Oncological safety of use of ultrasonic activated shears in gastric cancer surgery: Long-term results of randomized controlled trial
Su Mi Kim, Jae-Moon Bae, Min-Gew Choi, Jun Ho Lee, Tae Sung Sohn, Sung Kim
Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 06351, Republic of Korea
Abstract Objective: Ultrasonically activated shears (UAS) have been applied in open gastric surgeries with no or little evidence. It was previously reported about the surgical outcome and effectiveness of UAS based on a randomized controlled trial of 256 patients with gastric cancer. We aimed to clarify the long-term oncological safety of the use of UAS in the aspect of overall survival and recurrence.Methods: Gastric cancer patients who underwent gastrectomy with D2 lymph node dissection were enrolled and randomly assigned to either the conventional surgery group (n=125) or the UAS group (n=128). Survival,recurrence and long-term postoperative complications were compared between the two groups. The median follow-up period was 56 months.Results: Gastric cancer-related death was higher in patients of the UAS group compared with the conventional group (P=0.019). Overall survival rates stratified by stage were not significantly different between the two groups(P=0.170). Disease-free survival rates stratified by stage and recurrence-free survival rates of gastric cancer were similar between the conventional group and the UAS group (P=0.313 and 0.199, respectively). The postoperative complication rate was not significantly different between the groups (P=1.000).Conclusions: It is suggested that the use of UAS in gastrectomy for gastric cancer showed oncologically acceptable safety compared with conventional electric instruments even in long-term period.
Keywords: Stomach cancer; gastrectomy; ultrasonically activated shears (UAS); oncological safety
Introduction
Gastric cancer is the fifth most common cancer and the third leading cause of cancer-related death worldwide (1).International incidence rates of gastric cancer vary widely.The highest incidence rates of gastric cancer in the world occur in eastern Asian countries, including Korea and Japan (2-5).
Appropriated lymphadenectomy has been the mainstay for gastric cancer surgery because the number of metastatic lymph nodes is a strong prognostic factor (6-8). The development of surgical instruments has contributed to advanced surgical skills. Ultrasonically activated shears(UAS) have been widely used in various laparoscopic surgery and have been recently applied in open surgery.UAS facilitates the coagulation of vessels and dissection of tissues through high-frequency ultrasonic energy. UAS has several advantages that may make it useful for gastric cancer surgery, including reduced operating time, less intraoperative blood loss, and reduced leakage from the cut surface of organs in various surgical procedures such as cholecystectomy, gastrectomy, thyroidectomy, and pancreatic or hepatic resections (9-14).
Since UAS was first introduced with laparoscopic surgery(15), it has been also used in open surgery as well as laparoscopic and robotic surgery without concrete evidence of advantage. There have been few reports showing the use of UAS to reduce operative blood loss, postoperative lymphorrhea and hospital stay in gastrectomy (16). We previously reported the short-term surgical outcome and effectiveness of UAS through a randomized controlled trial of 256 patients with gastric cancer (14). In spite of advantage of UAS, there is a possibility that a high frequency vibrating energy from UAS makes a mist of cancer cells during lymphadenectomy which can be spread on surrounding tissues or peritoneal cavity resulting in early recurrence and poor survival rates. On the contrary, it can be also assumed that the high temperature heat and vibration from mechanical energy can eliminate microscopic cancer cells in lymphatics and small vessels during lymphadenectomy, which may result in less recurrence and good prognosis. There has, however, been no report of a long-term effect of UAS in the literature so far.
The study aimed to clarify the long-term oncological safety of the use of UAS by overall survival (OS) and recurrence rates after following up patients for more than five years.
Materials and methods
Patients and study procedure
The protocol was approved by the Institutional Review Board of the Samsung Medical Center, Seoul, Republic of Korea (IRB file No. 2009-08-089), and registered with ClinicalTrials.gov (No. NCT01179750). Written informed consent was obtained from all participants.
