Immunohistochemical expression of thymidylate synthase and prognosis in gastric cancer patients submitted to fluoropyrimidine-based chemotherapy
Marina Alessandra Pereira, Marcus Fernando Kodama Pertille Ramos, Andre Roncon Dias,Sheila Friedrich Faraj, Cinthya dos Santos Cirqueira, Evandro Sobroza de Mello, Bruno Zilberstein,Venancio Avancini Ferreira Alves, Ulysses Ribeiro Jr.
1Department of Gastroenterology, Cancer Institute, University of São Paulo Medical School, São Paulo 01246-000, Brazil; 2Department of Pathology, Cancer Institute, University of São Paulo Medical School, São Paulo 01246-000, Brazil; 3Department of Pathology, Instituto Adolfo Lutz, São Paulo 01246-000, Brazil
Abstract Objective: Adjuvant chemotherapy with 5-fluorouracil (5-FU) has been widely used in gastric cancer (GC)patients to prevent relapse after curative resection. 5-FU acts by inhibiting thymidylate synthase (TS), and high levels of TS correlate with resistance to treatment with fluoropyrimidines. The aim of this study was to evaluate the expression of TS in GC patients, and its relation with clinicopathological characteristics and prognosis in adjuvant chemotherapy with 5-FU.Methods: We retrospectively evaluated 285 patients who underwent D2-gastrectomy with curative intent. TS expression was determined by immunohistochemistry (IHC) in tumor cells by tissue microarray (TMA). TS level was evaluated according to the intensity and percentage of cells marked by a score system. Patients were divided in three groups according to their TS-score: negative, low and high.Results: TS expression was positive in 92.3% of GC. TS-high, TS-low and TS-negative were observed in 46.3%, 46.0% and 7.7% of patients, respectively. High-TS GC were associated with older age (P=0.007), high neutrophil/lymphocyte ratio (P=0.048), well/moderately differentiated histology (P=0.001), intestinal Lauren type(P<0.001) and absence of perineural invasion (P=0.003). Among 285 patients, 133 stage II/III patients (46.7%)received chemotherapy with 5-FU. In survival analysis, TS-high was associated with worse disease-free survival(DFS) in stage III GC patients who received 5-FU-based chemotherapy (P=0.007). Multivariate analysis revealed that total gastrectomy, poorly differentiated tumors and high TS-score were associated with worse DFS in stage III GC patients.Conclusions: High TS-score in stage III GC was associated with poor DFS in patients treated with fluoropyrimidine-based chemotherapy.
Keywords: Gastric cancer; adjuvant therapy; thymidylate synthase; 5-fluorouracil-based chemotherapy
Introduction
Gastrectomy with D2 lymphadenectomy is the mainstay treatment modality for advanced gastric cancer (GC) (1).Chemotherapy is indicated in selected patients to reduce recurrence and improve survival. Patients with pathological stage II, and III are eligible for adjuvant chemotherapy (1-3).
Distinct chemotherapeutic drugs and regimens have been recommended in clinical practice. Among them, the 5-fluorouracil (5-FU)-based therapy has been widely used for GC treatment to improve overall survival (OS) (4-8).
Thymidylate synthase (TS) is the target enzyme for 5-FU and others anti-folate agents, including capecitabine and S-1 (tegafur/gimeracil/oteracil). It is responsible for catalyzing the methylation of fluorodeoxyuridine monophosphate to deoxythymidine monophosphate, which is an important step involved in DNA biosynthesis (9).
The chemoresistance of some tumors to 5-FU therapy is an important clinical problem that has been reported in several gastrointestinal cancers (10). Studies have demonstrated that high TS levels correlate with resistance to fluoropyrimidine treatment (11-13). However, the clinical significance of TS expression and prognosis in GC remain unclear.
Thus, the aim of the current study was to investigate the TS immunoexpression and its relation to clinicopathological characteristics in GC, and evaluates the prognosis of patients who received 5-FU-based adjuvant chemotherapy.
Materials and methods
Patients
We retrospectively reviewed all gastric adenocarcinoma patients who underwent gastrectomy with curative intent between 2009 and 2016 from a prospective collected medical database.
Patients who underwent D2 lymphadenectomy due to gastric adenocarcinoma were eligible. Gastric stump tumors, palliative resections and patients without formalinfixed paraffin-embedded (FFPE) tissue available for analysis were excluded.
Surgical treatment was performed according to the guidelines of the Japanese Gastric Cancer Association (1).Surgical specimens were fixed in 10% buffered formalin or Carnoy’s Solution (14,15) and were evaluated according to the College of American Pathologists protocol (16).Immunohistochemistry (IHC) for cytokeratin was performed in some cases to detect lymph node micrometastasis (17). Tumor stage was defined according to the 8th edition of the TNM as proposed by the International Union Against Cancer (UICC) (18).
Clinical characteristics were analyzed according to the American Society of Anesthesiologists (ASA) (19)classification and comorbidities were classified at the time of surgery according to the Charlson Comorbidity Index(CCI) (20). CCI was considered without inclusion of the gastric cancer as comorbidity. The neutrophil-lymphocyte ratio (NLR) was calculated from blood tests collected before surgery.
