Overexpression of HSP27 and HSP70 is associated with decreased survival among patients with esophageal adenocarcinoma
Henna K Söderström, Juha T Kauppi, Niku Oksala, Timo Paavonen, Leena Krogerus, Jari Räsänen,Tuomo Rantanen
Abstract BACKGROUND Overexpression of heat shock proteins (HSPs) is associated with several malignancies and contributes to the development, progression, and metastasis of cancer, in addition to the inhibition of cellular death. In recent years, there has been active research into using HSP inhibitors in several malignancies. Due to the poor prognosis of esophageal adenocarcinoma (EAC), it would be valuable to find new biomarkers for the development of cancer treatments.AIM To evaluate the expressions of HSP27 and HSP70 and their effect on survival in EAC.METHODS Immunohistochemical analyses and evaluations of HSP27 and HSP70 expression were performed on all available samples from 93 patients diagnosed with EAC between 1990 and 2007 at two university hospitals. Fifteen cases with Barrett’s metaplasia and 5 control cases from the same patient population were included in the analysis. HSP expression was quantitatively assessed and classified as high or low. Kaplan-Meier analyses and Cox regression models adjusting for age and sex as well as tumor site, stage, and grade were used to evaluate the effect on survival.RESULTS Tumor stage and surgical treatment were the main prognostic factors. High HSP27 expression in cancer cases was a strong negative predictive factor, with a mean survival of 23 mo compared to the 49 mo in cases with a low expression (P= 0.018). The results were similar for HSP70, with a poorer survival of 17 mo in cases with high HSP70 expression, in contrast to 40 mo (P = 0.006) in cases with a low expression. A Cox regression survival analysis was performed, adjusting for possible confounding factors, and higher HSP27 and HSP70 expressions remained an independent negative prognostic factor. The HSPs’ correlation with survival was not affected by cancer treatments. When the analysis was adjusted for all factors, the odds ratios for HSP27 and HSP70 were 3.3 (CI: 1.6-6.6, P =0.001) and 2.2 (CI: 1.2-3.9, P = 0.02), respectively.CONCLUSION HSP27 and HSP70 overexpression is associated with poor survival in EAC, which is, to the best of our knowledge, reported for the first time.
Key words: Esophageal adenocarcinoma; Heat shock proteins; Heat shock protein 27;Heat shock protein 70; Overexpression; Survival
INTRODUCTION
Esophageal adenocarcinoma (EAC) is an aggressive disease with a poor prognosis,and its incidence is increasing in Western countries[1]. At the time of diagnosis, up to 70% of patients present with either local or distant metastasis, reducing the chances of curative treatment and resulting in a markedly impaired survival[2]. Over the past 10 years, however, we have seen an improvement in survival, especially in local and locally advanced disease[1], but the overall 5-year survival is nevertheless only 17%[2].
Heat shock proteins (HSPs) are classified according to molecular weight (i.e.,HSP27, HSP60, HSP70, HSP90, and HSP110) into distinct protein families, and they are present in all human cells[3]. They function as molecular chaperones in the processes of protein folding, cellular signaling, and apoptosis. Overexpression of HSPs is associated with several malignancies and contributes to the development,progression, and metastasis of cancer, in addition to the inhibition of cellular death. In other words, they are essential for cancer growth and tumor survival[3,4]. Conflictingly,in certain cancers, including esophageal squamous cell carcinoma (SCC)[5], elevated HSP27 and HSP70 expressions are associated with improved overall survival.Although lower HSP27 and HSP70 expressions have been found among patients with gastroesophageal reflux disease before and after antireflux surgery when compared to controls[6], no previous study exists on the association of HSP27 and HSP70 with survival among patients with EAC.
Since HSP expression is associated with cancer development and progression, it is an attractive target for cancer treatment. In recent years, there has been active research into using HSP inhibitors in several malignancies[4,7]. Because of the poor prognosis of EAC, it would be valuable to find new biomarkers for the development of cancer treatments[1].
We conducted the present study to analyze the expression of HSP27 and HSP70,and their association with survival, in patients with EAC.
MATERIALS AND METHODS
Patients
All patients who were surgically treated for EAC between 1990 and 2007 at Helsinki University Central Hospital (HUCH) and Tampere University Hospital (TAYS) were surveyed. In addition, patients who were diagnosed but not treated at TAYS during the same period were reviewed. All patients who had sufficient tissue material for analysis were included in the study. Control samples were obtained from the normal esophageal epithelium adjacent to the tumor. We also included 15 cases with Barrett’s metaplasia, with or without dysplasia.
Cancer stage and location were assessed in accordance with the American Joint Committee on Cancer 7thedition staging manual[8]. For statistical reasons, a modified staging system was implemented where Stage I consisted of stages I-IIA, Stage II of stages IIB-III, and Stage III of stages IVA and IVB.
Overall survival data was collected from patient records and Statistics Finland in December 2015.