The study design has been previously described (14).Patients were randomly assigned to the UAS or the conventional surgery group. We stratified patients according to gender, body mass index (BMI) and preoperative depth of tumor invasion (early or advanced gastric cancers based on endoscopic findings).Randomization lists were generated from an independent randomization group using a permuted block design of size four within each stratum. The assignment of each patient to one of the two surgery groups was shown to the surgeon at the time of surgery after the confirmation of inclusion.Figure 1summarizes the study scheme. The primary end points of the study were operative blood loss and operating time. The secondary end points were postoperative lymphatic drainage and postoperative complications. Based on the previous operation data, we calculated the sample size by estimating a 20% advantage of saving intraoperative blood loss and also operating time when using UAS. One hundred fifteen patients in each group were required to detect a difference in mean intraoperative blood loss of 184.5 mL of the conventional surgery group, with an estimated standard deviation (SD) of 99.7 mL, a power of 80% and a 5% risk of type 1 error. Fourteen patients in each group were required to detect a difference in mean operating time of 92.6 min, with an estimated SD of 17.1 min, a power of 80% and a 5% risk of type 1 error.We decided to randomize 128 patients per group considering an elimination rate of 10% (14). In brief,eligible patients underwent gastrectomy with D2 lymph node dissection for histologically confirmed gastric cancer at the Department of Surgery, Samsung Medical Center.Patients who had a history of other malignancy or bleeding disorder, coagulation disorder, chronic disease (heart failure and/or cardiovascular disease), active hepatitis, and previous abdominal surgery were excluded. They were randomly assigned to the UAS group or the conventional surgery group using only electrically coagulating instrument. All patients were divided according to gender,body mass index (BMI), and preoperative depth of tumor invasion (early or advanced gastric cancers based on endoscopic findings). A random permuted block design(block size of four) was used in each stratum.
Patient characteristics and clinical data
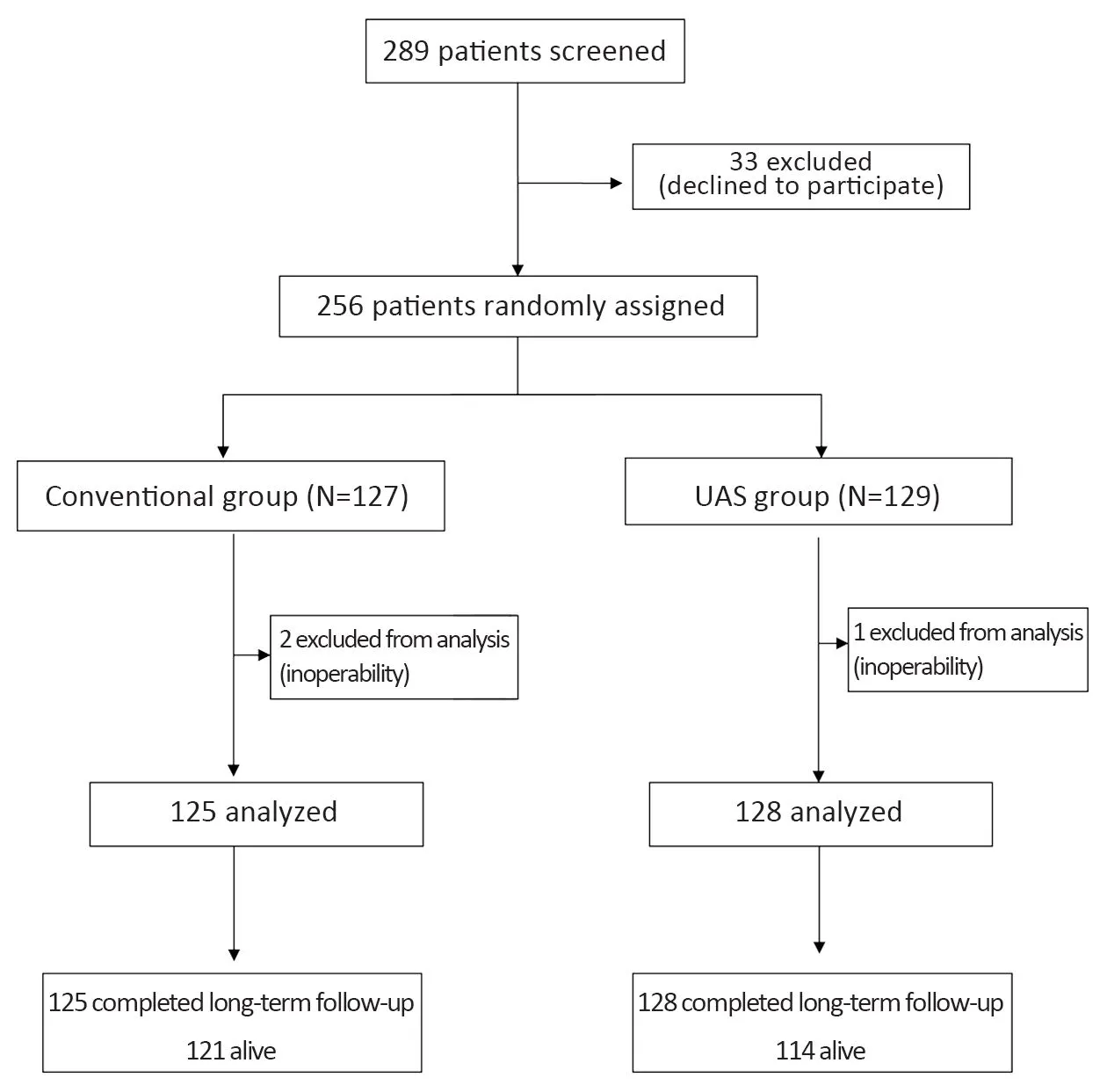
Figure 1 Schema of randomized controlled study. UAS,ultrasonically activated shears.
