Thermodynamic Analysis of Formation of Low-carbon Olefins via Coal Gasification Coupling C1 Reaction
Xu Jing; Tu Nan; Liu Tong
(College of Mechanical and Electrical Engineering, Xi’an Polytechnic University, Xi’an 710048)
Abstract: The complex reaction system of the coal gasification coupling C1 reaction was analyzed based on the principles of thermodynamics. The results show that an increase in the temperature is beneficial to the generation of hydrocarbons with high carbon-atom contents, in which the alkane yield is higher than the alkene yield. The complex reaction system consisting of C, H2O, CO, CO2, H2, C2H4, C3H6 and C4H8 was studied, and the obtained results indicated that when the maximum mole fraction content of C2―C4 olefins was regarded as the optimized objective function, the optimum temperature was approximately 648 K, the pressure was 0.1 MPa, the feed ratio was approximately 0.6, and the maximum mole fraction content of C2―C4 olefins was approximately 28.24%. The thermodynamic simulation and calculation of the complex reaction system can provide a basis for the determination and optimization of actual process conditions and are therefore of great theoretical and practical significance.
Key words: thermodynamic analysis; low-carbon olefins; coal gasification; coupling; C1 reaction
1 Introduction
Low-carbon olefins are important organic chemical materials[1-2]. These olefins can be used to produce a wide variety of synthetic materials such as plastics,synthetic resins, synthetic fiber and synthetic rubber[3].Currently, low-carbon olefins are mostly produced via the petrochemical route using the steam cracking reaction of light hydrocarbons (ethane, naphtha and light diesel fraction)[4]. With the rapid growth of the market demand for low-carbon olefins and the increasingly depleting global oil resources, major global petrochemical companies have been proactively developing a new alternative production route to replace the traditional route for olefin production[5-7]. Coal is a valuable organic carbon resource[8], and its utilization for producing lowcarbon olefins is a popular research direction[9-10]. This new route enjoys the advantages of simple processes flow diagram, low consumption of energy and coal, and good prospects in industrial application.
This paper studies the overlapped coupling between the coal gasification reaction and the C1synthesis reaction.Such coupling could induce the H2and CO generated in the coal gasification reaction to directly performing C1synthesis on the coal surface with its abundant pores to produce low-carbon olefins. Furthermore, the heat generated by C1synthesis could compensate for the heat consumption during coal gasification. The new production route not only can shorten the process flow diagram from gasification to C1synthesis, but also can save energy and reduce pollution to decrease the production cost.
The direct conversion of syngas (a mixture of H2and CO)into lower olefins based on the Fischer-Tropsch synthesis(F-T synthesis) process is known as an alternative process, in which syngas can be readily derived from the gasification of coal or biomass or from the reforming of natural gas[11-13]. Although coal gasification and C1synthesis have been studied systematically, there are few reports related with the research on the generation of lowcarbon olefins by the overlapped coupling between the coal gasification and C1synthesis reactions. Thus, it is of great theoretical and practical significance to perform simulations and thermodynamic calculations of the complex reaction system[14-17]. These reactions can also provide a basis for determining and optimizing the actual process production conditions.
2 Gibbs Free Energy Analysis of the Total Reaction System
2.1 Determination of components in the equilibrium system
The coal gasification coupling C1synthesis reaction system can be divided into two steps. The first step is the gasification reaction that generates CO, CO2and H2, and the second step is the C1synthesis (i.e., F-T synthesis)reaction[18-22]. Therefore, the reaction system is very complex, and many reactions may potentially be present.To simplify the calculations and facilitate the study of the relationship between the equilibrium composition of the key target product and the reaction conditions, we propose the following hypotheses:
(1) The pressure of the investigated reaction system is low, and its temperature is high. Therefore, each component in the system can be approximately treated as an ideal gas.
(2) Only a trace amount of hydrocarbons above C4is produced in the C1synthesis reaction[23], so the reaction products can be considered as the low-carbon C1―C4hydrocarbons.
(3) The effect of pulverized coal on the equilibrium composition is weak and thus can be ignored. The pulverized coal is mainly composed of carbon, and the side effects due to other elements are not considered[24].
According to the above hypotheses, the components of the simplified equilibrium reaction system cover C, H2O,CO, CO2, H2, CH4, C2H4, C2H6, C3H6, C3H8, C4H8, and C4H10.
2.2 Determination of independent reaction number in the reaction system
The atomic matrix method[24]is used to calculate the number of independent reactions in a complex reaction system. The rank of the atomic matrix for the coupled reaction system of coal gasification and C1synthesis is 3,and the component number of the matrix is 12. Therefore,the number of independent reactions is 9. A set of independent reaction equations for the system is derived according to the principle of atomic balance:

2.3 Analysis of the Gibbs free energy change in the reaction system
It is helpful to evaluate the thermodynamic feasibility of the reaction system, the reaction direction, and the process of each reaction from a macro perspective by studying the Gibbs free energy change in a complex reaction system. The ΔrGθTvalue of each independent reaction under different temperature conditions can be calculated according to the data presented in the reference[25]. The variation in ΔrGθTwith the temperature is shown in Figure 1.

Figure 1 Relationship between the Gibbs free energy change (ΔrGθT) and temperature (T) in the reaction system
The slope of each curve in Figure 1 indicates the temperature sensitivity of the Gibbs free energy change in each independent reaction. It can be seen from Figure 1 that the slope of Gibbs free energy change curve for all kinds of hydrocarbon reactions is negative, which shows that higher temperatures are favorable to the formation of hydrocarbons (ΔrGθTdecreases with higher temperatures and finally becomes less than 0). With the increase of the number of carbon atoms, the slope of the curves decreases as well, indicating that the temperature has a strong influence on the Gibbs free energy change of hydrocarbons with an increasing number of carbon atoms. For the various hydrocarbons with the same number of carbon atoms,alkane shows smaller Gibbs free energy change values than alkene. This difference leads to the conclusion that the yield of the alkane is much higher than that of the alkene.As shown in Figure 1, the Gibbs free energy change curves of C2—C4olefins form an intersection point at approximately 850 K, and those of the alkanes also form an intersection point at approximately 950 K, where the two intersection points act as the inflection points of the content, conversion and yield of each component. The hydrocarbons with more carbon atoms show a greater free energy change before the intersection, whereas the hydrocarbons with fewer carbon atoms demonstrate greater free energy after the intersection. In addition, the increase in temperature is beneficial to the generation of H2and CO; hence, the products of the reaction system contain a large amount of H2and CO.
2.4 Analysis of the system reaction conditions
The coal gasification coupling C1reaction system is a complex gas-solid mixture system. Because the pressure of the reaction system is in the range of 0.1—4 MPa, the gas mixture can be treated as an ideal solution when the relative pressure of each component of the system is less than 0.8. According to the chemical reaction equilibrium criterion ((dG)T,P=0), the equilibrium composition of a chemical equilibrium system that involves many reactions can be determined by the calculation of the minimum value of the Gibbs function G.
The chemical thermodynamic equilibrium system of the coal gasification coupling C1reaction can be analyzed by the above method. The range of key parameters is: the temperature range covering 500—1200 K, the pressure range covering 0.1—4.1 MPa, and the H2O/C mole ratio covering 0.5—2.0.
3 Equilibrium Analysis of the Reaction of C2―C4 Olefins Formation
3.1 Equilibrium composition analysis of the formation reaction of C2—C4 olefins
To more easily analyze the equilibrium state of the formation reaction of the C2—C4olefins and better study the yield of C2—C4olefins, the light hydrocarbons synthesized in the system are assumed to be C2—C4olefins only, and the reaction process is treated as an ideal one. According to the above hypothesis, the original reaction system includes only five independent reactions as shown in Equation 2.

