猪SPATA6基因的多态性与精液品质性状的关联分析
周佳伟 吴俊静 乔木 刘贵生 彭先文 梅书棋
摘要:公猪精液品质直接影响到猪场经济效益,通过转录组测序发现SPATA6基因在猪性成熟前后的睾丸组织中差异表达,并发现其内含子区域内存在多个SNP位点。通过SNaPshot的方法分别对杜洛克猪和大白猪中SPATA6基因的rs331255092和rs341061477位点进行分型,并与精液品质性状进行关联分析。结果表明,在杜洛克猪和大白猪中rs331255092和rs341061477位点均与精子畸形率显著相关,此外,在大白猪中rs331255092和rs341061477位点也与精液量显著相关。猪SPATA6基因的rs331255092和rs341061477位点对猪的精液品质存在潜在调控作用。
关键词:猪;SPATA6基因;SNP;精子畸形率;精液量
中图分类号:S828 文献标识码:A
文章编号:0439-8114(2019)24-0163-04
DOI:10.14088/j.cnki.issn0439-8114.2019.24.039 开放科学(资源服务)标识码(OSID):
Association analysis of SPATA6 gene polymorphism and semenquality in porcine
ZHOU Jia-wei,WU Jun-jing,QIAO Mu,LIU Gui-sheng,PENG Xian-wen,MEI Shu-qi
(Hubei Key Laboratory of Animal Embryo and Molecular Breeding/Institute of Animal Science and Veterinary Medicine,Hubei Academy of Agricultural Sciences,Wuhan 430064,China)
Abstract: The quality of boar semen directly affects the economic benefits of pig farms. Transcriptome sequencing showed that SPATA6 gene was differentially expressed in testicular tissues of porcine sexually mature and immature, and multiple SNP sites were found in its intron region. In this study, rs331255092 and rs341061477 loci of SPATA6 gene were identified by SNaPshot method in Duroc pig and Large White pig. The results showed that rs331255092 and rs341061477 were significantly correlated with sperm abnormality rate in Duroc pig and Large White pig. In addition, rs331255092 and rs341061477 were also significantly correlated with semen volume in Large White pig. In summary, rs331255092 and rs341061477 have potential regulatory effects on semen quality in pigs.
Key words: pig; SPATA6 gene; SNP; sperm abnormality rate; semen volume