All characteristics of patients were obtained from the prospectively collected database. The demographic characteristics were age, sex and BMI. Clinicopathological characteristics included the tumor location, size,differentiation, ulceration, depth of invasion and stage at diagnosis, as well as the presence of lymph node metastases.The stage at diagnosis was determined in accordance with the 7th edition of the Union for International Cancer Control (UICC)/American Joint Committee on Cancer classification system (AJCC) (17). In patients with multiple synchronous gastric cancers, the lesion with the deepest infiltration of the gastric wall was regarded as the main lesion and any others were regarded as accessory lesions.The clinicopathologic characteristics of the main lesion were used for analysis.
Operative procedures
All patients underwent open subtotal or total gastrectomy with at least a standard D2 lymph node dissection for gastric cancer by a single surgeon (JM Bae). A Harmonic Scalpel (Ethicon Endosurgery, Inc., Cincinnati, OH, USA)was used for the operation in the UAS group. The UAS was mainly applied to seal or divide lymphatic and vascular vessels during procedures such as omentectomy, lymph node dissection, and clearance for perigastric lymph node dissection around the gastric wall. The left and right gastric vessels and the left and right gastroepiploic vessels were ligated with a surgical tie or hemoclips. In the conventional group, a monopolar electric coagulator was used for omentectomy and lymph node dissection instead of a harmonic scalpel. Other instruments including surgical tie and hemoclips were used as the same between the two groups (14).
Outcomes
Recurrence rates, recurrence pattern and OS rates were analyzed. Locoregional recurrence was defined as recurrence at the anastomosis site, duodenal stump, tumor bed, or regional lymph nodes. Peritoneal seeding, hepatic metastasis, metastasis to extraperitoneal sites, and lymph nodes beyond the region were regarded as distant metastasis, as described in the 7th AJCC classification (17).Long-term complications were defined as complications that required a re-admission. The cutoff date for this final analysis was May 1, 2015. Postoperative complications included those that occurred after the discharge.
Statistical consideration
The data were statistically compared between the UAS and the control group using an independent samplet-test for continuous variables and the Chi-square test or Fisher’s exact test for categorical data analysis. P<0.05 was considered statistically significant. Comparisons of diseasefree survival (DFS) and OS between the two groups were performed using a two-sided log-rank test. Analyses adjusted according to stage were performed using the Cox regression model. Hazard ratios (HRs) with 95%confidence intervals (95% CI) were calculated according to the Kaplan-Meier method. Statistical analysis was performed using IBM SPSS Statistics (Version 21.0; IBM Corp., New York, USA).
Results
Between January 2010 and April 2011, 256 patients were enrolled and assigned to the conventional group (127 patients) or the UAS group (129 patients) (Figure 1). Two hundred fifty-three patients (125 patients in the conventional group and 128 patients in the UAS group)were included in the final analysis; three patients (two patients in the conventional group and one patient in the UAS group) were excluded for inoperable gastric cancer(Figure 1). Baseline characteristics were generally well balanced in each group, as previously reported (Table 1)(14). The distribution of postoperative stage was statistically uneven between the two groups, because participants were randomized by preoperative stage(P=0.029). All patients completed follow-up. The median period of follow-up was 56 (range, 43-62) months.