Samples
All tissue samples, from both endoscopy and surgery, were formalin-preserved and processed into paraffin blocks. All samples were re-evaluated according to WHO criteria by two highly experienced pathologists (Paavonen T and Krogerus L). Three histologically representative tumor sites (tissue cores) were selected from all surgically obtained samples and placed into tissue micro array (TMA) blocks (Beecher Instruments, Silver Spring, MD, USA). The diameter of the tissue cores was 1 mm.Endoscopic samples were processed as routine paraffin blocks.
Immunohistochemistry
The formalin-fixed and paraffin-embedded tissue samples and TMA blocks were cut into 5 μm sections and placed on Superfrost Plus microscope slides (Menzel-Gläser,Braunschweig, Germany). Monoclonal antibodies anti-heat shock protein 27 (anti-HSP27; clone G3.1, 1:400, Labvision corporation Neomarkers, FremontCalifornia,USA) and anti-heat shock protein 70 (anti-HSP70; clone C92F3A-5, 1:50, Stressgen Bioreagents, Michigan, USA) were used for immunohistochemical staining, which was done using a Ventana BenchMark LT Automated IHC Stainer (Ventana Medical System, Arizona, USA) with the Ultraview Universal DAB detection kit (catalogue No. 760-500, Ventana Medical System, Arizona, USA). The BenchMark LT instrument and preset was used at each step of the Ventana Ultraview Universal DAB detection kit to optimize the procedure. The Ventana High Temperature Liquid Coverslip (LCS,catalogue No: 650-010, Ventana) was applied when appropriate. The Ventana Trisbased reaction buffer (catalogue No. 950-300, Ventana) was used to rinse the slides between the steps. Deparaffinization was achieved using Ventana EZ Prep solution(catalogue No 950-100, Ventana), and epitope retrieval was performed using CC1:Tris-EDTA buffer pH 8.0 (catalogue No 950-124, Ventana) at 95-100 °C for 30 min with anti-HSP27, and for 60 min with anti-HSP70. UV-Inhibitor 3% H202 (Ventana) was used for 4 min at 37 °C to block endogenous peroxidase. The slides were incubated at 37 °C for 32 min with (the) specific antibodies, followed by the application of Ventana Ultraview HRP Universal Multimer (8 min at 37 °C). Diaminobenzidine was used as a chromogen to detect antibodies, and counterstaining with hematoxylin was performed before mounting. The above-mentioned process was fully automated for baking, deparaffinization and antigen retrieval.
Known positive tissue samples were used to confirm staining reliability in all staining patches[6].
Evaluation of HSP27 and HSP70 expressions
The expressions of HSP27 and HSP70 were evaluated by two experienced observers with no knowledge of clinicopathological data. In borderline cases, samples were reviwed by a higly experienced gastrointestinal pathologist and consensus was reached. Immunopositivity was measured from one (endoscopic biopsies) or three(TMA) tumor spots for each patient. The staining intensities were scored based on sample immunoreactivity as follows: 0 = no stained cells; 1 = faint, 2 = moderate; and 3 = strong positive staining. The percentages of positive epithelial cells (cell positives)were classified as 0 = no stained cells; 1 = 1%-25% positive cells; 2 = 26%-75% positive cells; and 3 ≥ 75% positively stained cells. The scores of staining intensity and positively stained cells were combined and ranged from 0 to 3. HSP expression is presented as low and high, with a cutoff point of 1.5, the median of the range[6]. In cases with multiple samples from one patient, the sample with the highest score was deemed representative.
Statistical methods
The statistical analysis was carried out using SPSS 21.0 (SPSS, Inc., Chicago, Il, USA).The patients’ age, sex, as well as tumor stage, grade, and site were used as variables.Kaplan-Meier and Cox regression survival models (adjusting for age and sex; age, sex,and stage; age, sex, and grade; age, sex, and site; age, sex, stage, grade, and site) were used for survival analysis. Chi-square test analysis was used on class parameters. The association between staining intensity and the different variables was analyzed using multinomial regression models. Correlating expression patterns were analyzed with Pearson’s correlation coefficient. A P value of < 0.05 was considered significant.Survival was calculated from the date of surgery. The survival rates are reported as means, unless stated otherwise. The statistical methods of this study were reviewed by Tuomas Selander from Kuopio University Hospital.
Ethics
The study has been approved by the Ethics Committee at HUCH.
RESULTS
In the selected time frame, there were 307 EAC cases. About 96 patients had available tumor samples, and a total of 151 specimens were located. Sufficient tissue material for analysis was available in 89 cases for HSP27 (131 specimens) and in 93 cases for HSP70 (136 specimens). In addition, we analysed 15 Barrett’s esophagus specimens,with or without dysplasia, from 12 cases. Analysis was also performed on 5 control samples. HSP27 and HSP70 staining was performed on all available cancer specimens;patient flow is demonstrated in Figure 1.