This new system is analyzed by the thermodynamic method introduced in the above section. Figure 2 shows the curve concerning the amount of each component in the equilibrium system related to the temperature under different pressures as obtained by thermodynamic calculations.
3.1.1 C2—C4 olefins line analysis
As shown in Figure 2, the trend for variation of each olefin is inconsistent. The molar fraction of C2H4increases uniformly and moderately with an increasing temperature and decreases slightly with an increasing pressure, and the overall trend is consistent with the conclusion obtained from Figure 1. The mole fraction of C3H6shows a weakly monotonic change with an increasing temperature, and the maximum value is achieved among the entire temperature range, because the curve of the Gibbs free energy of C2H4formation reaction intersects the curve of the Gibbs free energy of C3H6formation reaction at the extreme point.As shown in Figure 2, the maximum value for C3H6appears at approximately 700 K, 850 K, 900 K, and 950 K under a pressure of 0.1 MPa, 1.1 MPa, 2.1 MPa, and 3.1 MPa, respectively. This shows that the extreme point moves toward the direction of high temperature with an increasing pressure. The mole fraction content of C4H8is higher under lower temperature. Because the Gibbs free energy of C4H8formation reaction changes strongly with the change of the temperature and the difference in the chemical reaction stoichiometry coefficient is relatively high, the mole fraction of C4H8decreases more rapidly with an increasing temperature.
3.1.2 Analysis of H2O, H2, CO and CO2
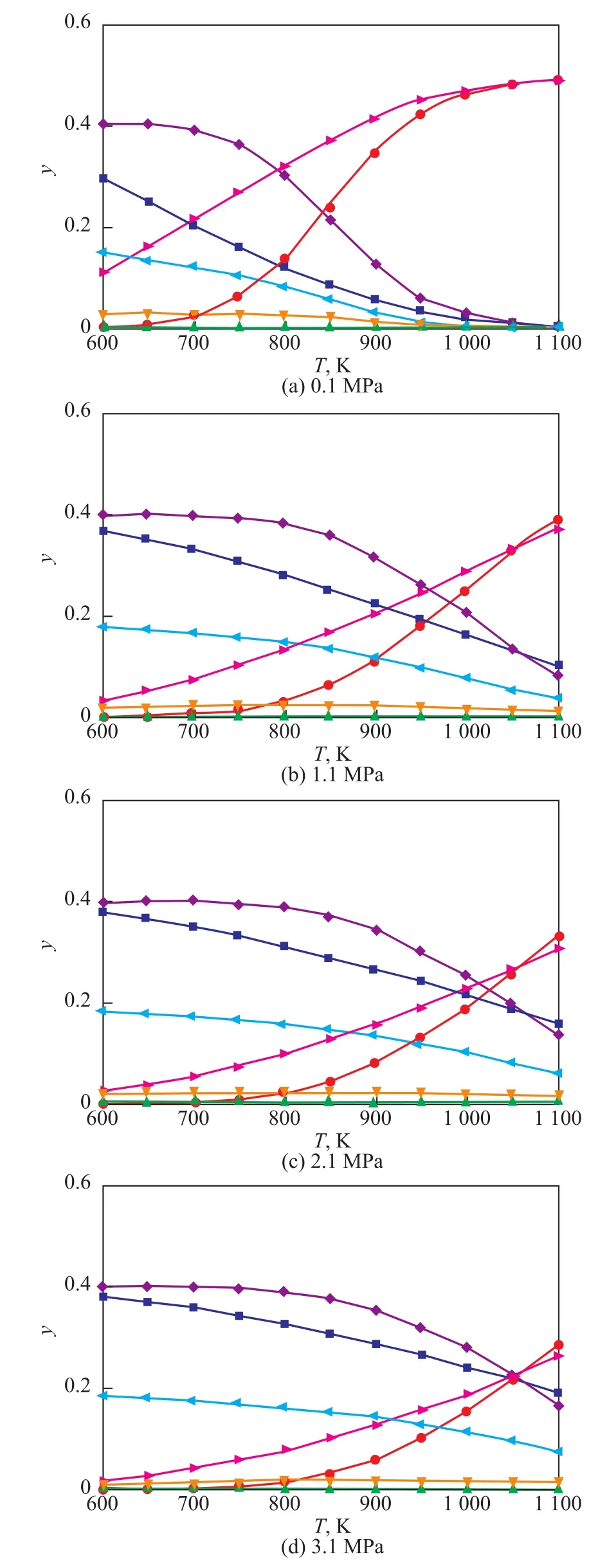
Figure 2 Equilibrium composition for the production of olefins
As shown in Figure 2, the mole fraction of H2O decreases and that of H2and CO increases with an increasing temperature, and the mole fraction of H2is higher than that of CO. In other words, coal reacts increasingly more easily upon water vapor with an increasing temperature.The mole fraction of CO2is very high when the reaction temperature is low but decreases very rapidly with an increase in the reaction temperature.
The general equation of the entire reaction system is as follows:

As shown in Eq. 3, the content of generated CO2is 0.5n times that of the olefins. The difference in the chemical reaction stoichiometry coefficient between the gas reactant and the gas resultant is as follows.

Therefore, an increasing pressure is not favorable to the formation of olefins. The relationship between Kyand P can be described as follows.

