Liquefaction of thermal extracts from co-thermal dissolution of a sub-bituminous coal with lignin and reusability of Ni-Mo-S/Al2O3 catalyst
-, , -,*, -, -, -, -, -, -, -, -, bao,2
(1.School of Chemistry & Chemical Engineering, Anhui Province Key Laboratory of Coal Clean Conversion and High Valued Utilization, Anhui University of Technology,Ma’anshan 243002, China;2.Department of Chemical and Biochemical Engineering, Western University, London, Ontario N6A 5B9, Canada)
Abstract: Four thermal dissolution soluble fractions (TDSFs) with different thermal dissolution soluble yields (TDSYs) obtained from thermal and co-thermal dissolutions (CTDs) of a Chinese sub-bituminous Shenfu (SF) coal and lignin were characterized by elemental analysis, FT-IR and synchronous fluorescence spectrum measurements. The hydro-liquefaction properties of the four TDSFs and SF raw coal with and without catalyst were compared and the recycled use property of the catalyst in hydro-liquefaction of the TDSF from CTD of SF coal and lignin was further probed. The results suggests that the TDSF from the thermal dissolution (TD) of SF coal contained much more amount of aromatic components and polyaromatic hydrocarbons (PAHs) with 4 and more rings than those from the CTD of SF coal and lignin at the same temperature. TDSFs gave much higher liquefaction conversions and oil yields than SF raw coal in hydro-liquefaction with or without catalyst. Almost all TDSF was converted with much high yield of oil and the TDSF from CTD of SF coal and lignin gave higher yield of oil than that from the TD of SF coal in hydro-liquefaction with Ni-Mo-S/Al2O3 catalyst which demonstrated a good reusability in the hydro-liquefaction of TDSF from the CTD of SF coal and lignin. Carbon deposition was hardly observed in the 4 times recycle used catalyst.
Key words: coal; lignin; co-thermal dissolution; liquefaction; catalyst reusability
The direct coal liquefaction (DCL) is still one of attractive choices to produce alternative transportation fuels from coal in China, who is limited in reserves of petroleum and natural gas but abundant in coal resources. However, in traditional DCL the catalyst used is often deposable with low catalytic activity because recovery of catalyst from the liquefied residue is very difficult. Therefore, the oil yield and liquefaction efficiency for the DCL are normally low. In our previous research we find that the thermal dissolution soluble fractions (TDSFs) from SF sub-bituminous coal have higher liquefaction activity than SF raw coal, and the solid acid catalyst used in the hydro-liquefaction of TDSF has a good reusability[1]. The results suggest that in the liquefaction of TDSF, a high activity hydro-liquefaction catalyst can be used due to its reusability in this case, therefore to realize high-efficiency coal liquefaction in economy. Then the focus will move to the issue that how to obtain the TDSF with high thermal dissolution soluble yield (TDSY) and liquefaction activity from low rank coals (LRCs).
Many literatures report that the TDSYs from LRC are dependent upon the thermal dissolution (TD) temperature, kinds of organic solvent and coal rank[2-5]. The TDSYs of LRCs in polar solvent crude methyl naphthalene oil (CMNO) are normally higher than that in the slightly polar solvent carbol oil or non-polar solvent 1-methyl naphthalene (1-MN)[6]. Addition of a small amount of methanol into 1-MN can greatly increase the TDSY due to methanolysis reactions[7]. In order to realize high efficient TD of LRC at mild temperature, co-thermal dissolutions (CTDs) of LRC and biomass have been carried out in our laboratory to obtain high TDSY and to decrease emission of greenhouse gases. A positive synergic effect was found in the CTD of coal and sawdust at lower temperature. The TDSY from the CTD was promoted 33.6% comparing to the weighted mean value of the individual TD of sawdust and coal at 320 ℃[8]. The co-thermolysis of biomass-related model compounds and coal suggested that addition of 10% benzyl phenyl ether could greatly promote the TD of SF coal at 360 ℃. This is because that the benzyloxy and phenoxy radicals formed from thermolysis of benzyl phenyl ether attack the coal molecules, and promoting depolymerization of coal[9]. Recently, we studied the CTD of SF coal and lignin, it was also found that pyrolysis of lignin at low temperatures could form some intermediates such as phenoxy radicals, which could further cause depolymeriaztion of coal, thus promoting the TD of coal[9].