About 45.4% of the patients had radical surgery and 5.4% recieved definitive oncological treatment with curative intent. 50.8% of the patients were referred to palliative treatment. The number of surgically treated patients is unusually high,because only operative cases were included from HUCH. Patient and tumor characteristics, surgical procedures, and causes of death are presented in Table 1.
Frequencies of HSP27 and HSP70 immunostaining
HSP27 expression was high in 53 (59.6%) and low in 36 (40.4%) of the cancer cases.There was no statistical difference in staining frequency in comparison to the Barrett’s esophagus and control cases. The expression of HSP70 was high in 28 (30.1%) and low in 65 (69.9%) of the cancer cases. The frequencies were significantly different in the BE cases, among which the expression was high in 53.3% of the cases (P = 0.025). There was a strong correlation between HSP27 and HSP70 staining intensities in the cancer cases (P < 0.001).
The frequencies of HSP27 and HSP70 immunostaining intensities by stage are presented in Table 2 and Table 3. There was no significant correlation between staining intensity and stage (P = 0.33 and P = 0.45)
Survival
The mean and median survival times for surgically treated patients were 98.4(58.5-138.2) and 58 (17.6-98.4) mo, respectively, while the respective rates of inoperable patients were 13.4 (8.6-18.1) and 8 (6.5-9.6) mo (P < 0.001).
Tumor stage was a strong prognostic factor - a higher stage was significantly associated with poorer survival (P < 0.001) independently of the HSP27 and HSP70 immunostainings (P < 0.001). Tumor stage did not correlate with HSP expression.There was a strong correlation between HSP27 and HSP70 immunostainings (P <0.001). We also found a correlation between HSP27/70 and tumor grade (P = 0.01 and P = 0.025, respectively), but the grade was not a prognostic factor. Conservatively treated patients presented significantly more often with high HSP27 and HSP70 expressions, compared to those whom underwent radical surgery. About 67.9% of all patients with high HSP27, and 75% of those with high HSP70, were inoperable at time of diagnosis (P < 0.01 and P = 0.01).
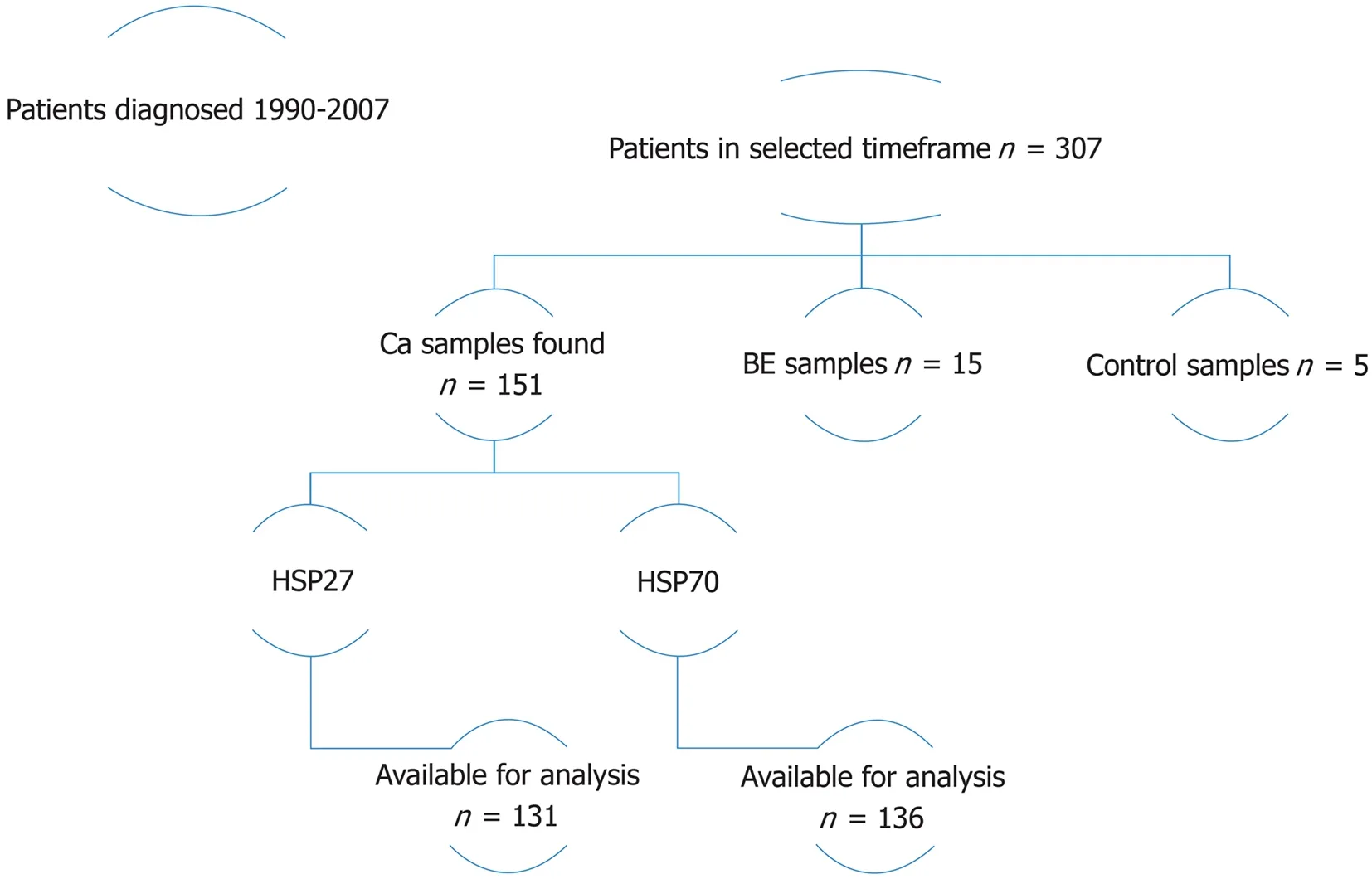
Figure 1 Patients in selected timeframe (n = 307). Number of tissue samples obtained. “Available for analysis” =number of samples with sufficient tissue material for heat shock protein analysis. BE: Barrett’s esophagus; HSP: Heat shock protein.