Formula 5 shows that the reactions generating different olefins have different “sensitivities” to pressure. In other words, the effect of pressure on the olefin-generating reactions is greater for olefins with more C atoms.Therefore, in this reaction system, the effect of the pressure on the reaction generating C2H4is relatively small, and the effect of the pressure on the reaction generating C3H6is intermediate, while the effect of the pressure on the reaction generating C4H8is greatest.However, under high pressure, the effect of the pressure on the reaction system is slight, and the reaction system is mostly affected by the temperature. Thus, the Gibbs free energy for olefin-generating reactions varies strongly with a change in the temperature, while the CO2yield varies strongly with a change in the temperature as well.
3.2 Analysis of yields of C2—C4 olefins
Figure 3 shows the relationship between the yields of C2—C4olefins and the reaction temperatures under different constant pressures. In Figure 3, the abscissa axis represents the reaction temperature, and the ordinate axis represents the yields of C2—C4olefins.
As shown in Figure 3, the influence of temperature on the olefins yields is quite strong when the reaction pressure is 0.1 MPa, and the yields change rapidly in the studied temperature range; when the reaction pressure is greater than 1.1 MPa, the influence of temperature on the olefins yields becomes weak and the yields also decrease, because a high temperature is unfavorable to the C1synthesis reaction.

Figure 3 Relationship between the yields of C2—C4 olefins and reaction temperatures under different constant pressures
Figure 4 shows the relationship between the yields of C2—C4olefins and the reaction pressures under different constant temperatures. In Figure 4, the abscissa axis represents the reaction pressure, and the ordinate axis represents the yields of C2—C4olefins.

Figure 4 Relationship between the yields of C2—C4 olefins and the pressure at different constant temperature
As shown in Figure 4, when the olefins yields increase with an increasing pressure, the trend of increase becomes mild at the same temperature. When the reaction pressure is greater than 1.1 MPa, the influence of the pressure on the olefins yields becomes weak. Therefore, it is helpful to increase the pressure for the reaction system, which can be only effective to an appropriate extent.
4 Analysis of Operating Conditions of Reaction Generating C2—C4 Olefins
According to the calculation and analysis described above, the synergies or restraining effects that exist between the operating parameters can affect the equilibrium composition of this reaction system. For example, the increase in temperature is beneficial to the formation of CO and H2, but the contents of CH4and CO2decrease rapidly; meanwhile, with an increasing pressure, the concentration of CO and H2decreases and the concentration of CO2and C2—C4olefins increases. The reaction temperature and pressure can be appropriately adjusted in order to obtain more C2—C4olefins, and the feed ratio also has a strong impact on the reaction equilibrium composition in the coal gasification reaction; therefore, we take the approach of fixing one parameter and discussing how the feed ratio and other parameters can impact the reaction equilibrium composition. Generally, chemical reactors can withstand only certain pressure values, and thus, the reaction pressure is usually the first determined factor.This section discusses the impact of the feed ratio and temperature on the reaction equilibrium concentration under a fixed pressure using the original software and analyzes the optimum operating conditions under different constant pressure values.
4.1 Interaction between the temperature and feed ratio
Figure 5 shows that the concentration of each component changes in the coal gasification coupling C1reaction system with a change in the temperature and feed ratio when the fixed pressure is 1.1 MPa.
As shown in Figure 5(a), the H2O content decreases and the conversion rate of H2O increases with an increasing temperature; the conversion rate of H2O decreases with an increasing feed ratio. The content of CO2decreases with an increasing temperature because a high temperature is unfavorable to the C1synthesis reaction, and the highest CO2content is achieved with an increasing feed ratio.When H2O/C ratio is equal to 0.8, the CO2content is higher than that of CO2obtained at other feed ratios,resulting in lower calorific values and C2—C4olefins contents in the entire reaction system, demonstrating that an appropriate feed ratio must be chosen.
As shown in Figure 5(b), the content of CO decreases and that of H2increases with an increasing feed ratio, and the highest CO content is achieved at a H2O/C ratio of 0.6.