Although co-liquefaction of coal with biomass has been studied extensively[10-16], and it is found that yields and quality of liquid products from the co-liquefaction under milder conditions are improved greatly[11-14]. But the catalytic hydro-liquefaction properties of the TDSF from the CTD of coal and biomass, especially reusability of the catalyst in this process have no report based on our knowledge. In this study, hydro-liquefaction behaviors of the TDSFs from CTD of SF coal and lignin and TD of SF coal were compared. The recycled use property of a high activity catalyst Ni-Mo-S/Al2O3in hydro-liquefaction of the TDSF from CTD of SF coal and lignin was also probed.
1 Experimental
1.1 Feedstocks and reagents
The feedstocks used are Shenfu (SF) coal-a Chinese sub-bituminous coal and lignin. Both were ground to the size less than 250 and 75 μm respectively, and dried under vacuum at 80 ℃ for 12 h before use. Table 1 shows the proximate and ultimate analyses of SF coal and lignin. All solvents used are commercial pure chemical reagent without further purification, and the purity is higher than 99.5%.
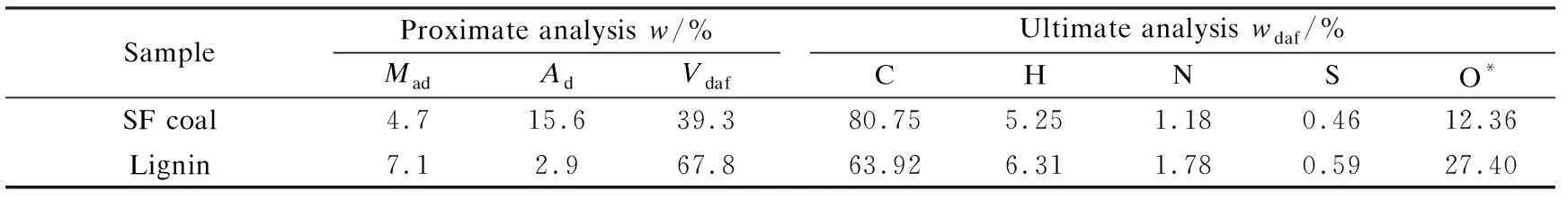
Table 1 Ultimate and proximate analyses of SF coal and lignin
*: by difference
1.2 Thermal dissolution
The descriptions of TD and CTD in details are shown elsewhere[5,8]. Briefly, about 0.15 g of SF coal or the mixture of SF coal and lignin (1∶1 by weight, TDSY giving the maximal value at the blending ratio) in TD or CTD was charged into a tuber cell. TD solvent was then pumped flowing through the cell in the rate of 1 mL/min at a required temperature for 1.5 h. The residual solid left in the cell was referred as TD insoluble fraction (TDIF) after washing and drying. The TDSF was precipitated from the TD solution by addition of excess ofn-hexane. TDSY is calculated by Eq. (1) based on the weight of TDIF.
TDSY = (Mf-Mr)/[Mf(1-Ad)]
(1)
whereMf(g),Mr(g), andAd(%, db) are the initial masses of the feedstock, TDIF, and the ash content of the feedstock, respectively.
1.3 Characterization of TDSF
The elemental analysis was carried out through an Elementar Vario EL III and duplicated. The experimental error was within 5% and the result is the mean value of two runs. A NICOLET6700 FT-IR spectrometer was used to the FT-IR measurement. The detection conditions are resolution, 4 cm-1; scan times, 32; scanning range, 4000-400 cm-1. The sample for FT-IR measurement was prepared by mixing 1 mg of sample with 100 mg of KBr and forming the mixtures into a pellet. A Hitachi F-4600 spectrophotometer was used for the synchronous fluorescence spectrum measurement. The TDSF was diluted in tetrahydrofuran to 5 mg/L and placed in a 1 cm path length quartz cell for the measurement. The excitation source was a 150 W xenon lamp. Emission and excitation wavelength difference was set as 13 nm. The spectrum was obtained by scanning from 200 to 900 nm at a rate of 240 nm/min.
