Treatment of Phenol-Contaminated Soil by Potassium Ferrate Based on pH Control
Lu Zheng; Zhang Yanqing; Jia Xin; Huang Jin; Xue Jianliang; Zhuang Hongli; Liu Guangmin
(1. College of Power and Energy Engineering, Harbin Engineering University, Harbin 150001;2. College of Material Science and Chemical Engineering, Harbin Engineering University, Harbin 150001;3. College of Chemical and Environmental Engineering, Shandong University of Science and Technology,Qingdao 266590; 4. Lanling Inspection and Testing Center, Linyi, Shandong 277731)
Abstract: This study aims to optimize the treatment of phenol-contaminated soil by potassium ferrate. Variations in pH value can accurately reflect the state and reaction status of the entire treatment process. Therefore, the pH value could be an important variable for optimizing the reaction conditions and achieving the automatic control of the process. About 99.89% of phenol was removed after 10 min of the pH-controlled reaction at a rotational speed of 40―70 r/min, with the initial phenol concentration equating to 10.0 g/kg and the total water consumption reaching 2.72 L (at a soil/water ratio of 1:0.68). The test results could provide a basis for practical application of automatic reaction control by pH value.
Key words: potassium ferrate; phenol contaminated soil; degradation; pH; automatic control
1 Introduction
Chemical leakage accidents frequently occur during storage, transport and use of hazardous chemicals and may result in serious point source soil pollution.Chemicals in soil not only can influence its biological,chemical and physical characteristics, but also can interfere with its natural productive capacity. In addition,soil pollution indirectly affects human health through consumption of poisonous grains, vegetables and fruits.Soil pollution can also influence social and economic development[1-2]. For example, phenol exerts a strong corrosive effect on skin and mucosa, causes liver damage and inhibits the central nervous system, thereby inducing hereditary toxicity and promoting carcinogenesis,teratogenesis, and mutagenesis[3]. Thus, the negative effects of chemical leakage must be eliminated by rapid on-site disposal of contaminated soil. Electrical methods are frequently used to treat chemical polluted soil with the advantages of easy installation and operation. Matta,et al. used the classic Fenton method to treat phenolcontaminated soil. By using a 10-g/L mixture of iron and iron oxide, and 155 mmol/L of H2O2at a pH value of 7, approximately 62% of phenol was mineralized to catecholamine and hydroquinone after 24 h[4].
Although the advanced oxidation technologies have negative effects on the original environment, and are not appropriate for low-permeability soils[5-6], they exhibit superiorities over other methods. Among them,potassium ferrate exhibits strong oxidative properties,and its oxidation product, namely Fe(III) is nontoxic and causes no secondary pollution to the environment[7].Therefore, potassium ferrate is a green chemistry oxidant[8]. Moreover, the colloidal Fe(OH)3exhibits a high adsorption performance and can flocculate most ions and the organic, inorganic and suspended solids over a wide range of pH[9]. Potassium ferrate can effectively oxidize not only organic compounds, such as ethanol, nitrogen oxides, sulfides, amino acids, phenols, nitrites, ammonia,glycol and hydrazine compounds, but also inorganic compounds such as cyanide, ammonia and hydrogen sulfide. Under certain conditions, potassium ferrate can even oxidize endocrine disrupting chemicals[10-14].
Automatic control of waste-water treatment processes has gained increasing attention[15]. Some researchers prefer the on-line measurements of pH value, the Oxidation-
Reduction Potential (ORP) and the Dissolved Oxygen(DO) because the real-time measurement of chemical compounds is neither simple nor economical[16-17]; the online ORP and pH monitoring are economical and effective techniques for controlling biological and chemical processes[18-21]. However, the pH control is difficult to achieve in practice considering the effect of lag factors,such as mixing and measuring in the reaction[22]. In addition, the pH control has few applications in polluted soil treatment but can be achieved at the anode during electro-kinetic removal of phenanthrene from kaolin soil[23]. Phenol is oxidized by potassium ferrate under neutral conditions. Thus, the automatic control of this process through on-line pH monitoring is convenient.
In this study, phenol was selected as the target oil contaminant. The effects of pH value for potassium ferrate were analyzed to control the system and maintain the optimal reaction conditions. Thereby, an effective method for treatment of chemical polluted soil and a remediation strategy for phenol polluted soil were concluded.
2 Experimental
(1) Materials and reagents
Potassium ferrate (K2FeO4, 86% purity) was used as received without further purification. Phenol was of analytically pure grade.
(2) Soil samples

