Development of Benzodithiophene-Based A-D-A Small Molecules with Different Acceptor End Groups for Efficient Organic Solar Cells
JIA Guoxiao , ZHANG Shaoqing ,*, YANG Liyan , HE Chang , FAN Huili ,*, HOU Jianhui ,,*
1 School of Chemical and Biological Engineering, University of Science and Technology Beijing, Beijing 100083, P. R. China.
2 State Key Laboratory of Polymer Physics and Chemistry, Beijing National Laboratory for Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, P. R. China
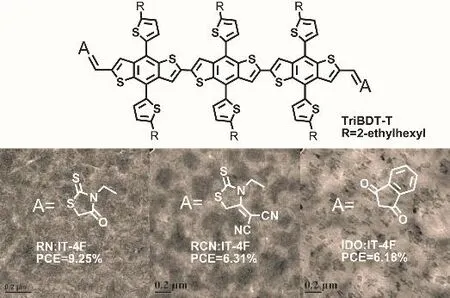
Abstract: In recent years, organic solar cells (OSCs) have attracted increasing attention, and the power conversion efficiency (PCE) of OSCs has markedly improved. To enhance the photovoltaic properties of OSCs, it is important to develop the donor materials in the light-harvesting layer, which mainly include conjugated polymers and small molecules (SMs). Compared with polymeric materials, small-molecule materials have been widely investigated for their superior characteristics, such as well-defined molecular structures that can provide good batch-to-batch reproducibility. In this work,we synthesized three SM donor materials with theacceptor-donor-acceptor(A-D-A) structure by employing the trialkylthienyl-substituted benzodithiophene (TriBDT-T) unit as the D-core unit, and rhodanine (RN), cyano-rhodanine (RCN), and 1,3-indanone (IDO)as the A end groups, respectively. The optical properties, molecular energy levels, and thermogravimetic characteristics of the three SMs were studied; moreover, the blend morphologies and photovoltaic properties of the devices by employing the non-fullerene (NF) acceptor, IT-4F, were systematically investigated. The results showed that 1) the three SMs exhibit good thermal stabilities as evinced by thermogravimetric analysis (TGA), and all decomposition temperatures exceeded 410 °C; 2) They all exhibit strong and broad absorption in the visible light range (300–700 nm), and show similar molar extinction coefficients; 3) the HOMO levels are -5.47 eV, -5.54 eV, and -5.44 eV for RN, RCN, and IDO, respectively,implying the clear influence of the different end groups for the energy levels of the A-D-A-type SMs; the slight differences in the optical and electrochemical properties of the corresponding donor material could be attributed to the different electron-withdrawing ability of the A-type end groups. When studying the photovoltaic properties, interestingly, the RN∶IT-4F blend was found to form fibrillar-like aggregates with appropriate size, and the corresponding devices exhibited desirable short circuit current (Jsc) and thus the highest PCE value of 9.25%; however, large-size aggregates were formed in the RCN∶IT-4F and IDO∶IT-4F blend films, resulting in a much lower Jsc and fill factor (FF), and the PCE of the corresponding devices were only around 6%. In summary, by introducing RN, RCN, and IDO as the A units, we synthesized a class of TriBDT-T based A-D-A type SMs. This study shows that terminal A units exert influence on the absorption spectra,molecular energy level, and morphologies after blending with the acceptor material, and hence, the corresponding devices exhibit significant differences in photovoltaic performance. This work also provides useful information for the molecular design of SM donor materials.
Key Words: Small molecule donor; Terminal modification; Organic solar cells; Non-fullerene acceptor; Acceptor-Donor-Acceptor structure