1.4 Preparation of Ni-Mo-S/Al2O3
The required amounts of Ni(NO3)2·6H2O and (NH4)6Mo7O24·4H2O were dissolved with distilled water, and then a certain amount of Al2O3was added into the solution. The solution was then evaporated at 60 ℃ with stirring. The precipitated product obtained was then dried at 110 ℃ overnight and calcined at 500 ℃ for 4 h. Then it was sulphided by CS2(0.02 mL/min) flow under H2atmosphere at 400 ℃ for 2 h and aged with N2(containing 1% O2) flow for 7 h. The catalyst obtained was called as Ni-Mo-S/Al2O3.
1.5 Liquefaction and product fractionation
The liquefactions of TDSFs and SF coal were carried out in a 30 mL tube reactor with shaking vertically. The descriptions about the hydro-liquefaction in details are shown elsewhere[1]. Briefly, 0.5 g of the dried coal or TDSF loaded with 0.05 g Ni-Mo-S/Al2O3catalyst or without catalyst together with 1 mL of tetralin was charged into the reactor. The hydro-liquefactions were carried out at 400 ℃ for 1 h with 5.0 MPa initial hydrogen pressure.
The fractionation of liquefaction product was made by Soxhlet solvent extraction. Tetrahydrofuran (THF),n-hexane and toluene were used as extraction solvent in turn. In this work oil is defined as then-hexane soluble fraction (HS), asphaltene (AS) is toluene soluble fraction, and preasphaltene (PA) is THF soluble fraction according to literature[1]. Gas yield was obtained from the material balance before and after liquefaction with gas released. The liquefaction conversion is defined as the summation of the THF soluble fraction and gas, and it is calculated from the THF insoluble residue (THFI). The fractionation procedure is shown in Figure 1.

Figure 1 Fractionation procedure of hydro-liquefaction product
Due to oil fraction may contain a certain amount of light constituents, which may be lost during the evaporation of solventn-hexane, therefore in this work the oil yield (including a small amount of water formed in the liquefaction) is calculated as:
oil(%) = conversion-PA-AS-gas (2)
The repeatability of the fractionation analyses is 1%.
2 Results and discussion
2.1 Preparation and characterization of TDSFs
In our previous work[5]we found that SF coal gave high yields of TDSF at the TD temperature 320-360 ℃. The TDSY decreased at 380 ℃, and it was lower when the TD temperature was less than 320 ℃. Therefore the TD temperatures of 320-360 ℃ were chosen in this study. Four kinds of TDSFs with different TDSYs were prepared, they were TDSF from the TD of SF coal in 1-MN at 360 ℃ (TD-1-MN-360), TDSFs from the CTD of SF coal and lignin in 1-MN at 360 ℃ (CTD-1-MN-360), 320 ℃ (CTD-1-MN-320) and 320 ℃ with 10% ethanol addition in 1-MN (CTD-1-MN+ET-320). Their TDSYs were 58%, 74%, 60% and 80% respectively. The TDSY from the TD of lignin in 1-MN at 360 ℃ was 83%, therefore the weighted mean value of TDSY in the CTD of SF coal and lignin (1∶1 in weight) was 70%. The TDSY increased to 74% in the CTD of SF coal and lignin at 360 ℃, which is higher than the weighted mean value of 70%, suggesting that depolymerization of lignin can promote the TD of SF coal[9]. Addition of 10% ethanol in 1-MN in the CTD at 320 ℃ increased TDSY greatly from 60% to 80% compared to that of without ethanol addition. This means that addition of a certain amount of ethanol in the non-polar solvent 1-MN is one of effective methods to increase the TDSY in CTD of SF coal and lignin at low temperature, and ethanolysis may be responsible for this promotion[7]. Table 2 shows the ultimate analysis and atomic ratios of the four TDSFs. The TDSF from the TD of SF coal contained more carbon and less hydrogen and oxygen, resulting in its lower H/C and O/C comparing to those from CTD of SF coal and lignin. This is because lignin contains less carbon and more hydrogen and oxygen than SF coal, and parts of lignin enter the TDSF in the CTD process. The elemental compositions and H/C, O/C atomic ratios of TDSFs from CTD of SF coal and lignin were similar.