公豬在养猪生产中发挥着重要作用,公猪精液质量直接影响猪场经济效益。精液量、精子密度、精子活力和精子畸形率是衡量精液品质的4个重要指标[1]。由于精液品质性状的遗传力低,很难通过常规育种手段进行遗传改良,而分子标记辅助选择法(Marker-assisted selection,MAS)是提高精液品质的一种有效方法[2]。部分学者通过候选基因法筛选出多个与精液品质性状相关的分子标记,例如ESR1、SPAG11、FST、PRLR、INHA、FSHβ、DAZL等[3-8],但依然有很多与精液品质相关的SNP位点有待挖掘。Song等[9]通过转录组测序发现精子发生相关基因6(Spermatogenesis associated 6,SPATA6)在大白猪性成熟前后的睾丸组织中差异表达,并存在多个SNP位点。SPATA6基因在睾丸组织中特异表达[10],在5周龄小鼠的睾丸中开始检测出SPATA6基因的表达,5周龄到15周龄表达量持续增加,直至63周龄均可检测出SPATA6基因的表达,且SPATA6基因主要在精母细胞中表达[11]。SPATA6基因的失活影响精子组装过程中的精子中段形成与头尾紧密连接,导致生成无头精子[12]。PMFBP1的突变会影响PMFBP1与SPATA6和SUN5基因的结合,引发无头精子症[13]。在少弱精子症患者中SPATA6基因的表达量显著低于正常男性,miR-23a/b-3p可以通过靶向SPATA6基因影响精子成熟[14]。
本研究以SPATA6基因为研究对象,探究SPATA6基因与精液量、精子密度、精子活力、精子畸形率相关的SNP位点,为公猪精液品质的遗传改良提供新的靶点。
1 材料与方法
1.1 试验样品
收集177头杜洛克猪和78头大白猪的精液样本。所有样品均记录了精液量、精子密度、精子活力和精子畸形率等4个指标。采用标准苯酚氯仿萃取法提取精液基因组DNA,保存于-20 ℃。
1.2 SNaPshot基因分型
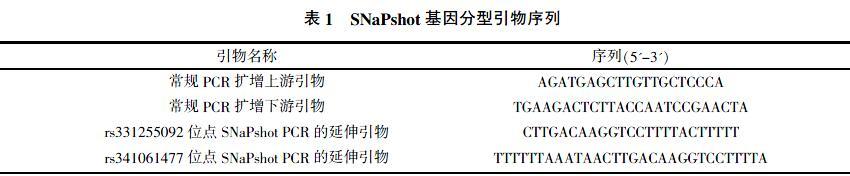
1.2.1 目的序列的常规PCR扩增 扩增含有rs341061477和rs331255092位点的DNA序列,PCR反应体系50 μL:金牌绿酶MIX试剂25 μL,gDNA模板1 μL,上、下游引物各2 μL(引物序列见表1),去离子水20 μL。由于rs341061477和rs331255092位点相聚较近,因此共用一对引物。PCR扩增程序为98 ℃预变性2 min;98 ℃变性10 s,60 ℃退火20 s,72 ℃延伸10 s,35个循环;72 ℃延伸1 min,4 ℃保存。
1.2.2 SNaPshot PCR 将PCR产物回收纯化,取15 μL回收纯化后的PCR产物,加入5 U SAP和 2 U Exo I,振荡混匀,37 ℃保温1 h,然后75 ℃保温 15 min以灭活SAP和Exo I酶;使用Applied Biosystems公司的SNaPshot Multiplex Kit将处理后的15 μL PCR产物吸出3 μL进行SNaPshot检测,PCR反应体系10 μL,Reaction Mix试剂5 μL,SAP和Exo I酶处理后PCR产物3 μL,rs341061477和rs331255092位点延伸引物各0.5 μL,去离子水1 μL,PCR扩增程序为96 ℃变性10 s,50 ℃退火5 s,60 ℃延伸30 s,25个循环,4 ℃保存。
1.2.3 SNP位点的读取与识别 将SNaPshot产物稀释20倍,稀释体系为Hi-Di Formamide 9.25 μL,GS-120LIZ 0.25 μL,SNaPshot产物0.5 μL,反应体系为95 ℃变性5 min,冰浴4 min;配制含有350 μL Hi-Di甲酰胺和50 μL Matrix标准品的混合液,95 ℃变性5 min,迅速冰冷5 min,平分2管,分装至上机板后对3730XL DNA Analyzer仪器进行光谱校正;使用3730XL DNA Analyzer对制备好的样品进行毛细管电泳并搜集信号[15];最后使用GeneMapper V4.0软件对结果进行分析。
1.3 连锁不平衡分析和群体关联分析
采用SHEsis在线软件(http://analysis.bio-x.cn/myAnalysis.php)进行连接不平衡分析[16,17]。采用SAS软件的一般线性模型将177头杜洛克猪和78头大白猪的SPATA6基因的rs341061477和rs331255092位点的基因型与精液品质性状进行关联分析。同时采用REG程序计算基因加性效应和显性效应,并进行显著性检验,所用模型为:
Yij=μ+Gi+Fj+eij
式中,Yij为性状表型值,μ为平均值,Gi为基因型效应(包括基因加性效应和显性效应;加性效应用1,0和-1分别代表TT、AT和AA基因型或TT、CT和CC基因型,显性效应用1,-1和1分别代表TT、AT和AA基因型或TT、CT和CC基因型);Fj为猪场综合效应;eij为残差效应。
2 結果与分析
2.1 rs341061477和rs331255092位点在杜洛克猪和大白猪中的等位基因频率
