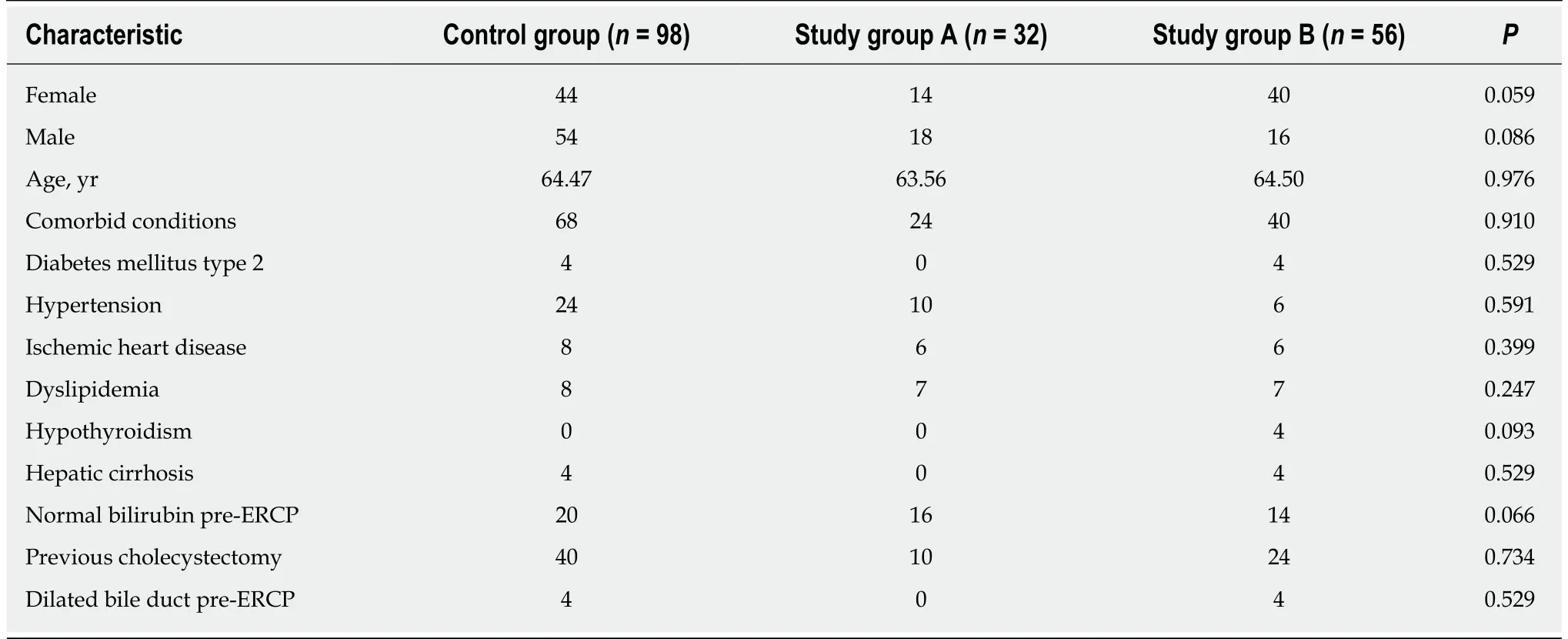
Table 1 Baseline characteristics of patients in the control group and the study groups
Resultf the comparative analysis of pancreatic enzymes and CRP at the two time points considered (6 h and 24 h post-ERCP) are summarized in Tables 3 and 4. In the Control group, 18.4% of the patients showed 3-fold ULN increase for amylasemia at 6 h post-ERCP, but only 8.2% of patients exhibited values that might be recorded as asymptomatic elevated enzyme levels, which are non-consistent with PEP, at 24 h post-ERCP. In Study group A, 25% of patients exhibited raised enzyme levels at 6 h post-ERCP and 12.5% of patients had amylase levels over the normal limit at 24 h post-ERCP; yet, none of these cases represented increases of more than 3-fold. In Study group B, 14.3% of patients exhibited asymptomatic elevated amylase levels at 6 h post-ERCP, which was similar to the percentages in the Control group and Study group A (P = 0.368), but the 28.6% of patients with this feature at 24 h post-ERCP represented a significantly larger pool compared to the other study arms (P = 0.019).This particularly worse evolution for the Study group A regimen at 24 h post-ERCP is an intriguing finding and unexplained in the scope of this study.