Postoperative complication was graded according to the Clavien-Dindo Classification (21), and major complications were defined as Clavien >II.
After surgical resection, adjuvant chemotherapy with a 5-FU-based regimen was administered to selected patients with stage II and stage III GC according to the institutional protocol.
Patients with chemotherapy with a 5-FU-based regimen,INT0116 (Macdonald regimen) (6), mFLOX (Classic Trial) (7), XELOX, Cisplatin plus XELODA and monotherapy with 5-FU were considered for the study analysis. Other chemotherapeutic regimens included irinotecan plus cisplatin (CPT-11/CDDP) (4).
The main clinical outcome considered was disease-free survival (DFS) and OS. Surgical mortality (Clavien V) was defined as death occurring within 1 month after surgery or during hospital stay after the procedure. Postoperative follow-up was performed on a quarterly basis in the first year and every 6 months in the following years. During the follow-up period, clinical physical examinations and hematological tests were performed in all patients. Patients were postoperatively evaluated for the presence of recurrence by imaging and upper gastrointestinal endoscopy according to clinical suspicion. Absence in medical appointment for more than 12 months was considered loss of follow-up.
The study was approved by Ethics Committee of Cancer Institute, University of São Paulo Medical School(NP771/2015) and is registered in the “Plataforma Brasil”(a National Research Council) under the CAAE number 43453515.6.0000.0065. Informed consent of patients was waived because of the retrospective nature of the study.
Tissue microarray (TMA)
All hematoxylin and eosin (HE)-stained slides were reviewed and representative tumor tissue samples were selected for each case. The selected area was marked for TMA construction: 3 tumor samples and 2 non-tumor tissue samples. The corresponding FFPE tissue blocks were also marked and arrayed in a precision mechanized system (Beecher Instruments, Silver Springs, MD, USA).Nine TMAs were constructed, and each one of them contained 3 tumor samples from each patient and 2 nontumor controls.
IHC staining
For IHC reactions, FFPE TMAs blocks were sectioned into 4-μm thickness. Sections were deparaffinized in xylene and rehydrated in serial graded alcohol. IHC staining of histologic sections was performed using a peroxidase technique. Antigen retrieval was performed with citrate buffer (pH=8.0) and endogenous peroxidase activity was blocked with hydrogen peroxide. The sections were stained for TS expression using a rabbit monoclonal anti-TS antibody (1:200, clone SP112; Spring Bioscience, CA,USA). The end products were visualized using diaminobenzidine tetrahydrochloride (DAB) and counterstained with hematoxylin.
TS immunoexpression and scoring system
Tumor cell immunostaining was assessed by a score system in a manner to reflect both the intensity of staining and percentage of cells stained.
The intensity of the staining was graded on a numerical scale ranging from 0 to 3, where 0 was no staining, 1 for low-intensity, 2 for moderate and 3 for strong. The percentage of cells that stained positive was also scored for each TMA sample spot (0 to 100%). The TS-score(range, 0-300) was generated based on the cross-product of the intensity score and the percentage of positive cells.The mean of the 3 TMA spots with tumor samples belonging to each particular case was obtained for the final TS-score value.
TS staining results were classified as TS-negative (0 cytoplasmic immunostaining), TS-low (<20% cytoplasmic immunostaining) and TS-high (≥20% cytoplasmic immunostaining). TS expression was based on the median value of the score. The assessment of both histology and staining was performed by two pathologists independently and the results, usually in good agreement, were discussed and sometimes corrected.
Statistical analysis
Descriptive statistics included frequencies with percent for nominal variables andor median for continuous variables. Categorized data were analyzed using the Chisquare test and one-way analysis of variance (ANOVA) was conducted to compare the mean values of the three groups.
Survival curves were estimated using the Kaplan-Meier method, and differences in survival were examined using the log-rank test. To determine factors associated with DFS, hazard ratios (HRs) with 95% confidence intervals(95% CI) were calculated by univariate and multivariate Cox proportional hazard regression models. Variables with P<0.05 on univariate analysis were included as co-variables in multivariate Cox regression to determine which variables independently affected prognosis.
DFS was calculated from the date of surgery to the date of relapse. OS was defined as the time between surgery and death of any cause or last follow-up. For the better comparative study of recurrence, postoperative mortality was excluded from the DFS analysis.
Statistical analyses were performed using the IBM SPSS Statistics (Version 20.0; IBM Corp., New York, USA).All tests were two-sided and P<0.05 was considered statistically significant.
Results
Patient characteristics
A total of 856 patients were surgically treated for primary gastric tumor in the study period. Of these, 393 patients underwent potentially curative gastrectomy with D2 lymphadenectomy. Gastric adenocarcinoma was diagnosed in 377 patients. Ninety patients were excluded because they did not have viable paraffin samples available for analysis.