Figure 1 Process of treating phenol point source polluted soil by potassium ferrate
Uncontaminated soil was collected from local land at Heilongjiang province in the northeast of China (at a depth of 0—15 cm, sandy loam). The soil is composed of 57.9% of sand, 25.8% of silt and 15.3% of clay and has a pH value of 8.15 in water. The uncontaminated soil was air-dried at room temperature, sieved to 1 mm size, sterilized at 170 °C for 3 hours and then mixed with phenol. A stock solution was prepared by dissolving phenol in 100 mL of hot water. The phenol concentration used in the experiments ranged from 0.1 g/kg to 20 g/kg.(3)Analytical methods
Phenol was extracted from the soil samples by adding tap water and 0.1 mol/L NaOH solution, and was then subject to oscillating for 30 min. External calibrations were performed by using standard phenol solutions with concentration ranging from 0.2 mg/L to 400 mg/L.
The concentrations of phenol and Fe (VI) in the aqueous solution were determined using a UV-vis spectrophotometer(TU-1810D, China) by monitoring the absorbance at 510 nm and 505 nm, respectively. The measured statistics were converted into the concentration by using a calibration curve.The pH value of the solution was determined by using a pH analyzer (DELTA320, China).
(4) Technological process
Potassium ferrate oxidizes phenol under neutral conditions,but the generated OH-molecules produce an alkaline environment during the reaction. Therefore, sulphuric acid should be added to the reaction tank to regulate the pH value of the soil and the reaction system. Adding acid to the system can improve the efficiency of potassium ferrate oxidation and enables direct discharge of soil in a neutral state. The process of treating the phenol point source polluted soil by potassium ferrate is shown in Figure 1.
The process of treating phenol-contaminated soil is described as follows:
(1) Oxidation treatment.
The phenol-contaminated soil with an effective volume of 1/4 to 1/2 that of the reactor was added to the oxidation reactor. Water was then added to achieve a soil to water mass ratio of 1:0.5 to 1:1. Potassium ferrate was continuously added to the reactor under continuous stirring at a speed of 40―70 r/min to allow the soil and water to be completely mixed. As the pH value of the mixture rose, a 2% sulfuric acid solution was added to the system to adjust the system pH value to 7.0―9.0 before more potassium ferrate was added. The oxidation reactor could degrade over 90% of the phenol under these operating conditions.
(2) Separation of soil and water in the oxidation unit.
The soil mixture produced during the oxidation treatment was transferred to a slurry separator. The liquid isolated by the separator was sent to the liquid reflux device and recycled by pumps in the oxidation reactor, and the isolated soil was sent to the reduction-neutralization unit for further treatment.
(3) Reduction neutralization reaction.
The FeSO4(0.1 mol/L) was added to the separated soil to remove the excess potassium ferrate. A 2% sulfuric acid solution was then added to neutralize the soil after complete reduction of potassium ferrate, which should keep the pH value of the soil between 6.0 and 9.0 after the treatment.
(4) Separation of soil and water from the reductionneutralization unit.
The soil mixture produced in the reduction neutralization process was transferred to a slurry separator. The liquid isolated by the separator was sent to the liquid reflux device and recycled by pumps in the reduction-neutralization reactor, and the isolated soil was then discharged.
(5) Automatic pH control process
The automatic pH control diagram is schematically illustrated in Figure 2. The soil and water in the reaction tank were thoroughly mixed by rapid stirring, while the real-time pH values were converted to electrical signals R(S) and sent as inputs to the microprocessor of the control system. Signals were then sent to the pH controller for comparison with the threshold value. If the signal exceeded the threshold value, the control program would command the execution unit to directly control the manipulating valves.

Figure 2 Automatic pH control diagram
3 Results and Discussion
3.1 Oxidation removal efficiency of phenol under different pH levels
Potassium ferrate was reduced to Fe (III) in the phenol degradation process. OH-ions, which could rapidly increase the pH value of the system, were also produced.By using pH control, different pH values were set to examine the phenol removal effect. The relationship between the phenol removal rate and the pH value of the system is presented in Figure 3.

Figure 3 The removal of phenol under different pH
Figure 3 reveals that the oxidation capability of potassium ferrate gradually weakens when the system pH rises.Therefore, the pH value increase caused by the reaction process is not beneficial to phenol degradation, and the treated soil cannot be safely discharged because of its high pH value.The existent forms of potassium ferrate at different pH value are as follows:
Alkaline conditions:

Acidic conditions:

The oxidation-reduction potential and stability of potassium ferrate is higher when the pH value is between 8 and 10. Therefore, the removal of phenol should be preferably realized under these conditions. However,the redox potential decreases and the oxidation capacity weakens with the increase of the pH value, resulting in the decrease of phenol removal rate.
In this study, the pH level could accurately reflect the state and reaction status of the entire process. When the reaction system was adjusted to be nearly neutral, the reaction could be carried out under optimal conditions.Additionally, the pH value could be used as a control variable to achieve optimal reaction conditions and enable automatic control of the whole process.
3.2 Relationship between the residual concentration of phenol and the pH value in the reaction process
Two samples of synthetic contaminated soil with an initial phenol concentration of 4 g/kg were prepared,with water being added to achieve a soil/water ratio(mass ratio) of 1:4, while potassium ferrate was added to achieve a potassium ferrate to phenol molar ratio of 7:1 and 20:1, respectively. The changes in the residual phenol concentration and the system pH value throughout the reaction are presented in Figure 4.