Lipase levels showed a similar evolution as amylasemia, with significant improvement in the Control group and Study group A (Tables 3 and 4). In the Control group, 30.6% of patients showed a 3-fold ULN increase at 6 h post-ERCP, but only 8.2% showed the same at 24 h post-ERCP. In Study group A, 31.3% of patients showed a 3-fold ULN increase at 6 h post-ERCP, dropping to 12.5% at 24 h post-ERCP. In Study group B, the percentage of patients with a 3-fold ULN increase in lipase levels remained unchanged at both time points (being 28.6% at 6 h and 24 h),with a statistically significant difference compared to the other two study arms (6 h: P= 0.040 and 24 h: P = 0.029).
Inflammatory status, evaluated by CRP values, varied remarkably during the study time course, but significant differences between the study arms occurred only at the 24 h post-ERCP time point (P = 0.046) (Figure 2, Tables 3 and 4).
DISCUSSION
Our study has clearly shown that there are no significant differences for PEP rates among patients treated with the standard NSAID prophylaxis, double NAC and NSAID prophylaxis, or split-NSAID regimens. Nevertheless, increased levels of PEP in both secondary or tertiary centers all around the world justify a continuous search for better prophylaxis protocols.
Acute pancreatitis remains the most frequent complication of ERCP and represents a significant cause of morbidity and mortality[3,12,26,27]. The reported incidence of thiscomplication has varied in different studies, according to the included patients’ risk level (i.e., mild or medium). A recent meta-analysis of 108 controlled randomized studies, including 13296 patients, found a global incidence of 9.7%, with a median incidence of 14.7% for the high-risk patients[28]. In the present study, the acute PEP incidence was 9.67% for our overall patient population; considering there was no inclusion selection factor related to risk level, the global incidence we found was similar to that of the previously reported data[3,12].
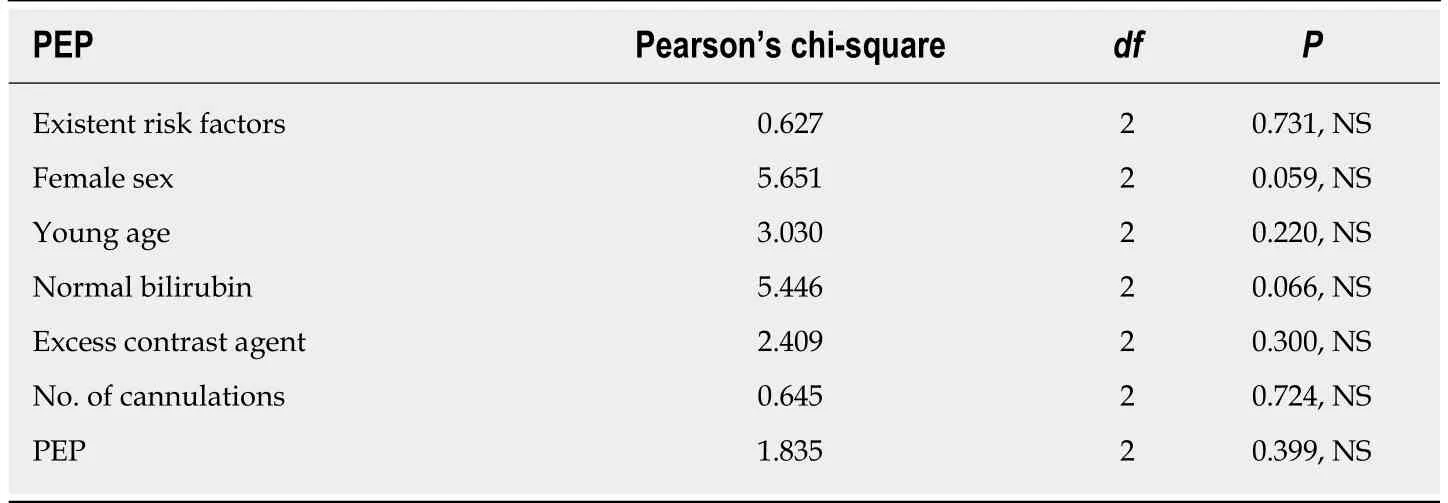
Table 2 Pearson’s chi-squared test to compare risk factors’ occurrence in the three study arms
A decrease in occurrence of acute PEP can be achieved by a thorough patient selection process, either by basing the choice of this procedure according to therapeutic use or by using special ERCP techniques[29,30]. There are several controlled studies and a meta-analysis that have demonstrated that use of prophylactic guidewire cannulation or prophylactic pancreatic duct stent might improve the incidence and seriousness of PEP, especially for high-risk patients[31-33].