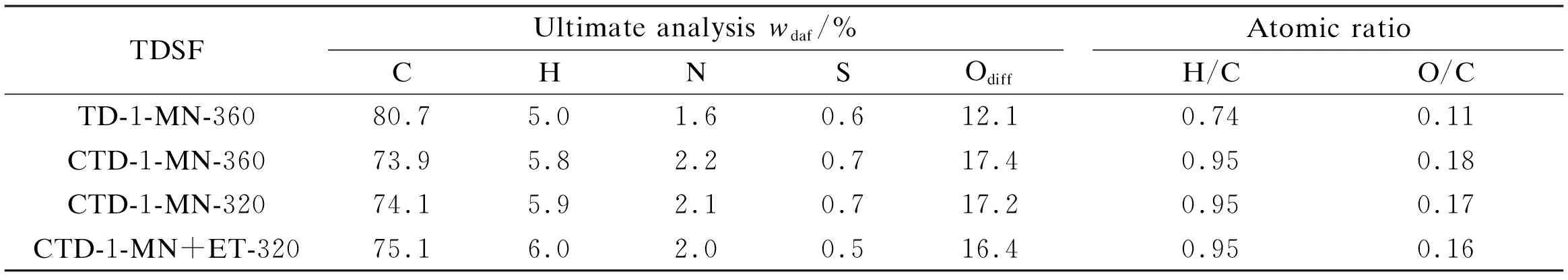
Table 2 Ultimate analysis and atomic ratios of TDSFs
Figure 2 shows the FT-IR spectra of the four TDSFs. The intensity of band near 3400 cm-1, which is assigned to self-associated OH hydrogen bonds[17], for the TDSF from the TD of SF coal at 360 ℃ (TD-1-MN-360) was much lower than those for the TDSFs from the CTD of SF coal and lignin. It is consistent with the lower O% and O/C in the TDSF of TD-1-MN-360 (Table 2). In the meantime, the intensity of band near 3050 cm-1, which is assigned to the aromatic CH bending[17], for the TDSF of TD-1-MN-360 was much stronger than those for the TDSFs from the CTD of SF coal and lignin. Comparing spectra of the three TDSFs from CTD, it can be found that a wide band attributed to C-O-C stretching (1200-1300 cm-1) and the band attributed to C=O stretching (1680 cm-1)[18]appearing in CTD-1-MN-360 became weaker, suggesting that partial ether and carbonyl oxygen groups were decomposed at 360 ℃ TD. This resulted in the lower content of ether and carbonyl oxygen groups in the TDSF of CTD-1-MN-360. The intensity of band near 3050 cm-1for the TDSF of CTD-1-MN-360 also became weaker, suggesting its low content of aromatic components compared to the TDSFs obtained in CTD at 320 ℃. This was also reflected by the almost disappeared bands of 750-900 cm-1, which are attributed to aromatic out-of-plane CH bending bands in the TDSF of CTD-1-MN-360[19]. The spectra of CTD-1-MN-320 and CTD-1-MN+ET-320 are similar, suggesting their similar structure of the two TDSFs.