Figure 4 The changes in pH value and residual phenol concentration in different time
Figure 4 reveals that the pH value increased rapidly as the reaction proceeded, and the residual phenol content gradually decreased until the pH value was stabilized when the potassium ferrate/phenol molar ratio was 7:1,which indicated a lack of potassium ferrate. The residual phenol content was less than 10% after 10 min, and the pH value increased slowly thereafter when the molar ratio was 20:1.
These results indicate that changes in the pH directly reflect the status of the reaction: the system pH value increases rapidly and then stabilizes, and phenol cannot be completely degraded at a complete lack of potassium ferrate; the system pH values increases slowly, and phenol can be completely degraded with excessive supply of potassium ferrate.
3.3 Effect of potassium ferrate addition on the pH value of the system
The experiment was conducted under the conditions covering: an initial phenol concentration of 10.0 g/kg,a synthetic contaminated soil volume of 5.00 kg; and an initial water volume of 20 L. The influence of the potassium ferrate dosage on the pH value is shown in Figure 5.

Figure 5 The pH change with different potassium ferratedosage
Figure 5 shows that the system pH value increases rapidly with an increasing potassium ferrate dosage. The logarithmic relationship between the change in pH value and the potassium ferrate dosage is as follows:

where, x is the potassium ferrate dosage, kg; y is the pH value.
In this experiment, 2 g of synthetic contaminated soil with a phenol concentration of 4 g/kg and 2 g of clean soil were used to conduct a comparative test. Water was added to achieve a soil-water mass ratio of 1:4, while a constant amount of potassium ferrate was added. The change in the residual potassium ferrate fraction and the system pH value during the reaction is shown in Figure 6.

Figure 6 The pH change and residual concentration ofpotassium ferrate at different time
Figure 6 reveals that the pH value rose rapidly during the first 3 min and increased less rapidly over the next 3 min,and then stabilized until the end of the reaction during the process of phenol degradation by potassium ferrate. At the end, no residual potassium ferrate was observed, and the following reaction was completely carried out:

The pH value increased rapidly throughout the first 30 min and more slowly over the subsequent 30 min during the self-decomposition of potassium ferrate. Approximately 45% of the potassium ferrate remained after 60 min.The following potassium ferrate decomposition reaction occurred:

Therefore, the rate of phenol oxidation by potassium ferrate is much faster than the rate of potassium ferrate decomposition. Additionally, the change in the pH value is directly related to the residual potassium ferrate content,and the system pH value stabilizes after complete decomposition of potassium ferrate. The system pH value quickly reaches its maximum value and then stabilizes when the reaction system lacks potassium ferrate, but the pH value slowly increases at a rate of 0.01―0.03 per minute when excessive potassium ferrate is present.
3.4 Control mode and treatment effect of pH value during continuous oxidation experiment
3.4.1 Control mode of pH value
The threshold pH value of the oxidation reactor: The interval control method was chosen to keep the system pH value in the range of 7.0―9.0, which could represent the optimal reaction condition. Control procedures were determined by setting a threshold pH value for the oxidation process in a bid to monitor the system pH value in real-time. The control program’s commands were transformed to electrical signals by the execution unit to control the solenoid valve on the acid tank in the oxidation treatment unit, which could determine the rate of acid addition. Three threshold parameters were selected: A1(pH=13.0), A2 (pH=10.0) and A3 (pH=7.0).
The threshold pH value of the reduction-neutralization reactor:The safety regulations required the pH value of the discharged soil to be within 6.0―9.0. The threshold setting A4 (pH=6.0) was therefore chosen as the threshold of the reduction-neutralization unit. The program’s commands were transformed to electrical signals by the execution unit to control the solenoid valve on the acid tank in the reduction neutralization unit, which could determine the rate of acid addition to the reduction-neutralization reaction tank and adjusted the pH value of the discharged sludge.
3.4.2 Treatment effect of continuous oxidation experiment
Synthetic contaminated soils with different phenol concentrations were treated in the reaction system under the automatic control conditions described above. The treatment results are presented in Table 1.
Table 1 demonstrates that the molar ratio of potassium ferrate to phenol decreases gradually as the initial phenol concentration increases to achieve the same removal rate after pH adjustment. The phenol removal rate reached 99.89% after 10 min under the conditions covering:an initial phenol concentration of 10.0 g/kg, a total consumption of 2.72 L of water (at a soil/water ratio of 1:0.68), a total amount of 0.61 kg of potassium ferrate,and a potassium ferrate to phenol molar ratio of 7.2:1.The potassium ferrate consumption was greatly reduced,the reaction time was decreased, and the operational efficiency was improved.
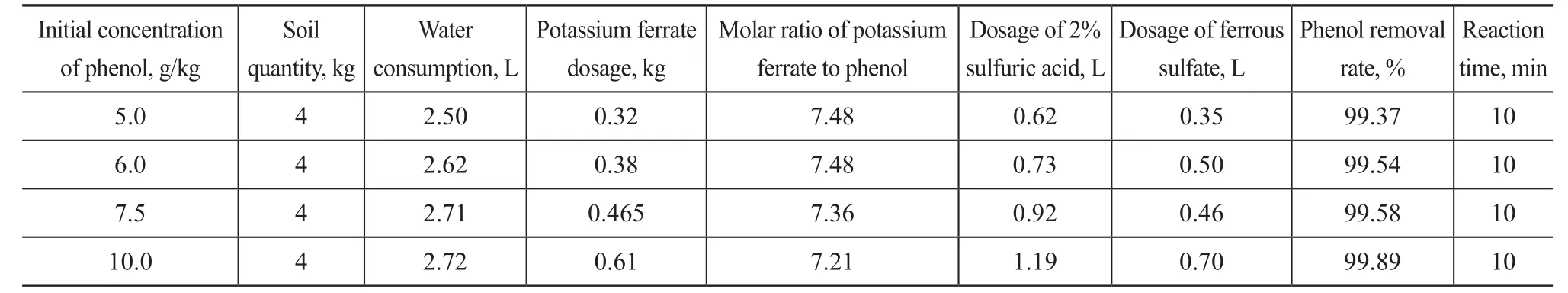
Table 1 The effect of the continuous oxidation experiment
4 Conclusions
Variations in the pH levels of the phenol oxidation system could accurately reflect the status of the entire process,so a set of threshold pH values was used to control the system and maintain the optimal reaction conditions.Automatic control of the entire process was achieved.The interval control method, in which a threshold value was chosen, was used to achieve automatic control of the process while considering the characteristics of pH control and the reaction mechanism between potassium ferrate and phenol. This method could reduce the difficulty of pH control and was easy to implement. Four threshold parameters were selected: A1 (pH=13.0), A2(pH=10.0), A3 (pH=7.0), and A4 (pH=6.0). At a rotational speed of 40―70 r/min, an initial phenol concentration of 10.0 g/kg, and a total water consumption of 2.72 L (with the soil/water ratio equating to 1:0.68), 99.89% of phenol was removed after 10 min of the pH-controlled reaction process. The potassium ferrate consumption was greatly reduced, while the reaction time was decreased and the operational efficiency was improved.
Acknowledgments:This research was financially supported by the National Key R&D Plan of China (2017YFC1404605),the Natural Science Foundation of China (Grant No. 51579049 and 51509044) and the High-Tech Ship Program. The authors gratefully acknowledge the Excellent Subject Leaders’Foundation of Harbin Science and Technology Bureau, the Key Laboratory of Superlight Materials and Surface Technology of the Ministry of Education, and the Harbin Engineering University for their support. This research was also supported by the Open Research Fund Program of Shandong Provincial Key Laboratory of Oilfield Produced Water Treatment and Environmental Pollution Control (SINOPEC Petroleum Eaguieering Corporation) (No.201801)
- 中国炼油与石油化工的其它文章
- Application of Big Data Technology in Evaluation of Operating Status of High-pressure Hydrogenation Heat Exchanger
- Identification of NixSy on Industrial Spent S Zorb Sorbents by Using XPS and TPR-MS
- Thermodynamic Analysis of Formation of Low-carbon Olefins via Coal Gasification Coupling C1 Reaction
- Conversion of Biomass to Hydrocarbon-rich Bio-oil via Microwave-assisted Catalytic Pyrolysis: A Review
- Comparison of Lubricities of Two Novel Benzotriazole Derivatives Used as Additives in Water-Glycol Hydraulic Fluid
- Structural Characterization of Petroleum Molecules by CID FT-ICR MS with Narrow Isolation Window