Thus, a total of 287 patients met the inclusion criteria and were selected for the study. Two patients whose samples were lost during histological sections were excluded and 285 patients remained for analysis. Of those,167 (58.6%) were male and 118 (41.4%) were female; the mean age was 61.6 (range, 25-87) years. Subtotal gastrectomy was performed in most cases (60.3%) and the mean number of lymph nodes retrieved was 41.8±17.7(median, 40). Pathological stage was I/II in 156 cases(54.7%) and stage III/IV in 129 cases (45.3%).
TS expression was positive in 92.3% of the patients(Figure 1). Based on the cut-off value, 131 (46.0%) patients were classified as TS-low and 132 (46.3%) as TS-high.
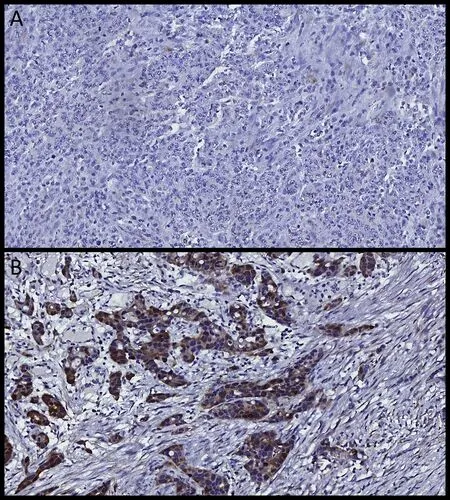
Figure 1 Representative images of immunohistochemical results.(A) Thymidylate synthase (TS)-negative case; (B) TS-positive gastric adenocarcinoma.
The clinicopathological characteristics according to TS-score are shown inTable 1. There were no differences between the groups regarding body mass index (BMI) (TS-negative=23.2 kg/cm2; TS-low=25.2 kg/cm2; TS-high=41.2 kg/cm2; P=0.602), ASA (I/II: TS-negative: 100%; TS-low=88.5%; TS-high=87.9%; P=0.274) and Charlson score(P=0.758). High IHC scores for TS significantly correlated with older age (P=0.007), higher NLR (P=0.048),well/moderately differentiated histology (P=0.001), Lauren intestinal type (P<0.001) and the absence of perineural invasion (P=0.003). There were no associations between TS-score and tumor invasion (pT) (P=0.469), number of harvested lymph nodes (TS negative=37.5; TS-low=43.5 and TS-high=40.8; P=0.234), lymph node metastasis (pN+)(P=0.234) or pTNM stage (P=0.064).
Survival analyses
The median follow-up period after resection was 35.9(mean, 37.6; range, 1.0-102.9) months. Recurrence occurred in 79 (27.7%) patients (35 regional, 32 peritoneal and 32 distant; 18 had recurrence in more than one site)and 95 (33.3%) patients died.
Among the 94 (33.0%) pTNM I/IV patients, 13 underwent chemotherapy (9 with 5-FU-based and 4 with other regimens); and 81 received surgery only (Figure 2).
Regarding the other 191 (67.0%) cases, which correspond to stage II/III GC, a total of 133 (46.7%)patients received 5-FU-based adjuvant chemotherapy: 40 were stage II and 93 stage III. Of the other stage II/III patients, 11 received other chemotherapy regimen and 47 did not receive adjuvant chemotherapy.
In the survival analysis of the entire population, no statistically significant effect of TS-score on DFS and OS was observed (P=0.149 and P=0.316, respectively) (Table 2).
Considering the pTNM II/III patients who underwent surgery only (did not received fluoropyrimidine therapy),TS-score was not significantly correlated with DFS and OS.
When survival was analyzed separately for stage II and III GC, there was no significant difference in OS and DFS rates for pTNM II patients who received 5-FU-based therapy according to TS-groups.
The pTNM III 5-FU-based group with TS-high had worse DFS compared to TS-negative (P=0.050). The median DFS for low- and high-TS was 48.2 and 15.4 months,respectively. Regarding OS in stage III 5-FU-based therapy group, slightly depressed curves were observed for the TS-high/low compared with TS-negative. The median OS was 51.4 months for TS-low and 40.9 months for TS-high group (Figure 3).
Univariate and multivariate analyses were performed to evaluate the prognostic factors affecting DFS for pTNM III+5-FU-based therapy (Table 3). Multivariate analysis identified total gastrectomy, poorly differentiated histologic and TS-high as independent factors associated with lower DFS.
Discussion
This study evaluated the influence of TS expression on the outcome of patients who received 5-FU-based therapy after D2 gastrectomy with curative intent. In the adjuvant setting, we found that high TS-score was associated with poor prognosis for advanced GC stage III compared with low TS levels.
Several studies have evaluated the association between tumor TS expression and clinical outcome for gastrointestinal cancer patients. Most of them reported that patients with high TS levels have poor prognosis with regard to FU-based chemotherapy (11,22). However, the relation between TS expression and outcomes in GC patients remains controversial.
In the current study, TS-high was associated with well/moderately differentiated histology and intestinal type.This topic is controversial; some had similar results (23),while others observed the opposite (24).