From the perspective of the involved pathophysiology of PEP, many studies have evaluated the efficacy of various agents (i.e., NSAIDs, octreotide, antioxidants), in various dosages, with oral, intrarectal or parenteral administration routes, given before or after the procedure[14-20]. These studies have yielded conflicting results.Although many have demonstrated that intrarectal administration of NSAIDs before or after ERCP is efficient in preventing PEP - and as a consequence many of the international guidelines do recommend NSAIDs as a primary prophylaxis measure - a series of more recent studies has generally contradicted those previous findings[19-22].Moreover, although the role of oxidative stress in the pathophysiology of this process has been clearly stated, the efficacy of various antioxidant agents in preventing PEP has not been confirmed as of yet[23-25].
On the other hand, as previously mentioned, there has been some pharmacological research for PEP prophylaxis. Most of the studied molecules specifically interfere with different steps of the inflammatory cascade. The most convincing results appear to be those related to intrarectal administered NSAIDs[16,34-36]. The efficacy has been studied according to NSAID type (indomethacin or diclofenac), dose (50 mg indomethacin vs 100 mg indomethacin), route of administration (intrarectal, oral, or intravenous), time of administration (before or after the ERCP), and in comparison to placebo. Several meta-analyses have shown a risk reduction related to moderate or severe PEP occurrence and a similar efficiency of the rectal administration route, immediately before or after ERCP[3,35,37].
The most cited study belongs to Elmunzer et al[36]and it reported the efficacy of 100 mg indomethacin suppository administered intrarectal immediately after ERCP.Although most studies have reported favorable results, a recent prospective, doubleblind, placebo-controlled study showed no benefit in indomethacin administration for PEP prophylaxis or as it being protective against PEP in high-risk patients as well as in average-risk patients[22,38]. Another recent study published by Yang et al[29]showed efficiency of NSAIDs prophylaxis for acute PEP but reported its relation to the moment of administration, either before or after the procedure. On the other hand,some recently published papers have suggested the efficient use of other drug classes(e.g., nitroglycerin, antioxidants, somatostatin, antibiotics) that might be useful in PEP prophylaxis[23,39-42].

Figure 1 Percentages of patients with acute pancreatitis in the three groups. PEP: Post-endoscopic retrograde cholangiopancreatography pancreatitis.
Considering the hypothesis these studies suggest, our study evaluated the efficacy of two regimens with indomethacin: One with administration of indomethacin suppository, before and after ERCP, and the other with administration of NAC(antioxidant) associated with indomethacin before and after. Our study design included both of these being compared to the standard regimen recommended by the European guidelines (i.e., indomethacin suppository immediately after ERCP). Upon analyzing the efficacy of these regimens for potential correlation with the incidence and seriousness of PEP we found no superiority of any regimen used (indomethacin suppository administered before and after ERCP, and indomethacin associated with NAC) compared to the regimen using indomethacin monotherapy after ERCP (χ2=2.793, P = 0.247). In addition, our analysis of the serum pancreatic enzymes (amylase and lipase levels) showed that the mixed regimen had superior efficacy. Finally, the criteria for evaluating inflammatory syndrome revealed a similar evolution as the pancreatic enzymes, showing a significant improvement. Similar results have been reported in recent studies or meta-analysis[23,24].