High HSP27 expression was a strong negative predictive factor, with a mean survival of 23 mo, compared to the survival rate of 49 mo in cases with negative and low expressions (P = 0.018). The results were similar for HSP70 immunostaining -cases with a high expression had an inferior survival rate of 17 mo in comparison to the rate of 40 mo for those with negative or low expression (P = 0.006). Kaplan-Meier survival functions for HSP27/70 are shown in Figure 2. Patients at risk at 6 mo, 12 mo,and 36 mo are presented in Table 4.
Subgroup survival analysis was done according to tumor stage. Among HSP27 cancer cases, survival data was available for only six patients with stage I disease.There was no difference in survival between high (4 cases) and low (2 cases)expressions (P = 0.77). 44 patients with stage II cancer had available survival data, and survival among patients with high HSP27 expression (27 cases) was 23.9 mo compared to 53.2 mo for low expression (17 cases). This finding was statistically significant (P = 0.044). Among patients with stage III cancer, 15 had high HSP27 expression and only three had low expression, with survival times of 8.8 mo and 22.3 mo, respectively (P = 0.097).
Similar results were obtained for HSP70. Among the seven stage I cancer patients with available survival data only one had high HSP70 expression and was still alive at the end of follow-up. About 47 patients had stage II disease and there was a statistically significant difference in survival between the 16 cases with high HSP70 expression (17.8 mo) compared to the 31 cases with low expression (43.0 mo) (P =0.018). About 18 patients had stage III disease with an equal distribution of high and low expressions, but the difference in survival (14.9 mo and 7.2 mo, respectively) was not significant (P = 0.137)
Cox regression survival analysis was performed adjusting for age, sex, cancer stage,tumor grade, and tumor site. Higher HSP27 and HSP70 expressions remained an independent negative prognostic factor, and cancer stage was the only analyzed confounder that was significantly associated with survival. Age, sex, and tumor site did not affect survival. The HSPs’ effect on survival was not associated with cancer treatments. When adjusting for all factors, the hazard ratios for HSP27 and HSP70 were 3.3 (95%CI: 1.6-6.6, P = 0.001) and 2.2 (95%CI: 1.2-3.9, P = 0.02), respectively.
DISCUSSION
The main finding in the present study was a significant positive association between elevated HSP27 and HSP70 expressions and poor survival in EAC. To the best of ourknowledge, this is reported herein for the first time. Higher expressions were also associated with higher tumor grade, but there was no association with pathological TNM stage or tumor site.