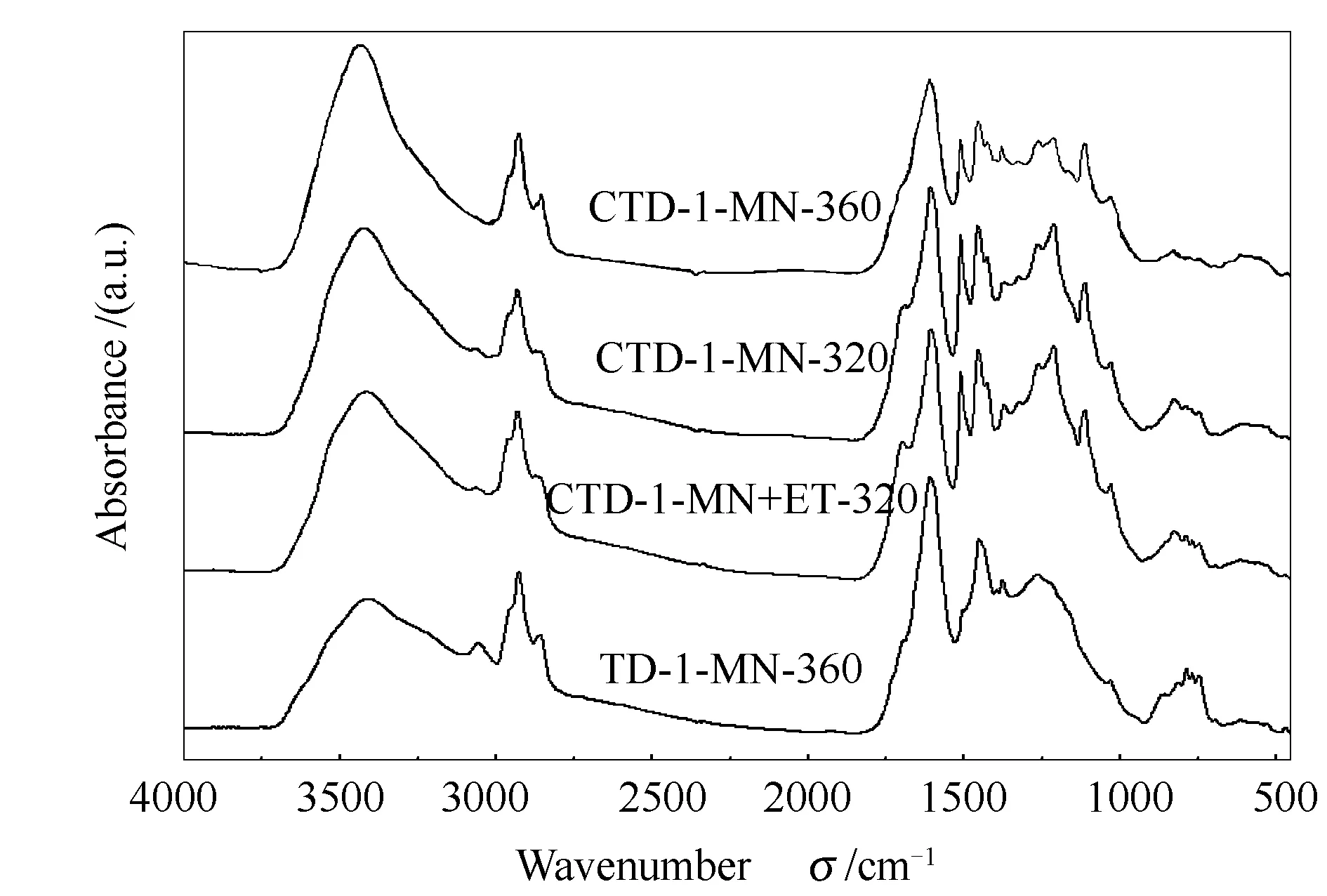
Figure 2 FT-IR spectra of the four TDSFs
Figure 3 shows the synchronous fluorescence spectra of the four TDSFs (THF soluble fractions). In general, the synchronous fluorescence spectrum peaks at 280-300, 300-340, 340-400 nm, and more than 400 nm were attributed to the polyaromatic hydrocarbons (PAHs) containing 1, 2, 3, and 4~ rings, respectively[20,21]. The TDSFs of TD-1-MN-360 and CTD-1-MN-360, which were obtained at high TD temperature 360 ℃, contained much amount of PAHs with 4 and more rings. Comparing with the TDSF of TD-1-MN-360, the TDSF of CTD-1-MN-360 contained much more amount of PAHs with 1 and 2 rings. This means that the TDSF from the CTD of SF coal and lignin contains much more amount of low ring’s PAHs than the TDSF from TD of SF coal at the same TD temperature. The ring’s distributions of PAHs in the TDSFs of CTD-1-MN-320 and CTD-1-MN+ET-320 were similar as shown in Figure 3.