Considering the absence of any significant difference in the prevention of PEP through different pharmacologic regimens alongside the relatively low morbidity and mortality associated with PEP in our patient groups, the main strength of our study resides in its prospective character, especially considering the current paucity of prospective trials in ERCP. Moreover, our results suggest that various pharmacologic pathogenic preventive regimens locally available may be used for PEP prophylaxis,with similar efficacy and safety profiles. Nevertheless, the main limitation of this study is that, despite the availability of at least two alternative pharmacologic preventive measures (either intravenous NSAIDs or aggressive intravenous hydration), no such alternative regimens were tested. Furthermore, in this respect, a double-blind prospective randomized controlled trial would offer stronger evidence.
The particularity of our study is that it evaluated two pharmacological therapeutic approaches (one using both NSAIDs and NAC, and the other including the usual dose but with split administration of NSAIDs) and compared these regimens with the standard therapy (recommended by the European guidelines) and not to placebo. The results obtained demonstrate that both split-dose administration of indomethacin (50 mg pre- and post-ERCP) and combined administration of 600 mg NAC before the procedure with per-rectum administration of 100 mg indomethacin post-ERCP have similar efficacy in preventing PEP, as compared to the standard guidelinerecommended regimen (per-rectum administration of 100 mg indomethacin post-ERCP). However, our study also shows that the post-ERCP biological response,reflected by the number of patients developing asymptomatic hyperamylasemia and pronounced inflammatory syndrome, was attenuated in the study group receiving the combination therapy (NSAIDs associated with NAC), with a faster improvement of the biological response.
Considering these findings, further research in the field - eventually, through multicentric studies, enrolling high numbers of patients and modulating the antioxidant dose - could lead to development of a more efficient prophylactic pharmacological approach, with a satisfactory safety profile and tenable costs.
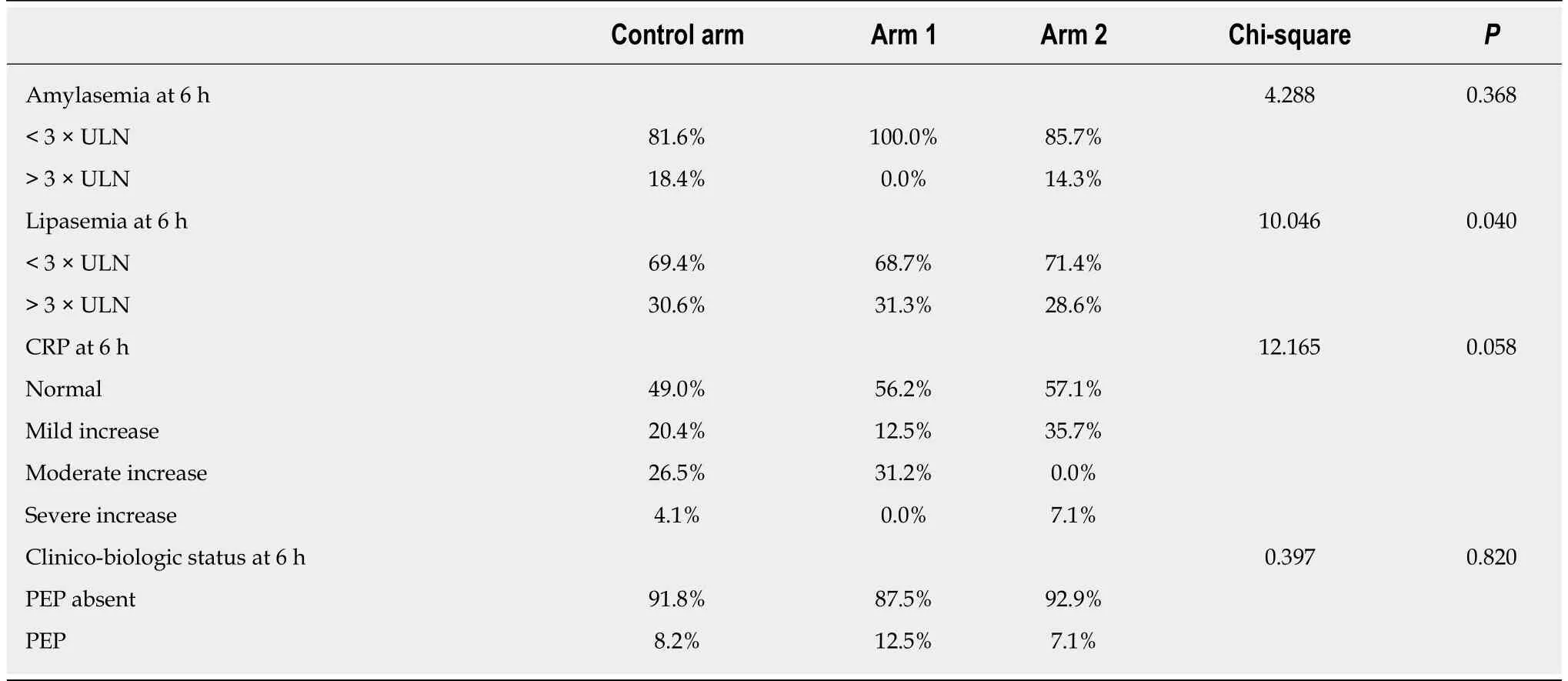
Table 3 Pearson’s chi-squared test comparison between the three study arms’ clinical and laboratory factors at 6 h
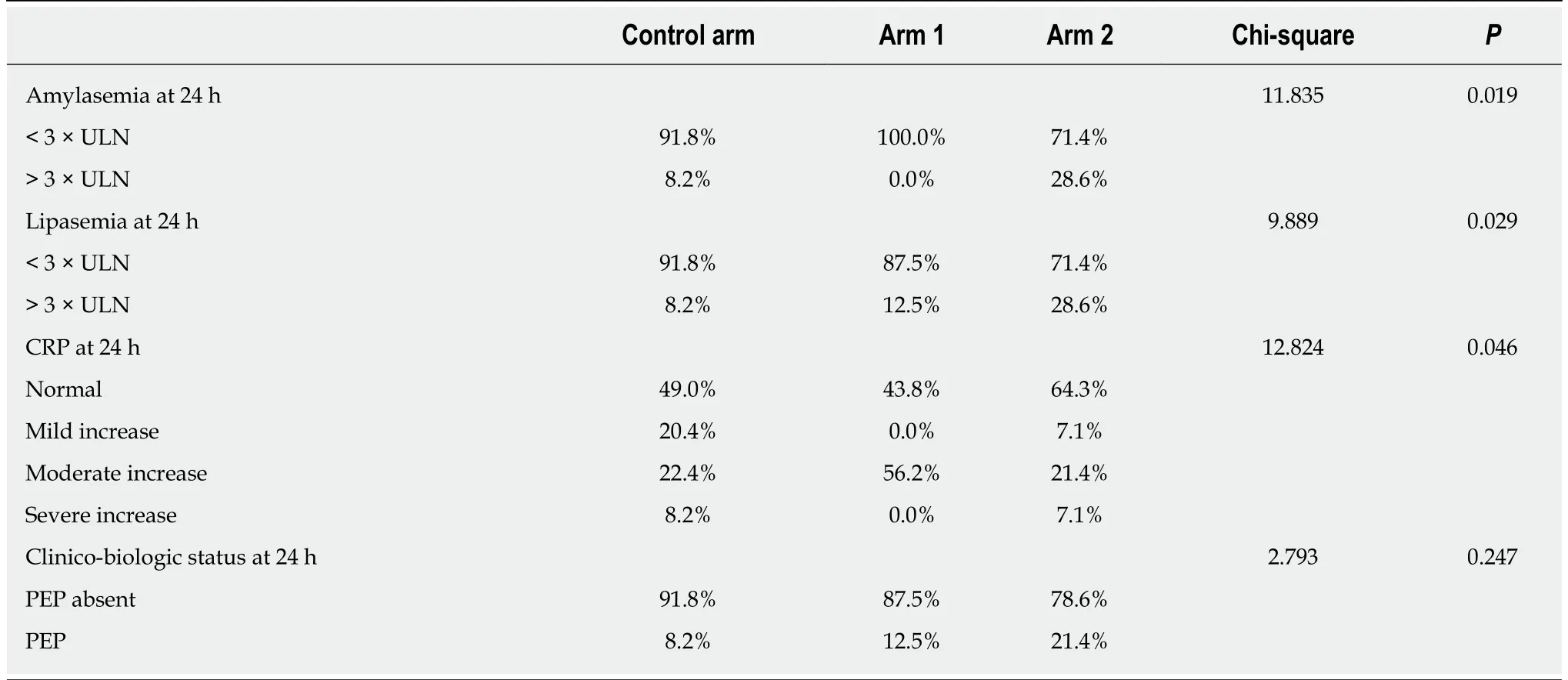
Table 4 Pearson’s chi-squared test comparison between the three study arms’ clinical and laboratory factors at 24 h
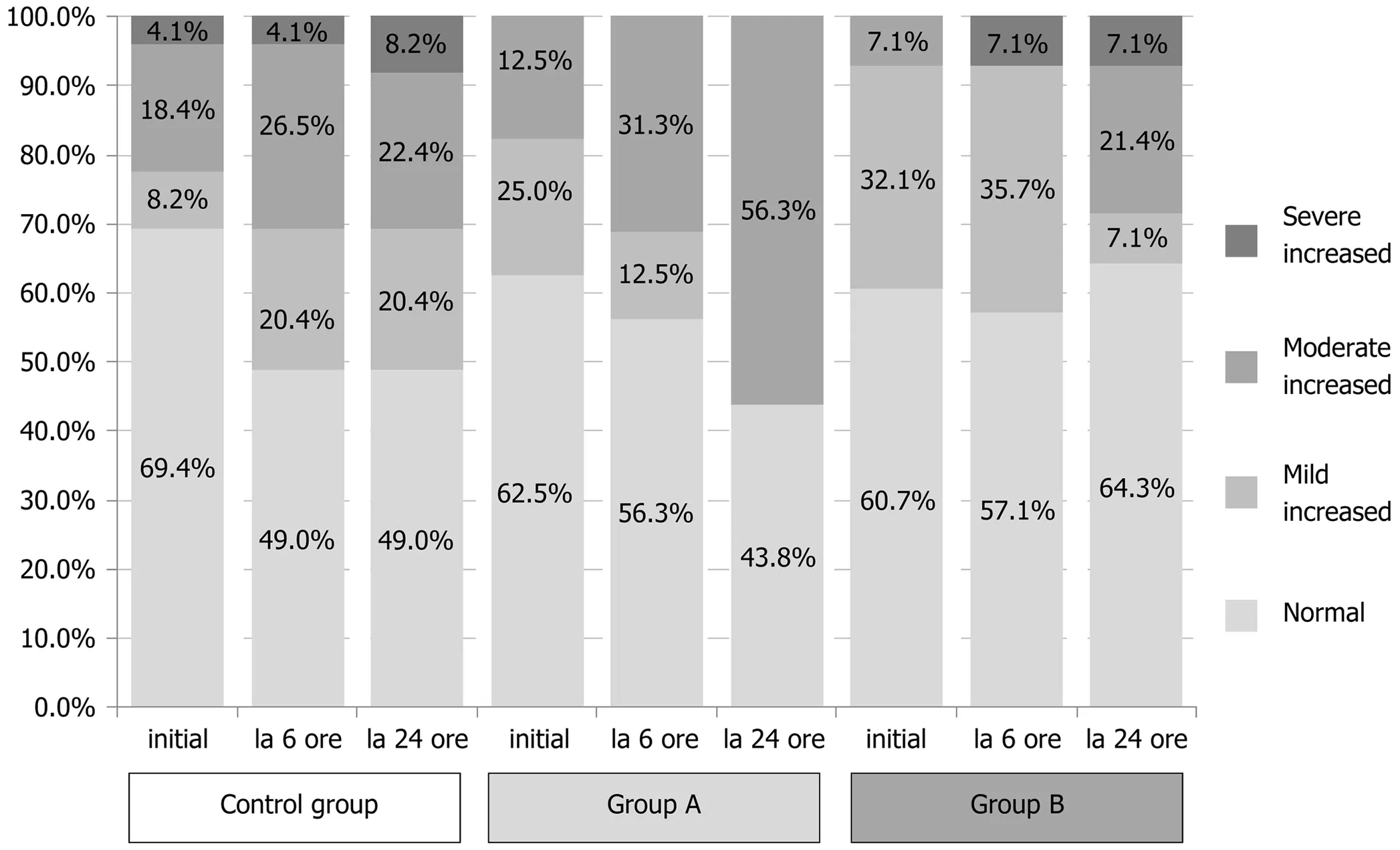
Figure 2 C-reactive protein evolution from baseline, at 6 h and at 24 h, compared in the three study arms.
ARTICLE HIGHLIGHTS
Research background
Endoscopic retrograde cholangiopancreatography (ERCP) represents a complex endoscopic technique, which is almost exclusively used nowdays for therapeutic intent. Although ERCP should be a safe procedure for the vast majority of patients, postprocedural complications may arise, among which post-ERCP pancreatitis (PEP) is the most frequently reported.