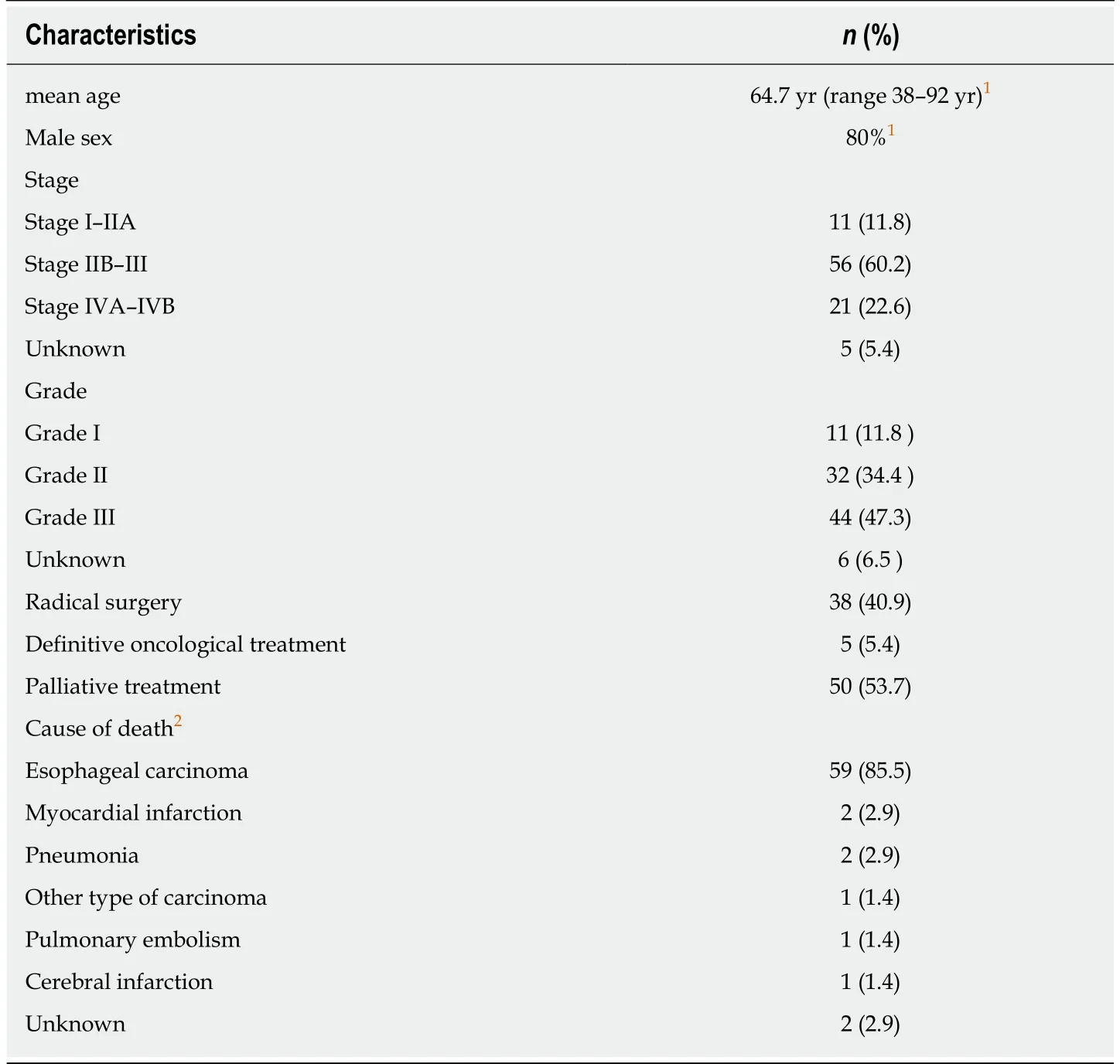
Table 1 Patient and cancer characteristics
Conservatively treated patients had higher HSP27/70 expressions than those for whom radical surgery was an option. This is consistent with the finding that overexpression is associated with higher grade, i.e., more aggressive disease, and therefore more likely to be inoperable at time of diagnosis. We found that patients with stage II EAC and high HSP27/70 expressions had significantly poorer prognosis,making them a high risk subgroup.
Previous studies have shown similar results in connection with other adenocarcinomas, such as colon and gastric carcinoma, for both HSP27[9,10]and HSP70[9,11]. Giaginis et al[10]found no effect on survival, but high HSP27 expression was significantly associated with higher T, N, and M stages, signaling more aggressive disease. Lee et al[11]employed study methods similar to those used in the present study, and while HSP70 was not associated with survival when analyzing the entire patient population, there was a significant correlation between high expression and poor survival in adenocarcinomas of the cardia and those of the intestinal type.
There are several studies linking high HSP27 and HSP70 expressions in esophageal SCC with better overall survival and less aggressive disease[12,13]. It stands to reason that the expressions in other adenocarcinomas of the gastrointestinal tract would have similar HSP expressions as in EAC, explaining our opposing findings compared to SCC. In a meta-analysis conducted in 2015[5], the authors found 5 eligible studies on HSP27/70 and survival, only one of which was regarding EAC (HSP27)[14]. One study did not differentiate between EAC/SCC (HSP70)[15]and is therefore not comparable to the present study. The remaining three were studies on SCC.
Studies regarding HSP27/70 and EAC survival are very scarce. We found only four studies on the topic, all conducted by the same German research group[14,16-18]and none of them with the same principal method of analysis as ours, rendering them noncomparable to present study as such. The main focus in the German group’s studies has been to identify and verify different protein expression profiles using reverse phase protein arrays (RPPA), in which HSP27 and HSP70 are a part of a palette ofseveral proteins. These expression profile analyses did not correlate with the group’s immunohistochemical (IHC) results[17]. None of the three studies showed a correlation between IHC HSP27/70 and survival, nor any prognostic factors including TNM stage. One of the studies with RPPAs did find that a combination of low HSP27 and high HER2 correlated with better overall survival in EAC, but no IHC was performed,therefore yielding results that are not comparable to ours[14]. Two of the studies focused on expression profiles and chemotherapy responses, and the results contradicted each other[16,17].