It is worth mentioning that TS is not a biomarker of tumor aggressiveness, it was not associated with lymphnode metastasis, tumor invasion and advanced cancer stage.Despite other reports (23,25) , a meta-analysis observed no association between TS levels and advanced tumor stage (11).
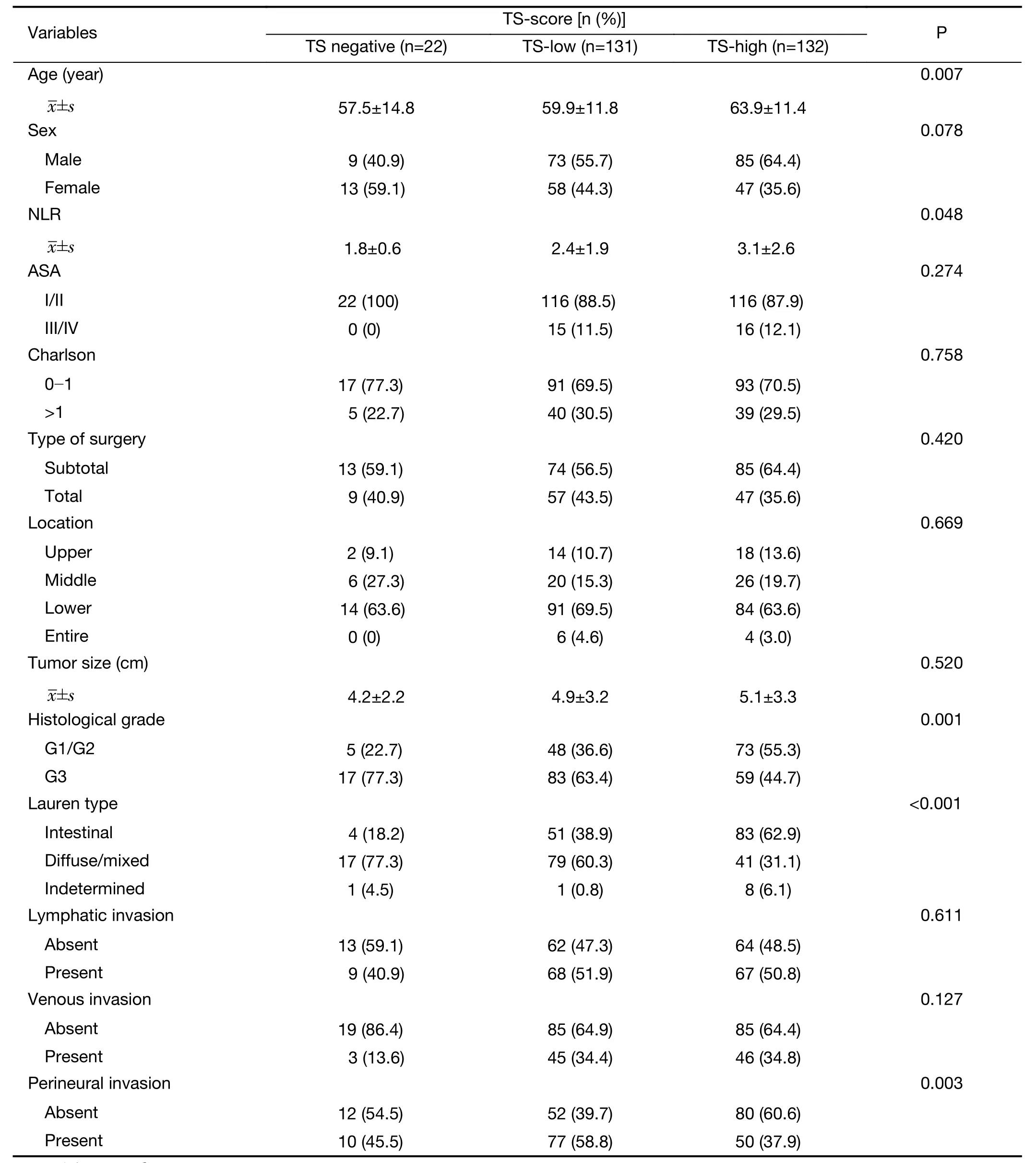
Table 1 Clinicopathological characteristics according to immunohistochemical scores for TS (TS-score) (N=285)
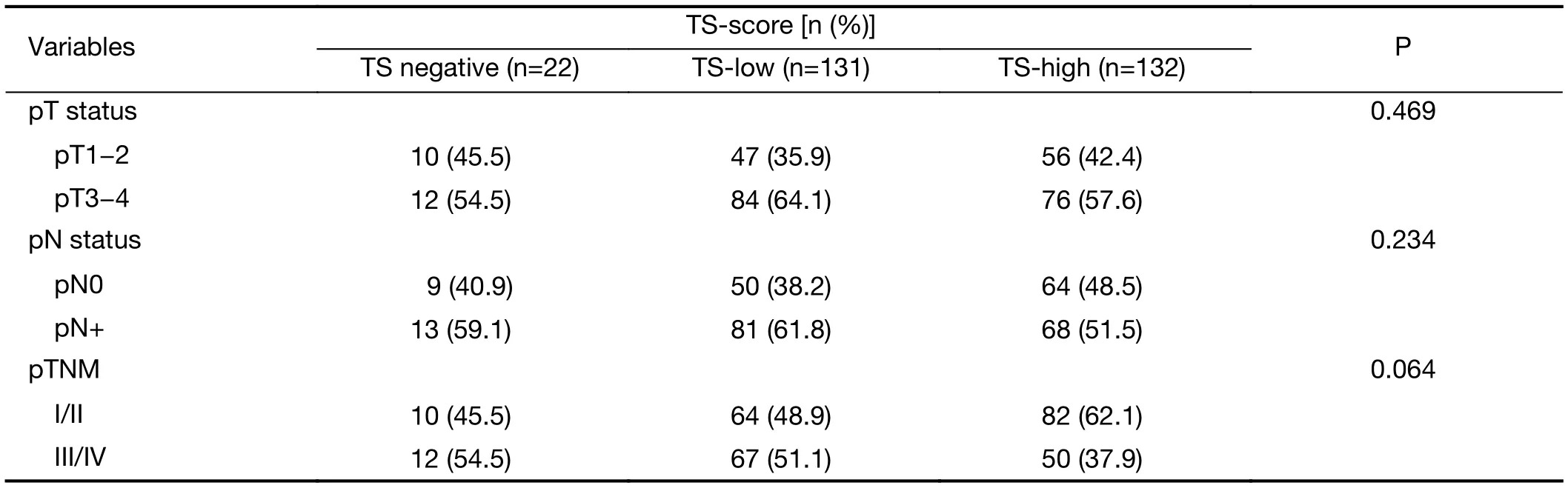
Table 1 (continued)
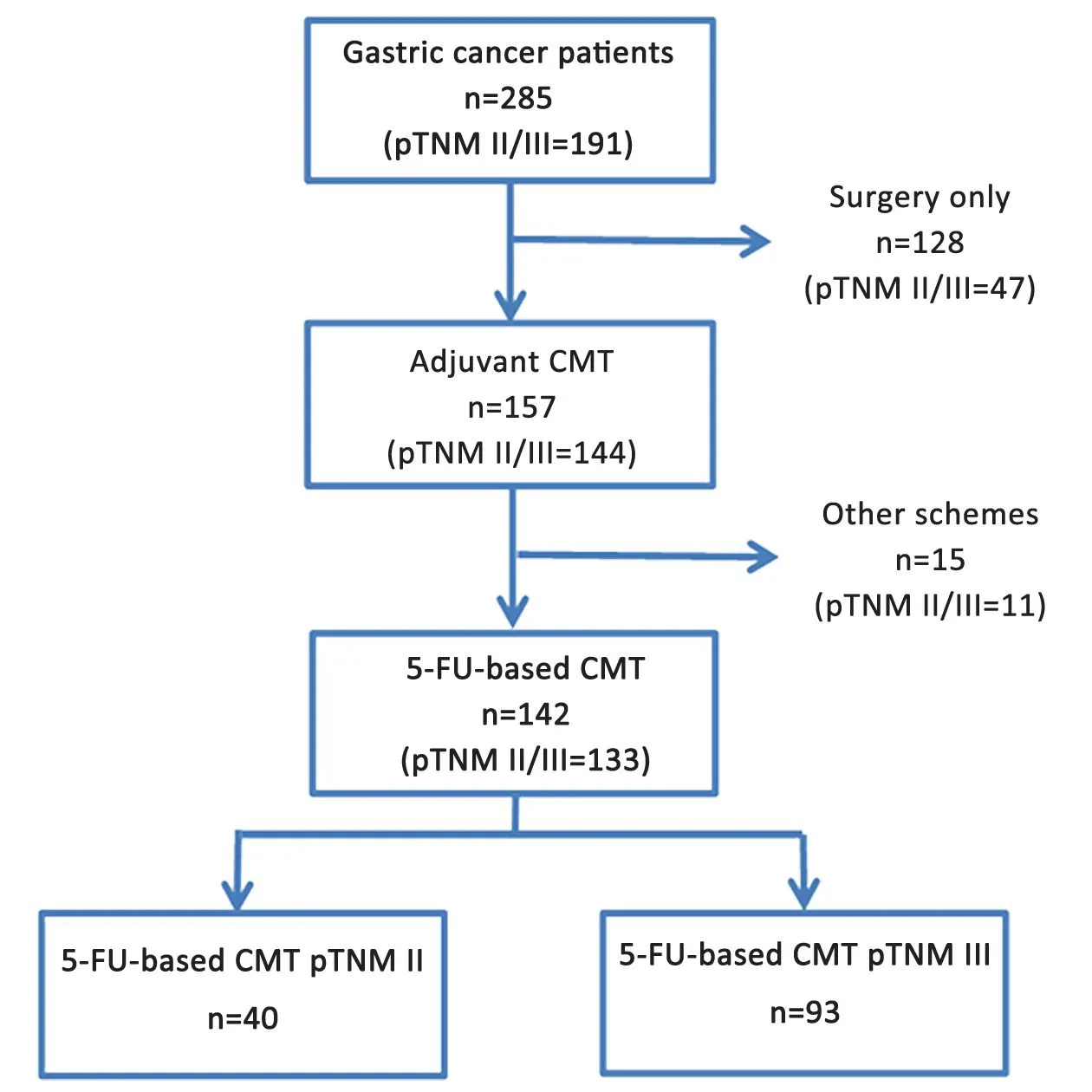
Figure 2 Flowchart of patient selection. CMT, chemotherapy.