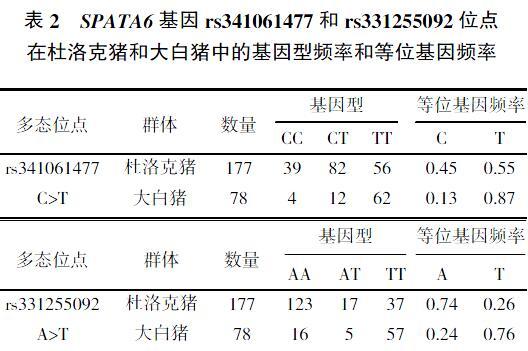
Song等[9]通过大白猪性成熟前后的睾丸组织的转录组测序发现SPATA6基因差异表达,且SPATA6基因的rs341061477和rs331255092位点存在多态性。SNaPshot法检测177头杜洛克猪和78头大白猪的精液样本中SPATA6基因的rs341061477和rs331255092位点的基因型频率和等位基因频率结果如表2所示。rs341061477位点在大白猪中等位基因T的频率为0.87,是优势等位基因,但在杜洛克猪中差异不显著;rs331255092位点在杜洛克猪中等位基因A的频率为0.74,是优势等位基因;而在大白猪中等位基因T的频率为0.76,是优势等位基因。
2.2 rs341061477和rs331255092位点的连锁不平衡分析
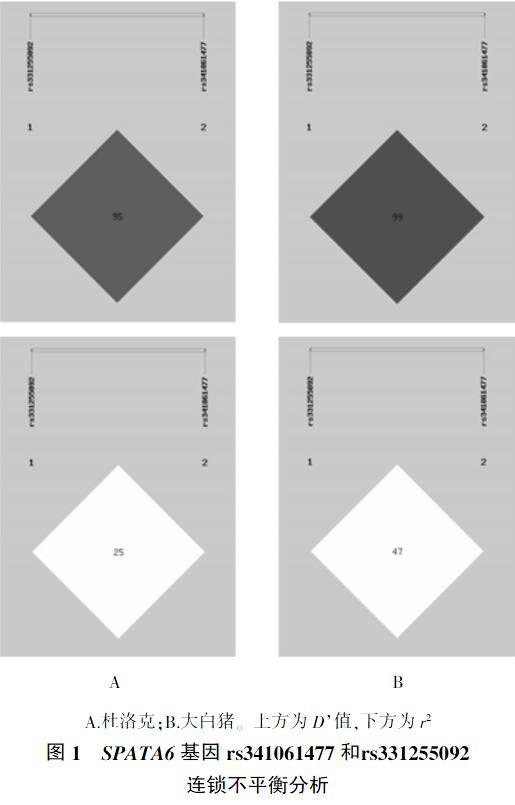
通过SHEsis在线软件对SPATA6基因的rs341061477和rs331255092位点进行连锁不平衡分析。结果表明,这两个多态位点在杜洛克猪和大白猪群体中的r2分别为0.25(图1A)和0.47(图1B),而当r2<0.8时认为两个突变是不连锁的[18],所以SPATA6基因rs341061477和rs331255092位点在杜洛克猪和大白猪群体中为不连锁位点。
2.3 rs341061477和rs331255092位点与精液品质的关联分析
SPATA6基因在睾丸组织中特异表达,研究表明SPATA6基因的失活会影响精子发生过程,导致生成无头精子[10,12,13]。GWAS分析发现SPATA6基因的遗传变异会影响小鼠睾丸重量[19]。SPATA6基因的多态位点与杜洛克猪和大白猪的精液品质性状关联分析结果见表3。如表3所示,rs341061477位点在杜洛克猪中TT基因型的精子畸形率显著高于CT基因型(P<0.05);在大白猪中TT基因型和CT基因型的精子畸形率极显著高于CC基因型(P<0.05),且加性效应达到1.39%(P<0.01),TT基因型的精液量极显著低于CT基因型(P<0.01)。如表4所示,rs331255092位点在杜洛克猪中AT基因型的精子畸形率显著高于AA基因型(P<0.05);在大白猪中AT基因型精子畸形率也显著高于AA基因型(P<0.05),AA基因型的精液量极显著高于TT基因型(P<0.01),且加性效应达到19.10 mL(P<0.01)。结果表明,在杜洛克猪和大白猪中,SPATA6基因的rs341061477和rs331255092位点与精子畸形率和精液量显著相关。
3 小结与讨论
公猪的生育力是影响养猪业生产效率的重要因素,而精液品质是衡量公猪生育力的重要指标。本研究以177头杜洛克猪和78头大白猪的精液样本为试验材料,鉴定影响精液品质性状的关键SNP位点,为公猪遗传改良提供有效的分子育种标记。
GWAS分析发现SPATA6基因的遗传变异可能会影响睾丸重量[19],在营养不良组和营养良好组的绵羊睾丸组织中SPATA6基因发生不同情况的可变剪接事件[20]。因此,鉴定SPATA6基因中影响精液品质的SNP位点对于改良精液品质以及解析SPATA6基因的可变剪切事件具有重要意义。本研究选取位于SPATA6基因第十内含子的rs341061477和rs331255092位点,通过SNaPshot法鉴定基因型,并将其与精液品质性状进行关联分析。
杜洛克猪中rs341061477位点的TT基因型的精子畸形率显著高于CT基因型,大白猪中rs341061477位点的TT基因型和CT基因型的精子畸形率显著高于CC基因型;在杜洛克猪和大白猪中rs331255092位点的AT基因型的精子畸形率均显著高于AA基因型;此外,在大白猪中rs331255092和rs341061477位点也与精液量显著相关。
本研究表明SPATA6基因的rs341061477和rs331255092位點显著影响精子畸形率,为进一步探究SPATA6基因在精子发生过程中的作用奠定了基础,也为公猪精液品质的遗传改良提供了新的靶点。
参考文献:
[1] XU X,POMMIER S,ARBOV T,et al. In vitro maturation and fertilization techniques for assessment of semen quality and boar fertility[J].J Anim Sci,1998,76(12):3079-3089.