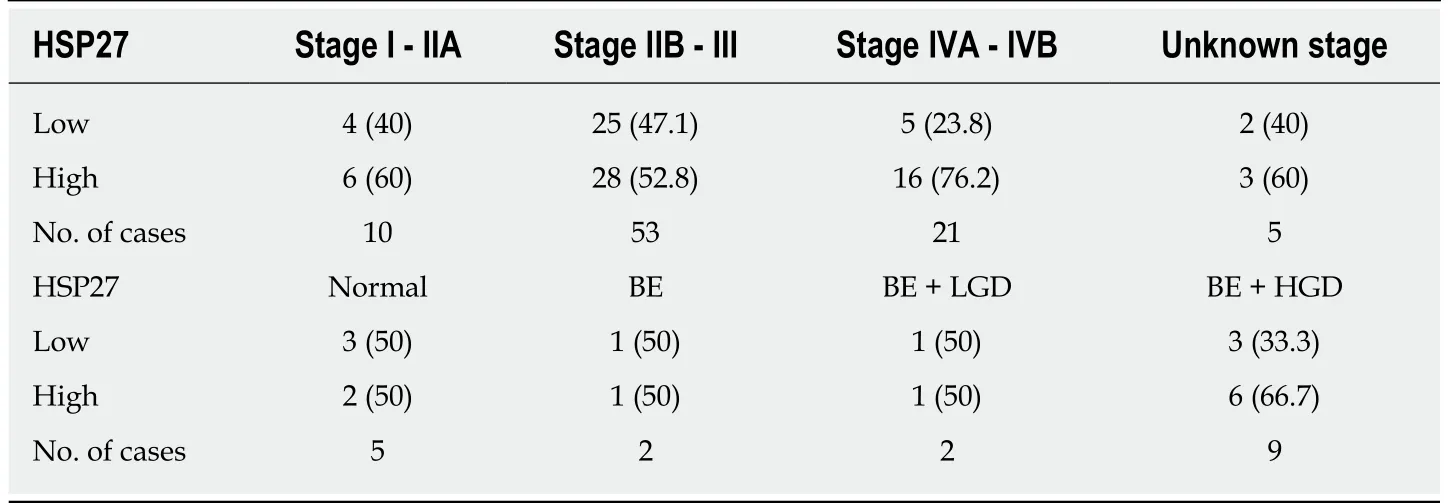
Table 2 Frequencies of heat shock protein 27 immunostaining n (%)
A study by Soldes et al[19]analyzing 22 EAC patients with or without Barrett’s metaplasia showed that elevated levels of HSP27 were present in normal esophageal mucosa, while the expression was significantly lower in Barrett’s esophagus and carcinoma samples. The authors speculated that one mechanism of carcinoma development would be the replacement of protective mucosa expressing high levels of HSP27 with metaplasia expressing low levels of HSP27. In the present study, 54.1%of the cancer cases showed high HSP27 levels, with no statistical difference in comparison to Barrett’s esophagus or control samples, which differs considerably from the results of Soldes et al[19]. The study by Soldes et al[19]did not include survival analysis.
The main limitations of the present study are its retrospective nature and the relatively small sample size. The samples are from 1990-2007 when oncological treatment and the surgical approach differed slightly from today’s treatment options,so the overall survival analysis may not be completely in line with current survival data. This, however, did not limit the analysis of the HSPs’ prognostic impact, which was independent of treatments.
In conclusion, we report, for the first time, an association between higher HSP27/70 expressions and poor survival in EAC. The expressions did not differ from the Barrett’s esophagus or control samples, rendering them insignificant as diagnostic markers, but potentially useful as prognostic tools. We found that patients with stage II EAC and high HSP27/70 expressions had significantly poorer prognosis, making them a possible target group for HSP inhibitors. Further studies on HSP27/70 expressions in EAC are required to evaluate their feasibility and utility as therapeutic targets.