Recurrence
Three (2.4%) out of 125 patients in the conventional group had experienced recurrence, compared with 10 (7.8%) out of 128 patients in the UAS group (P=0.084). The estimated 5-year recurrence-free survival rate was 96.8% for the conventional group versus 92.2% for the UAS group(P=0.199). Gastric cancer recurrence occurred at the following sites: locoregional, peritoneum, liver, and distant lymph nodes. There was significant difference in overall locoregional recurrence between the two groups (P=0.029)(Table 2), but stage-related locoregional recurrence rates were not significantly different between two groups(Table 3). There were more patients with higher postoperative stage in the UAS group.
One out of four patients with recurrence died of recurrence of gastric cancer and the other three patients inthe conventional group remained alive. In the UAS group,all ten patients with recurrence had gastric cancerrelated death.
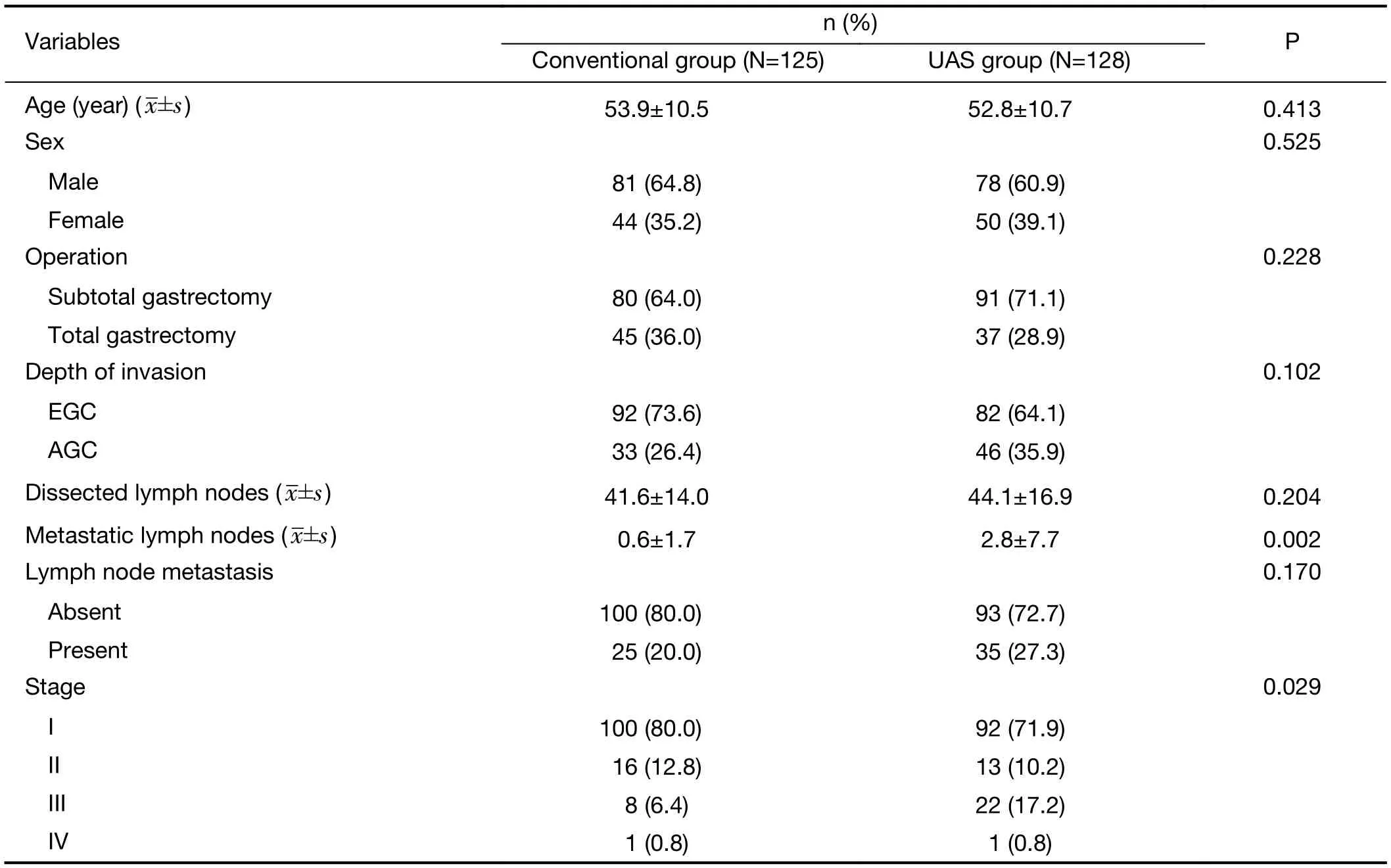
Table 1 Clinicopathologic features of UAS group and conventional electrosurgery group (N=253)
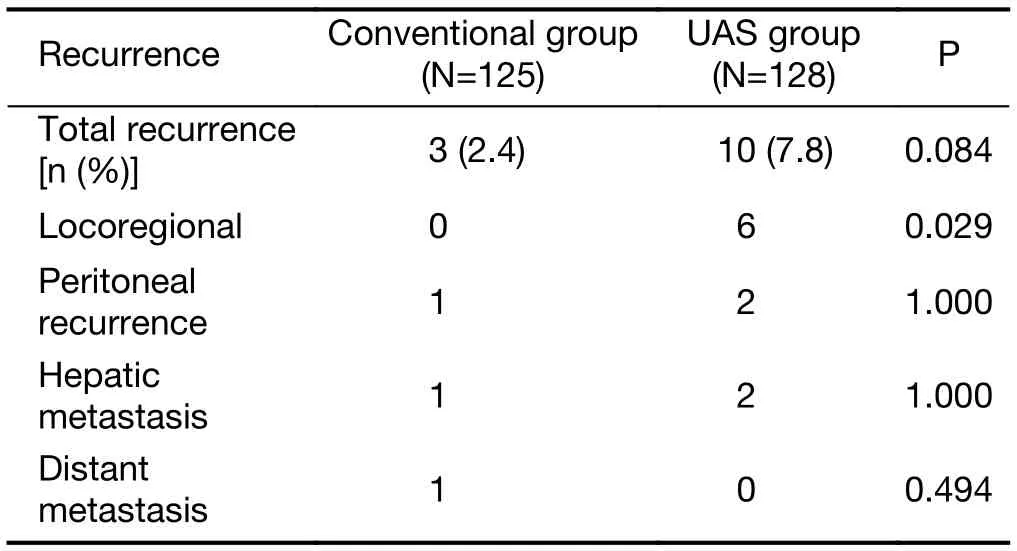
Table 2 Patterns of recurrence after gastrectomy (N=253)
OS
After follow-up for five years, four (3.2%) out of 125 patients in the conventional group died compared with 14(10.9%) out of 128 patients in the UAS group (P=0.025). In the conventional group, two patients died of gastric cancer,one from recurrence, the other from progression, and another two patients died of problems unrelated to the gastric cancer such as liver cirrhosis or unknown other causes. In the UAS group, 11 patients died of gastric cancer; that is, 10 patients had recurrences while one patient with stage IV did not have remission. The other three patients in the UAS group died of cerebral hemorrhage (one patient) or unknown causes (two patients).