Interestingly, the rate of NLR was significantly higher in the TS-high group. An elevated NLR has been associated with worse prognosis and survival in several tumors,including GC (26,27). In addition, it is believed that NLR may have potential usefulness in the prediction of chemotherapy responses, where patients with lower NLR before second cycle of chemotherapy had an improved progression-free survival when compared with patients with higher NLR, which could explain why the high NLR value was observed in our TS-high group (28).
Currently, adjuvant chemotherapy with FU has been widely used for treating advanced GC in order to improve outcome (2,4,5,29). As mechanism of action, FU acts by binding to TS. This connection prevents the TS binding to its substrate (deoxyuridine monophosphate), thereby inhibiting DNA synthesis (9). This may explain why the increase in TS expression would be associated with tumor resistance to 5-FU-based therapy.
In this study, TS levels did not influence the survival of the general population. However, high TS-scores were associated with poor DFS in stage III GC, which consists of the main group of patients who received adjuvant chemotherapy with 5-FU (30). So, this result reflects that the worse prognosis related to TS should be considered in the context of response to chemotherapy with FU.
Similarly, it has also been reported that there was no association between TS expression and outcomes in both the surgery-only group and the group with all population(30). However, some authors reported that prognostic advantage may be different according to the type of chemotherapy, and adjuvant 5-FU chemotherapy could prolong the survival of patients even with high TS mRNA levels, as in the case of administration of some combined chemotherapy regimens (31).
Although it presents some varied results, the predominantly established concept is still that patients with high TS expression treated with 5-FU had worse survival,and the mechanisms related to tumor resistance would be responsible for these results. In a study on metastatic GC,considering S-1-alone group, the response rate in the low TS group was 50%, but only 8% in the high TS group(P<0.05) (32). In GC patients treated with 5-FU andleucovorin, the median OS was shorter for patients with high TS expression compared to the low TS expression(4 monthsvs.10 months, P<0.01) (13). In addition to lower postoperative survival rates for positive-TS compared with negative-TS (P=0.048), a significantly higher lymph node recurrence in stage IIIB GC has already been observed in patients followed by adjuvant chemotherapy with 5-FU(26.7%vs.8.3%, P=0.046) (33).

Table 2 Univariate analysis of DFS and OS for gastric cancer patients
In the present cohort, it has been demonstrated that 5-FU based-chemotherapy after surgery for locally GC staged as pTNM III with TS-high resulted in differences for DFS compared with negative-TS. Accordingly, to evaluate the effect of clinicopathological variables on survival, Cox regression analysis was performed for DFS in this specific group of patients. The analysis showed that total gastrectomy, poorly differentiated type and TS-high were independent risk factors for lower DFS. Therefore,TS may serve as a predictive and prognostic marker for FU-based treatment in pTNM III GC.
However, results must be interpreted with caution.Different methodologies are available to determine TS status in tumor cells, such as IHC and reverse transcriptase polymerase chain reaction (RT-PCR), which may contribute to the heterogeneity among studies. Moreover, a great variety of cutoff values have been used, which might be another source of bias. Studies use median value (32),IHC intensity thresholds (13), H-score and median (34), to categorize results in negative and positive (33) or use percentile as the cutoff point (30). So, this emphasized that additional studies with consistent methodology are needed to define the precise predictive value of TS. Differences can also be attributed to the type of chemotherapy schemes adopted, since monotherapy with 5-FU and combined chemotherapy regimens may contribute to the difference in response rates (4,32).
There are some limitations in our study that should be addressed. Results were obtained from a single cohort. The interpretation of the impact of TS-score in stage II cases was limited due to absence of a control group (TS-negative). Due to its retrospective design, different 5-FU-based regimens were administered and chemotherapy dose cannot be well controlled. Furthermore, we cannot determine if 5-FU-based therapy would still benefit patients even with high TS rates compared with other non-5-FU regimens, because in our study only a small number of cases used other chemotherapeutic regimens that did not involve FU. Also, some patients did not undergo adjuvant therapy, despite stage II and III, due to intolerance to the chemotherapeutic regimen (elderly patients/comorbidities cases) or nonadherence to treatment.