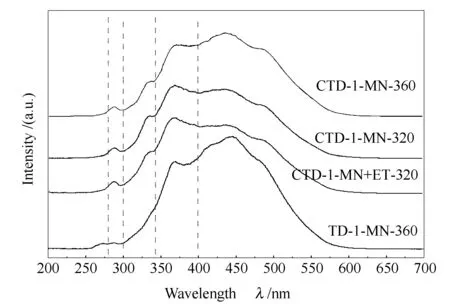
Figure 3 Synchronous fluorescence spectra of the four TDSFs
2.2 Hydro-liquefaction of SF coal and its TDSFs
Table 3 shows the hydro-liquefaction product distributions of SF raw coal and its four TDSFs without catalyst.
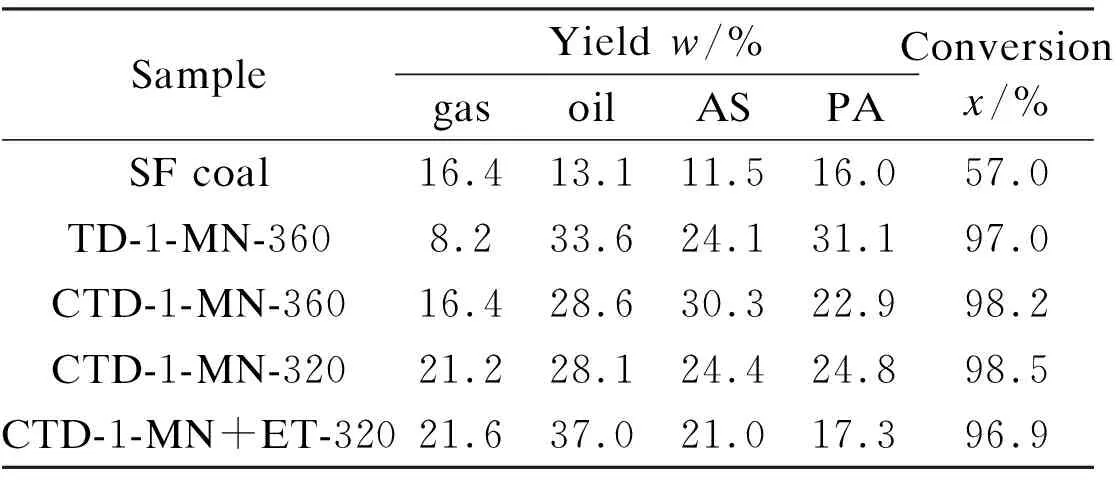
Table 3 Hydro-liquefaction of SF raw coal and its TDSFs without catalyst
The hydro-liquefaction activities of the four TDSFs are much higher than that of SF raw coal. The liquefaction conversions of the TDSFs were more than 96%, but the liquefaction conversion of SF raw coal was only 57%. In coal liquefaction, oil should be the most valuable fraction in the liquefaction products. Table 3 shows that the oil yield from SF coal was 13.1%, which is less than the half of that from TDSFs. For the four TDSFs, their liquefaction conversions are similar, but their liquefied product distributions are quite different. The liquefaction of TD-1-MN-360 gave lower gas yield and higher oil yield than that of CTD-1-MN-360. This means that the TDSF from the CTD of SF coal and lignin, which contains more light constituents comparing to that from TD of SF coal mentioned above, is easy to pyrolyze and to form more amounts of gases. It is interesting that liquefaction of CTD-1-MN+ET-320 gave more oil yield than that of CTD-1-MN-320. This suggests that the additional increased parts of TDSF by ethanolysis in the CTD with the addition of ethanol in 1-MN can be converted into oil in the hydro-liquefaction without catalyst.