A multivariate analysis using a Cox regression model revealed that higher tumor stage (more than stage III)significantly correlated with OS (HR, 20.832; 95% CI,6.534-66.419; P<0.001) and DFS (HR, 17.360; 95% CI,6.359-47.392; P<0.001). Age, sex, and UAS use were not associated with OS and DFS (Table 3).
The five-year survival rates were 96.8% and 89.0% in the conventional and UAS groups, respectively (P=0.012).Although the distribution of stage between the two groups was statistically different (P=0.029), DFS stratified by stage was similar between the conventional group and the UASgroup (P=0.313) (Figure 2). The OS rates stratified by stage was also not significantly different between the two groups(P=0.170) (Figure 3).

Table 3 Multivariate analysis for OS and DFS
Delayed postoperative complication
The long-term postoperative complication rate was not significantly different between the two groups (4.0% in the conventional groupvs. 4.7% in the UAS group,respectively, P=1.000) (Table 4). The most common complication in the UAS group was ileus (5 patients),followed by incisional hernia. In the conventional group,only one patient had ileus; however, there was no significant difference in the occurrence rate of ileus between the two groups (P=0.213). Three patients had incisional hernia, and one patient underwent the operation due to desmoid tumor in the small bowel. Three patients in the conventional group suffered from morbidity unrelated to the gastrectomy including cerebral infarction, superior mesenteric artery infarction, and gallbladder perforation.These all occurred in several years after the gastrectomy.