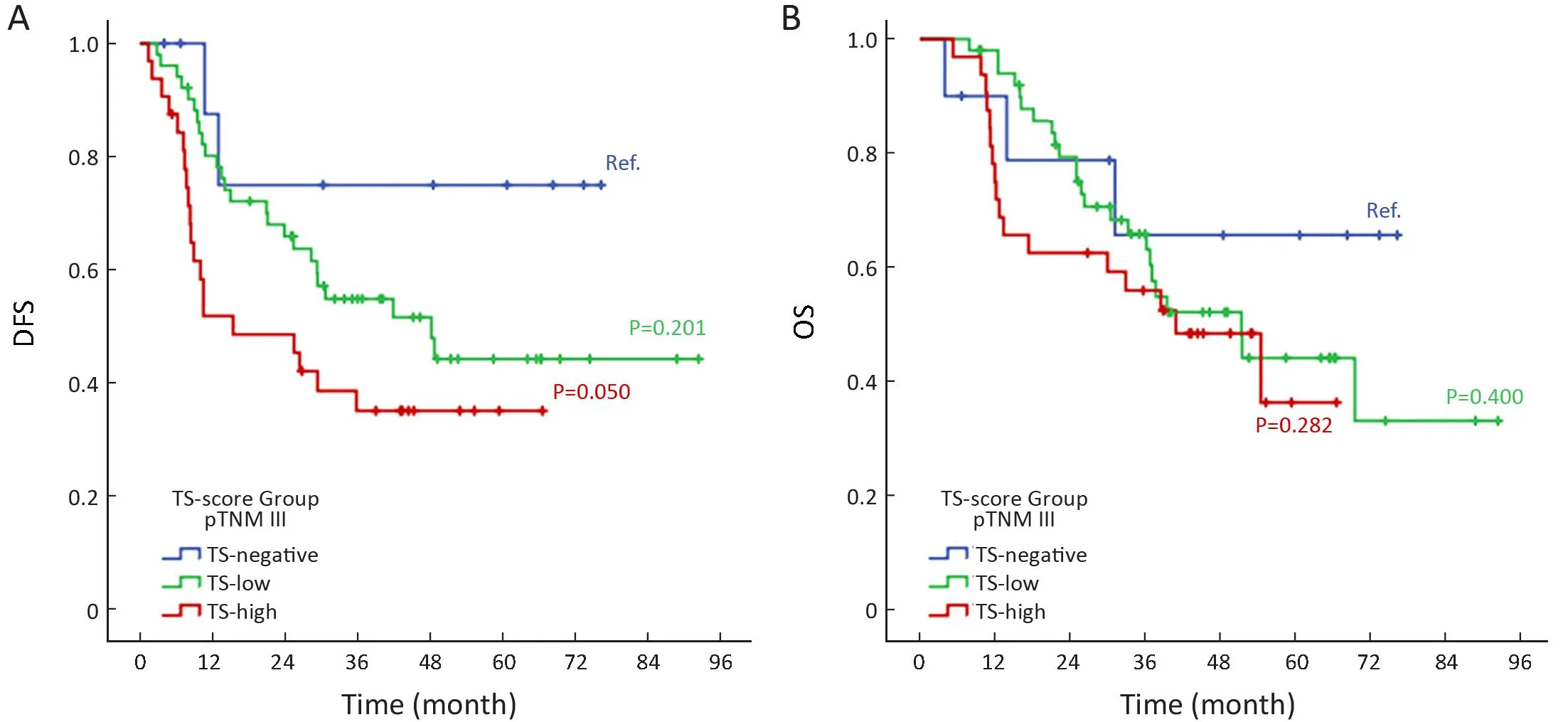
Figure 3 Kaplan-Meier survival curve analysis. (A) Disease-free survival (DFS) and (B) overall survival (OS) of pTNM III gastric cancer(GC) patients who received 5-FU-based adjuvant chemotherapy according to thymidylate synthase (TS)-score groups (n=93). Ref., TS-negative cases were considered as reference group.