Table 4 shows hydro-liquefaction product distributions of SF raw coal and the four TDSFs with Ni-Mo-S/Al2O3as catalyst. Comparing with Table 3 it can be observed that the conversion and oil yield of SF raw coal increased greatly with the catalyst. The conversions of the four TDSFs were more than 99%, and almost all TDSF was liquefied with the catalyst. Also oil yields from liquefaction of the four TDSFs were much higher than that of SF raw coal. Table 4 shows that liquefaction of the three TDSFs from the CTD of SF coal and lignin gave much higher oil yields than that of the TDSF from TD of SF coal. It is interesting that gas yields of the TDSFs from the CTD under Ni-Mo-S/Al2O3decreased compared to those without catalyst, suggestting the catalyst is favorable to formation of oil fraction in the hydro-liquefaction. The TDSF of CTD-1-MN-320 gave higher oil yield than that of CTD-1-MN-360. This is because that CTD-1-MN-360 contained much more amount of PAHs with 4 and more rings mentioned above. Comparing with CTD-1-MN-320, CTD-1-MN+ET-320 gave more gas yield. In our previous work, we found that the series reactions of coal →PA → AS → oil+gas were the main reactions of SF coal catalytic liquefaction[22]. Table 3 shows that hydro-liquefaction of CTD-1-MN+ET-320 without catalyst gave more oil yield than that of CTD-1-MN-320. Therefore comparing with CTD-1-MN-320, more AS fractions from CTD-1-MN+ET-320 in the hydro-liquefaction were converted into gas fraction with the catalyst due to the different compositions of the two TDSFs.

Table 4 Hydro-liquefaction of SF raw coal and its TDSFs catalyzed by Ni-Mo-S/Al2O3
Figure 4 shows synchronous fluorescence spectra of the liquefied products of AS and PA from hydro-liquefaction of the four TDSFs.
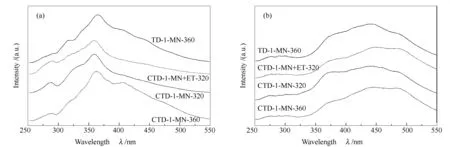
Figure 4 Synchronous fluorescence spectra of the liquefied products of AS (a) and PA (b) from the liquefaction of the four TDSFs
Figure 4 (a) indicates that the main peak located near 363 cm-1for the AS from liquefaction of TDSF of TD-1-MN-360 shifts to lower wavenumbers compared with that from liquefaction of TDSFs obtained by CTD. This suggests that the PAHs in ASs from liquefaction of TDSFs obtained by CTD changed to low ring’s numbers compared to that in AS from liquefaction of TDSF obtained by TD of SF coal. This is consistent with the ring’s distributions of PAHs in the TDSFs. The TDSFs from the CTD of SF coal and lignin contained much more amount of low ring’s PAHs than the TDSF from TD of SF coal as mentioned above. The results suggested the TDSF from CTD of SF coal and lignin had higher hydro-liquefaction activities than that from TD of SF coal alone. The synchronous fluorescence spectra of PA from liquefaction of the four different TDSFs were similar (Figure 4 (b)), suggesting their similar ring’s distributions of PAHs.
2.3 Recycle use of catalyst in hydro-liquefaction of TDSF
The reused property of catalyst is one of the key factors to affect the cost of high efficient hydro-liquefaction of coal. Koyano et al[23]found that a Ni-Mo/Al2O3catalyst could be recycled five times and its deactivation was not detected in hydro-liquefaction of TDSF from a subbituminous coal. We also found that a solid acidic catalyst BF3/SBA-15 could be recycle used at least 4 times for liquefaction of TDSF from SF coal, and the reused catalyst had higher catalytic hydro-liquefaction activity compared to the fresh one[1]. This means that the catalyst used in liquefaction of TDSF is reusable, therefore a high effective catalyst can be used in hydro-liquefaction of TDSF although it is more expensive. Table 4 shows that conversions of the four TDSFs are all more than 99%, and only less than 1% of THFI is left and mixed with the catalyst. Therefore the catalyst used should still keep high activity. The TDSF of CTD-1-MN+ET-320 is obtained at mild temperature 320℃ with high TDSY of 80%, it can be used as hydro-liquefaction material to probe the recycle use property of the Ni-Mo-S/Al2O3catalyst. In the reusability of catalyst measurements, the THFI and catalyst were not separated after the former hydro-liquefaction, and were directly used in the next run. This means that the used catalyst does not have a regenerating treatment, and it should be more effective to evaluate the reusability of the catalyst.