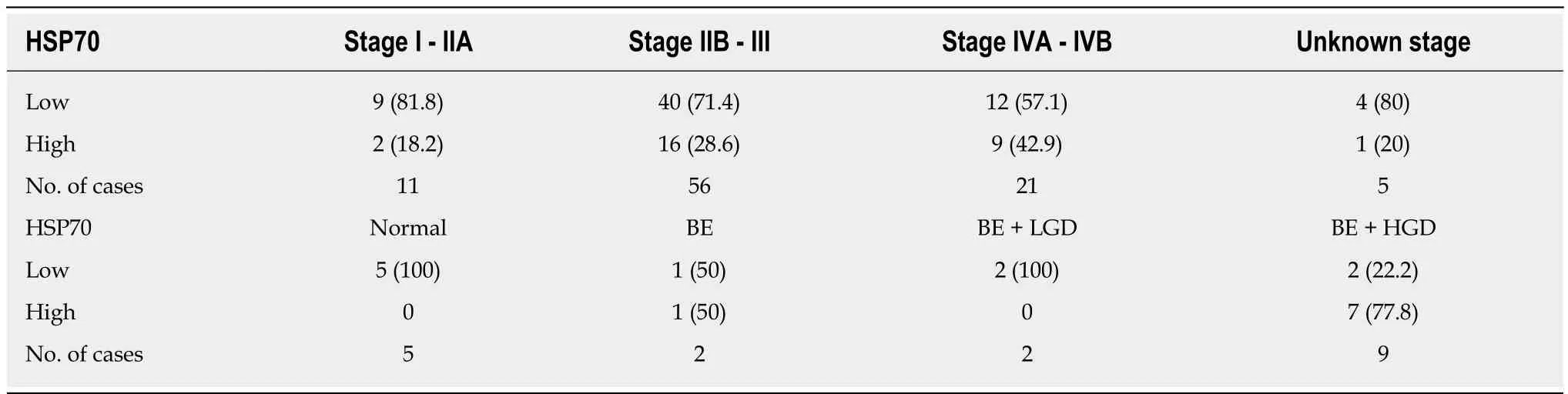
Table 3 Frequencies of heat shock protein 70 immunostaining n (%)
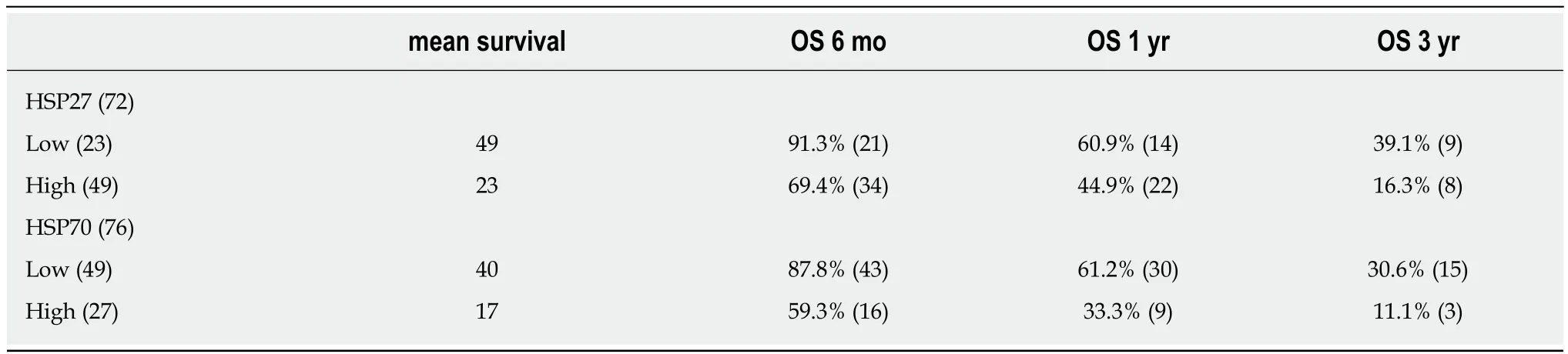
Table 4 Overall survival and (number of patients at risk) at 6, 12 and 36 mo
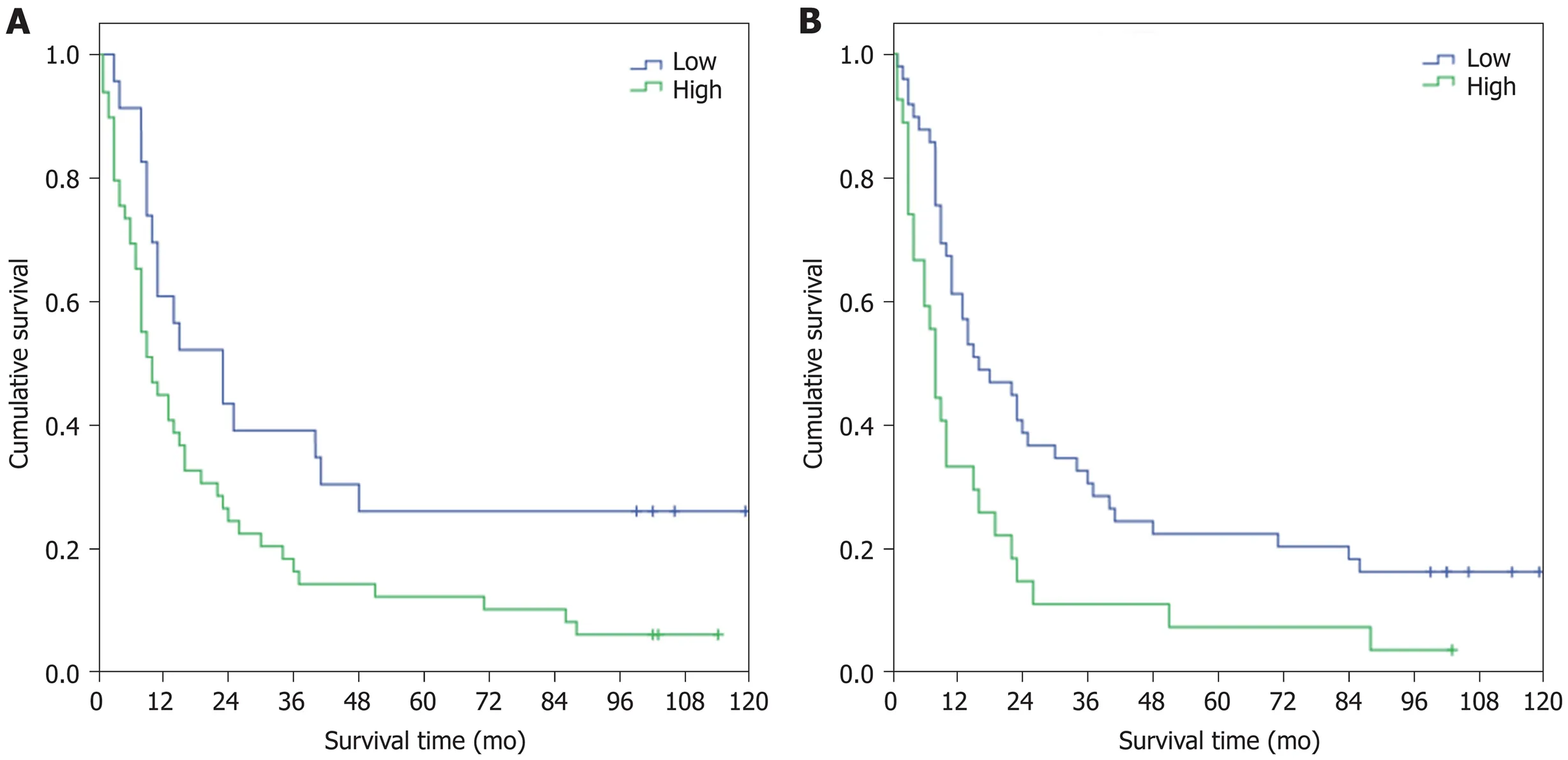
Figure 2 Survival (mo) associated with heat shock protein 27 (A) and heat shock protein 70 (B) expression.
ARTICLE HIGHLIGHTS
Research background
Overexpression of heat shock proteins (HSPs) is associated with several malignancies. It contributes to the development, progression, and metastasis of cancer, and to the inhibition of cellular death. There has been active research into using HSP inhibitors in several malignancies in recent years.
Research motivation
Because of the poor prognosis of esophageal adenocarcinoma (EAC), it would be valuable to find new biomarkers for the development of cancer treatments.
Research objectives
In the present study, the authors intend to analyze the expression of HSP27 and HSP70, and their association with survival, in patients with EAC.
Research methods
Immunohistochemical analyses and evaluations of HSP27 and HSP70 expression were performed on all available samples from 93 patients diagnosed with EAC. From the same patient population, 15 cases with Barrett’s metaplasia and 5 control cases were included in the analysis.HSP expression was quantitatively assessed and classified as high or low. Kaplan-Meier analyses and Cox regression models were used to evaluate the effect on survival.
Research results