Research motivation
PEP still remains an important concern with respect to its prophylaxis and management,especially considering the paucity of prospective trials in ERCP regarding this complication.
Research objectives
This study aimed to evaluate the efficacy of three prophylactic approaches for preventing PEP,using pharmacologic agents with different mechanisms of action (indomethacin in various doses with or without an extra dose of N-acetylcysteine (NAC) for prevention of acute pancreatitis in patients with choledocholithiasis and indication for undergoing ERCP.
Research methods
The study design was that of a prospective, single-blind randomized trial for comparing and dynamically evaluating the efficacy of the three pharmacological combination therapies.
Research results
Both split-dose administration of indomethacin (50 mg pre- and post-ERCP) and combined administration of 600 mg NAC before the procedure with per-rectum administration of 100 mg indomethacin post-ERCP showed similar efficacy in preventing PEP, as compared to the standard, guideline-reccommended regimen (per-rectum administration of 100 mg indomethacin post-ERCP).
Research conclusions
The results of this prospective randomized control trial support the potential use of various pharmacologic pathogenic regimens for the prophylaxis of PEP, showing similar efficacy and safety profiles. However, considering other potential prophylactic approaches, using either intravenous nonsteroidal anti-inflammatory drugs or aggressive intravenous hydration, doubleblind prospective randomized controlled trials would offer stronger evidence for establishing prophylactic strategies in PEP.
Research perspectives
Considering these findings, further research in the field (eventually, through multicentric studies, enrolling high number of patients and modulating the antioxidant dose) could lead to developing more a efficient prophylactic pharmacological approach, with a satisfactory safety profile and tenable costs.
 World Journal of Clinical Cases2019年3期
World Journal of Clinical Cases2019年3期
- World Journal of Clinical Cases的其它文章
- Overexpression of HSP27 and HSP70 is associated with decreased survival among patients with esophageal adenocarcinoma
- Incidence, risk factors, and outcome of BK polyomavirus infection after kidney transplantation
- Influencing factors of postoperative early delayed gastric emptying after minimally invasive Ivor-Lewis esophagectomy
- Celiac crisis, a rare occurrence in adult celiac disease: A systematic review
- Hand-assisted laparoscopic splenectomy is a useful surgical treatment method for patients with excessive splenomegaly: A metaanalysis
- Successful treatment of obstructing colonic cancer by combining self-expandable stent and neoadjuvant chemotherapy: A case report