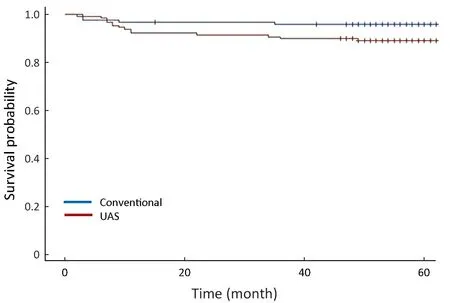
Figure 2 Disease-free survival (DFS) rates in conventional group and ultrasonically activated shears (UAS) group (P=0.313,stratified by stage).
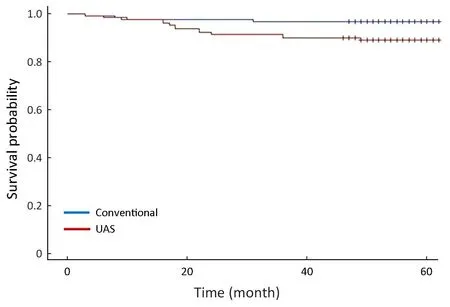
Figure 3 Overall survival (OS) rates in conventional group and ultrasonically activated shears (UAS) group (P=0.170, stratified by stage).
Discussion
Although there is constant improvement and application of surgical instruments in clinical practice, there have been few studies demonstrating the efficacy for surgical outcome and feasibility with surgical instruments. UAS is used for hemostasis by generating high mechanical energy. Thefriction heat that is generated by a vibration blade in very high frequency denatures the protein in the tissue and forms a sticky coagulum that seals the vessel lumen. A mist is usually produced during the transfer of high vibrating energy to tissues to evaporate water. Abeet al. had reported that UAS could occlude veins and lymphatic vessels, as well as arteries, so as to be considered for lymph node dissection for malignancy (18). There have been some studies about the advantages of UAS compared with conventional electric cautery instruments in various types of surgery (11,19-21).In our previous study (14), authors showed reduced operative time and similar short-term surgical outcomes,including operative blood loss, amount of postoperative drainage, and postoperative short-term complications between the UAS group for patients with gastrectomy and the conventional group, by a prospective randomized controlled trial (RCT). To the best of our knowledge, there has not been any study in the literature on the long-term outcomes after using of UAS. From the actual follow-up data of more than five years in the present study, it was found that gastric cancer-related death rates were higher in patients of the UAS group compared to the conventional group. It may be assumed that radical lymph node dissection might open lymphatic channels and disseminate viable tumor cells into the peritoneal cavity followed by local recurrence (22,23). The usage of UAS might make more evaporation of tumor cells, owing to the high vibrating energy and temperature compared to electrocautery during operation. It was, however, demonstrated that viable airborne colon cancer cells were not released after tumor ablation with UAS or electrocautery in rats(24). In the present study, there was no statistical difference in overall local recurrences between the UAS group and the conventional group, although the event of recurrence was more frequent in UAS group. It is thought that statistical difference was not shown because the number of local recurrence was relatively small compared to the number of each group. Our previous study suggested that UAS might also have the advantage of preventing possible tumor cell spillage via the lymphovascular channels by more secure coagulation (14). UAS was considered to have sufficient bursting pressure in lymphatic vessels (18). It, however, did not seem to clinically affect local recurrence compared to conventional electrocautery.