Tumor stage and surgical treatment were the main prognostic factors. High HSP27 expression in cancer cases was a strong negative predictive factor, with a mean survival of 23 mo vs the 49 mo in cases with a low expression. The results were similar for HSP70, with a poorer survival of 17 mo in cases with high HSP70 expression, in contrast to 40 mo in cases with a low expression.Higher HSP27 and HSP70 expressions remained an independent negative prognostic factor. The HSPs’ correlation with survival was not affected by cancer treatments.
Research conclusions
To the best of our knowledge, reported for the first time, HSP27 and HSP70 overexpression is associated with poor survival in EAC.
Research perspectives
To evaluate their feasibility and utility as therapeutic targets, further studies on HSP27/70 expressions in EAC are required.
 World Journal of Clinical Cases2019年3期
World Journal of Clinical Cases2019年3期
- World Journal of Clinical Cases的其它文章
- Incidence, risk factors, and outcome of BK polyomavirus infection after kidney transplantation
- Influencing factors of postoperative early delayed gastric emptying after minimally invasive Ivor-Lewis esophagectomy
- Split-dose or hybrid nonsteroidal anti-inflammatory drugs and Nacetylcysteine therapy for prevention of post-retrograde cholangiopancreatography pancreatitis Laura Pavel, Gheorghe Gh Bălan, Alexandra Nicorescu, Georgiana Emmanuela
- Celiac crisis, a rare occurrence in adult celiac disease: A systematic review
- Hand-assisted laparoscopic splenectomy is a useful surgical treatment method for patients with excessive splenomegaly: A metaanalysis
- Successful treatment of obstructing colonic cancer by combining self-expandable stent and neoadjuvant chemotherapy: A case report