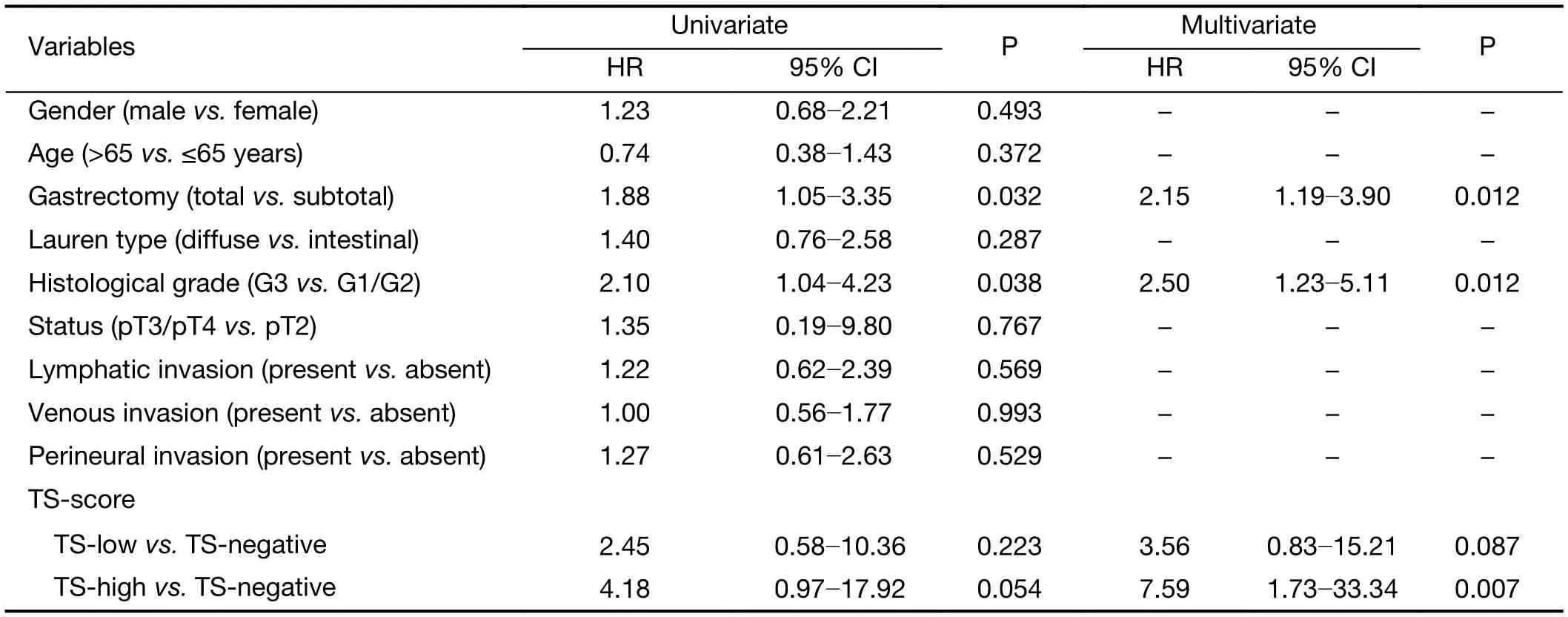
Table 3 Univariate and multivariate analysis of factors influencing DFS in stage III gastric cancer patients receiving gastrectomy and 5-FU-based adjuvant chemotherapy with negative, low and high TS-score (N=93)
As strengths, our analyses are based on a population of GC patients submitted to potentially curative surgery by highly specialized surgeons in the treatment of GC. D2 lymphadenectomy was performed in all cases, and the number of lymph nodes retrieved was high — which testifies the quality of the surgery and reduces the risk of substaging. Additionally, the results may provide useful information for selecting adjuvant treatment and determine which group of patients would have a greater chance of recurrence and could benefit from closer monitoring after adjuvant 5-FU-based therapy according to the TS-levels using IHC analysis. In this context, some advantages can be attributed to IHC. This methodology is routinely performed, and allows the evaluation the intratumoral heterogeneity of TS expression. Moreover, most tumors available for analysis are paraffin-embedded samples, and large retrospective studies using archival material could be performed. Furthermore, previous study showed that IHC scores for TS correlated with RT-PCR results (30).
Recently, the GC characterization in molecular subgroups has provided tools which will assist in the development of targeted therapy (35), and studies based on protein expression have contributed to the development of more individualized treatment in clinical practice (36,37).
Thus, the research of potential markers may provide a better understanding of tumor biology to determine in the future which patients would actually benefit from specific treatments, and which patients should not receive adjuvant therapy.
Conclusions
TS-high was an independent factor associated with significantly worse DFS in stage III patients treated with 5-FU-based chemotherapy. This finding suggests that prognosis of GC treated with FU-related regimens was influenced by TS, and its level may be used to predict the treatment outcomes.
Acknowledgements
This work was supported by Fundacao de Amparo a Pesquisa do Estado de São Paulo (FAPESP agency) (No.2016/25524-0).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
 Chinese Journal of Cancer Research2018年5期
Chinese Journal of Cancer Research2018年5期
- Chinese Journal of Cancer Research的其它文章
- Validation of clinical significance of examined lymph node count for accurate prognostic evaluation of gastric cancer for the eighth edition of the American Joint Committee on Cancer (AJCC) TNM staging system
- Oncological safety of use of ultrasonic activated shears in gastric cancer surgery: Long-term results of randomized controlled trial
- Anatomical variation of infra-pyloric artery origination: A prospective multicenter observational study (IPA-Origin)
- Feasibility of personalized treatment concepts in gastrointestinal malignancies: Sub-group results of prospective clinical phase II trial EXACT
- Oxaliplatin plus S-1 or capecitabine as neoadjuvant or adjuvant chemotherapy for locally advanced gastric cancer with D2 lymphadenectomy: 5-year follow-up results of a phase II-III randomized trial
- A comparative study of totally laparoscopic distal gastrectomy versus laparoscopic-assisted distal gastrectomy in gastric cancer patients: Short-term operative outcomes at a high-volume center