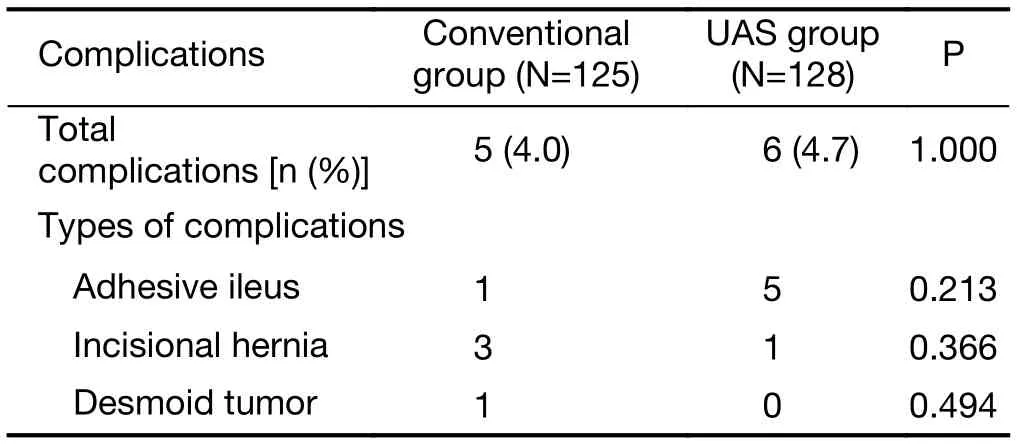
Table 4 Long-term postoperative complications after gastrectomy(N=253)
Unfortunately, the UAS group had more cases with advanced stage compared to the conventional group in the present study. The randomization was performed based on preoperative diagnosis by esophagogastroduodenoscopy(EGD) and computerized tomography (CT). The clinical staging would have discrepancy with actual staging by pathologic results after the surgery, although CT coupled with EGD was a good modality in the preoperative diagnosis and staging for gastric cancer (25). The five-year recurrence rates for gastric cancer have been reported to be 1.6% to 2.8% in the early stage (26,27), and as high as 27%to 46% in the advanced stage (28). Because there were 192(75.9%) patients with early stage gastric cancer in our study, the number of events for analysis of recurrence was very small, which could induce misleading analytical results. In the present study, most recurrences occurred in cases with advanced stage (2 recurrences in stage Ivs. 13 recurrences in stage II or higher), which strongly suggested that the recurrence rate was related to the stage and not to only the type of surgical instruments. Further studies with a larger cohort of gastric cancer patients with advanced stage would be more valuable.
Some studies have suggested the association of postoperative adhesion and UAS (29-31). Sasiet al. assessed perioperative outcomes after laparoscopic cholecystectomy comparing monopolar energy dissection with ultrasonic energy, and showed that the ultrasonic energy group had significantly superior perioperative outcomes for operating time, postoperative pain, length of stay, and time to return to work, in comparison to the monopolar energy group(29). It was speculated that decreased operative inflammation resulted in lower postoperative adhesion rates. Brokelmanet al. (32) also showed that ultrasonic scalpel dissection had significantly lower peritoneal total and active transforming growth factor β1 levels, suggesting a reduced risk of formation of peritoneal adhesions compared to the surgery using electrocautery. Although UAS had been reported to be associated with less postoperative adhesion formation, a study using a rabbit model showed no clinical difference in adhesion scores between the ultrasonic energy and monopolar electrosurgical energy in acute and late (8 weeks) postoperative periods (33,34). The present study also showed that there was no significant difference in postoperative adhesive ileus between the two groups, although there were more adhesive ileus cases in the UAS group.
Patients in the UAS group had more local recurrence although it had not been statistically significant and they had more advanced stage gastric cancer. In addition, the UAS group showed significantly worse DFS and OS compared with those in the conventional group, although DFS and OS were not significantly different if adjusted by stage between two groups. One out of four patients with recurrence died of gastric cancer recurrence in the conventional group, all ten patients with recurrence had gastric cancer-related death in the UAS group. These results might be induced by too small number of cases or unbalanced pathologic stage in spite of randomization.There is little possibility that the use of UAS might be one of causes for local recurrence, however, the influence of UAS for local recurrence and gastric cancer related-death cannot be totally excluded.
Conclusions
It is suggested that the use of UAS in gastrectomy for gastric cancer showed oncologically acceptable safety compared to conventional electric instruments even in long-term period.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
 Chinese Journal of Cancer Research2018年5期
Chinese Journal of Cancer Research2018年5期
- Chinese Journal of Cancer Research的其它文章
- Validation of clinical significance of examined lymph node count for accurate prognostic evaluation of gastric cancer for the eighth edition of the American Joint Committee on Cancer (AJCC) TNM staging system
- Anatomical variation of infra-pyloric artery origination: A prospective multicenter observational study (IPA-Origin)
- Feasibility of personalized treatment concepts in gastrointestinal malignancies: Sub-group results of prospective clinical phase II trial EXACT
- Oxaliplatin plus S-1 or capecitabine as neoadjuvant or adjuvant chemotherapy for locally advanced gastric cancer with D2 lymphadenectomy: 5-year follow-up results of a phase II-III randomized trial
- Immunohistochemical expression of thymidylate synthase and prognosis in gastric cancer patients submitted to fluoropyrimidine-based chemotherapy
- A comparative study of totally laparoscopic distal gastrectomy versus laparoscopic-assisted distal gastrectomy in gastric cancer patients: Short-term operative outcomes at a high-volume center