[2] MARQUES D B D,LOPES M S,BROEKHUIJSE M L W J,et al. Genetic parameters for semen quality and quantity traits in five pig lines[J].J Anim Sci,2017,95(10):4251-4259.
[3] LIU X,JU Z,WANG L,et al. Six novel single-nucleotide polymorphisms in SPAG11 gene and their association with sperm quality traits in Chinese Holstein bulls[J].Anim Reprod Sci,2011, 129(1-2):14-21.
[4] GUNAWAN A,KAEWMALA K,UDDIN M J,et al. Association study and expression analysis of porcine ESR1 as a candidate gene for boar fertility and sperm quality[J].Anim Reprod Sci,2011,128(1-4):11-21.
[5] SATO Y,HASEGAWA C,TAJIMA A, et al. Association of TUSC1 and DPF3 gene polymorphisms with male infertility[J].J Assist Reprod Genet,2018,35(2):257-263.
[6] WIMMERS K,LIN C L,THOLEN E,et al. Polymorphisms in candidate genes as markers for sperm quality and boar fertility[J].Anim Genet,2005,36(2):152-5.
[7] LIN C L,PONSUKSILI S,THOLEN E,et al. Candidate gene markers for sperm quality and fertility of boar[J].Anim Reprod Sci,2006,92(3-4):349-63.
[8] MA C,LI J,TAO H,et al. Discovery of two potential DAZL gene markers for sperm quality in boars by population association studies[J].Anim Reprod Sci,2013,143(1-4):97-101.
[9] SONG H,ZHU L, LI Y,et al. Exploiting RNA-sequencing data from the porcine testes to identify the key genes involved in spermatogenesis in large white pigs[J].Gene,2015,573(2):303-309.
[10] OH C,AHO H,SHAMSADIN R,et al. Characterization,expression pattern and chromosomal localization of the spermatogenesis associated 6 gene (Spata6)[J].Mol Hum Reprod,2003,9(6):321-330.
[11] YAMANO Y,OHYAMA K,SANO T,et al. A novel spermatogenesis-related factor-1 gene expressed in maturing rat testis[J].Biochem Biophys Res Commun,2001,289(4):888-893.
[12] YUAN S,STRATTON C J,BAO J,et al. Spata6 is required for normal assembly of the sperm connecting piece and tight head-tail conjunction[J].Proc Natl Acad Sci U S A,2015,112(5):430-439.
[13] ZHU F,LIU C,WANG F,et al. Mutations in PMFBP1 Cause Acephalic Spermatozoa Syndrome[J].Am J Hum Genet,2018, 103(2):188-199.
[14] ABU-HALIMA M,AYESH B M,HART M,et al. Differential expression of miR-23a/b-3p and its target genes in male patients with subfertility[J].Fertil Steril,2019,S0015-0282(19)30306-1.
[15] BUJALKOVA M,ZAVODNA K,KRIVULCIK T,et al. Multiplex SNaPshot genotyping for detecting loss of heterozygosity in the mismatch-repair genes MLH1 and MSH2 in microsatellite-unstable tumors[J].Clin Chem,2008,54(11):1844-1854.
[16] YONG Y,HE L. SHEsis,a powerful software platform for analyses of linkage disequilibrium,haplotype construction,and genetic association at polymorphism loci[J].Cell Res,2005,15(2):97-98.
[17] SU L,MEI S,TAO H,et al. Identification of the promoter region and genetic mutations of the porcine GALP gene[J].Mol Biol Rep,2013,40(4):2821-2827.
[18] FR?魻JD?魻 S,SJ?魻LIND L,PARKKONEN M,et al. Polymorphisms in the gene encoding angiotensin I converting enzyme 2 and diabetic nephropathy[J].Diabetologia,2005,48(11):2278-2281.
[19] YUAN J T,GATTI D M,PHILIP V M,et al. Genome-wide association for testis weight in the diversity outbred mouse population[J].Mamm Genome,2018,29(5-6):310-324.
[20] GUAN Y,LIANG G,MARTIN G B,et al. Functional changes in mRNA expression and alternative pre-mRNA splicing associated with the effects of nutrition on apoptosis and spermatogenesis in the adult testis[J].BMC Genomics,2017,18(1):64.