The hydro-liquefactions were carried out at 400 ℃ for 60 min with 5 MPa H2initial pressures. The results are summarized in Figure 5. Hydro-liquefaction conversions kept at more than 99% for the fourth use of catalyst in hydro-liquefaction; the THFI was less than 0.5% and not deposited in the catalyst. The results demonstrated that accumulation of THFI on the reused catalyst did not occur, and the recycled catalyst kept the high hydro-liquefaction activity. However, with the increase of the recycle time of catalyst used, the oil yield decreased obviously although no carbon deposition was observed in the reused catalyst. The fresh catalyst gave the high oil yield of 65.9%, and then the oil yield decreased to 54.7%, 46.8% and 46.7% in the second, third and 4th recycle use. This suggests that hydro-liquefaction activity of the catalyst decreased in a certain degree for the reused catalyst. After the 3rd use the catalytic activity became stable.

Figure 5 Liquefied product distributions of hydro-liquefaction of TDSF of CTD-1-MN+ET-320 using recycled Ni-Mo-S/Al2O3 catalyst
It is proved that carbon deposition in the reused catalyst slowly takes place, and BF3/SBA-15 catalyst used in the catalytic liquefaction of TDSF from TD of SF coal has a good reusability[1]. This work also suggests that the Ni-Mo-S/Al2O3catalyst used in hydro-liquefaction of TDSF from the CTD of SF coal and lignin has a good reusability and the carbon deposition in the catalyst during this process is negligible.
Based on our results, it can be found that the TDSF from CTD of coal and lignin has a good hydro-liquefaction activity, and it gives much more amounts of oil yield than that from TD of coal. The catalyst used in hydro-liquefaction of TDSF from CTD of coal and lignin is reusable. Therefore, it supplies a new pathway to realize high-efficiency liquefaction of coal in economy, which is firstly coal and biomass are co-thermally dissolved at a mild temperature to obtain TDSF with high TDSY, and then the TDSF is catalytically hydro-liquefied with a high efficient and reusable catalyst to give more oil yield.
3 Conclusions
The TDSF from TD of SF coal contained more amounts of aromatic components and PAHs with 4 and more rings comparing with those from the CTD of SF coal and lignin at 360 ℃. The contents of aromatic components, ether and carbonyl oxygen groups in the TDSFs from CTD of SF coal and lignin increased with decreasing CTD temperature. Addition of 10% ethanol into 1-MN increased the TDSY greatly, but the TDSF structure was similar with that of TDSF obtained without ethanol addition at 320 ℃.
The TDSFs from TD and CTD had much high hydro-liquefaction activity. They gave much higher liquefaction conversions and oil yields than SF raw coal. Ni-Mo-S/Al2O3catalyst could greatly promote hydro-liquefactions of SF raw coal and TDSFs. Almost all TDSF was converted with much high yield of oil and the TDSF from CTD of SF coal and lignin gave higher yield of oil than that from TD of SF coal in the catalytic hydro-liquefaction.
Ni-Mo-S/Al2O3catalyst used in hydro-liquefaction of TDSF from the CTD of SF coal and lignin had a good reusability and there was hardly carbon deposition in the catalyst. Therefore, obtaining the TDSF with high yield from CTD of coal and biomass at a mild temperature, and producing more oil from hydro-liquefaction of the TDSF with high efficient and reusable are one of new pathways to realize the high-efficiency liquefaction of coal in economy.