Figure 5 The concentration of each component with a change in the temperature and feed ratio when the fixed pressure is 1.1 MPa
As shown in Figure 5(c), the total content of the C2—C4olefins decreases with the increasing temperature and feed ratio; in other words, a lower temperature and feed ratio can help to improve the total content of the C2—C4olefins. At a temperature of 748 K and a H2O/C ratio of 0.6,the total content of the C2—C4olefins reaches a maximum value of 27.9%, and the conversion rate of water is 100%.This means that under a fixed pressure, the selection of the appropriate reaction temperature and feed ratio can achieve a higher selectivity of olefins.
Figure 5(d) shows the content of C2H4, C3H6and C4H8.It can be seen from Figure 5(d) that a small amount of water vapor can improve the total content of the C2—C4olefins due to the easier generation of hydrogen and saturated hydrocarbons when the water vapor content is high coupled with an abundance of hydrogen atoms in the system.
4.2 Analysis of optimum operating conditions
As shown in Figure 4, the pressure also has an effect on the reaction equilibrium contents and the yields of olefins. Therefore, it is necessary to analyze the optimum operating conditions by taking the reaction temperature and feed ratio as the optimized independent variables and the maximum mole fraction content of C2—C4olefins as the optimized objective function under different reaction pressures.
(1)Under 0.1 MPa
Figure 6 shows that for a reaction pressure of 0.1 MPa,the optimum operating conditions of the equilibrium system are as follows: a H2O/C feed ratio of 0.6, a reaction temperature of 648 K, a maximum mole fraction of C2—C4olefins of 28.24%, and a H2O conversion rate of 100%.
(2)Under 2.1 MPa

Figure 6 Operating conditions of the equilibrium system under 0.1 MPa
Figure 7 shows that under a reaction pressure of 2.1 MPa the optimum operating conditions of the equilibrium system cover: a H2O/C feed ratio of 0.6, a reaction temperature of 798 K, a maximum mole fraction content of C2—C4olefins of 27.83%, and a H2O conversion rate of 99%.

Figure 7 Operating conditions of the equilibrium system under 2.1 MPa
(3)Under 3.1 MPa
Figure 8 shows that under a reaction pressure of 3.1 MPa,the optimum operating conditions of the equilibrium system cover: a H2O/C feed ratio of 0.6, a reaction temperature of 748 K, a maximum mole fraction content of C2—C4olefins of 27.79%, and a H2O conversion rate of 100%.
In summary, when taking the reaction temperature and feed ratio as the optimized independent variables and the maximum mole fraction content of C2—C4olefins as the optimized objective function under different reaction pressures, the optimum operating conditions include: a reaction pressure of 0.1 MPa, a H2O/C feed ratio of 0.6,a reaction temperature of 648 K, and a maximum mole fraction content of C2—C4olefins of 28.24%. However,during the actual reaction, the reaction temperature is generally higher than 750 K on account of achieving an appropriate gasification rate.

Figure 8 Operating conditions of the equilibrium system under 3.1 MPa
5 Conclusions
Thermodynamic analysis of the formation of low-carbon olefins via the coal gasification coupling C1reaction can be used to obtain the system’s thermodynamic state parameters. The results can help to correctly predict the performance of the gasification coupling reactor and provide an important basis for the reactor structure design and the determination of the optimal technological conditions. These results can provide an important guidance for researchers regarding the potential of catalysts by obtaining the ideal yields of low-carbon olefins and the optimum operating conditions. This study can offer a measure of the gap between the existing catalysts and the ideal catalysts, thereby enabling researchers to avoid unguided, trial-and-error searches for high-efficiency catalysts. In this paper, synergy or constraints are observed among the operating parameters, namely, the pressure, the temperature, and the feed ratio. When one parameter is fixed to analyze the impacts of the others, we not only can analyze the relationships of the mutual synergy or constraints among the operating parameters, but also can obtain the information for the optimized operating conditions of the reaction system. Therefore, thermodynamic study is of important theoretical and practical significance.
Acknowledgements:This research project is supported by the National Natural Science Foundation of China(NSFC Grant No. 51706168).
- 中国炼油与石油化工的其它文章
- Application of Big Data Technology in Evaluation of Operating Status of High-pressure Hydrogenation Heat Exchanger
- Removal of Basic Nitrogen Compounds from Coker Diesel by Eutectic Ionic Liquid
- Structural Characterization of Petroleum Molecules by CID FT-ICR MS with Narrow Isolation Window
- Conversion of Biomass to Hydrocarbon-rich Bio-oil via Microwave-assisted Catalytic Pyrolysis: A Review
- Comparison of Lubricities of Two Novel Benzotriazole Derivatives Used as Additives in Water-Glycol Hydraulic Fluid
- Identification of NixSy on Industrial Spent S Zorb Sorbents by Using XPS and TPR-MS

