Identification of NixSy on Industrial Spent S Zorb Sorbents by Using XPS and TPR-MS
Yuan Hui; Qiu Limei; Xu Guangtong; Xin Mudi; Shi Yanqiang; Chen Shuai; Zou Kang
(SINOPEC Research Institute of Petroleum Processing, State Key Laboratory of Catalytic Materials and Reaction Engineering, Beijing 100083)
Abstract: Three industrial spent S Zorb sorbents extracted from production line were studied with XRD, TPR-MS and XPS.The characterization results of XPS and TPR-MS identified the existence of amorphous NixSy on industrial spent S Zorb sorbents, while the existing XRD quantitative analysis methods can only provide the long-range order in phase information and the grain size of Ni metal. XPS is a powerful tool to investigate the chemical states of nickel atom and the depthwise distribution of nickel species on S Zorb sorbent. NixSy and Ni metal species coexist on the industrial spent sorbents, and their percentages to total nickel slightly change with the operating conditions in the surface layer. It proves that NixSy is a stable intermediate product rather than a transition state. The information can contribute to the better elucidation of S Zorb desulfurization mechanism and offer a new direction for selectivity optimization of industrial S Zorb sorbents.
Key words: S Zorb; sorbent; nickel sulfide; reaction mechanism; X-ray photoelectron spectroscopy
1 Introduction
Deep removal of sulfur from the gasoline has effectively been driven by the needs to protect the environment and to meet the upgraded fuel quality standards[1-2]. The S Zorb adsorptive desulfurization process, which can keep the S content of gasoline to be less than 10 μg/g along with a minimum octane loss and a highest liquid yield,is becoming a major technology for the production of clean gasoline with an ultra-low sulfur content. Since the technology was licensed by ConocoPhillips in 2007 and improved further by SINOPEC, more than 30 industrial plants have been put on stream[3-4]. To solve the problems appearing along with the operation evolution aimed at securing the smooth running of the plants, the SINOPEC Research Institute of Petroleum Processing analyzed the cause of sorbent deactivation[5], developed the rapid quantitative phase analysis method, set up the activity evaluation model[6-7], and successively investigated the distribution and phase transformation of zinc and sulfur in the sorbent[8-12]. The active phase of S Zorb sorbent involves ZnO and Ni metal particles supported on perlite.Based on the overall mechanism tentatively described by Babich[13], the Ni metal of S Zorb sorbents promotes the decomposition of organic sulfur compounds and transfers S to ZnO, which acts as an acceptor in the reaction unit. To date, few studies were concentrated on the characterization of Ni in the S Zorb sorbent and even less was on the specific industrial S Zorb system[5,11].So far the rapid quantitative phase analysis method by X-ray powder diffraction (XRD) is the only way to get the phase information of Ni on the industrial S Zorb sorbents. According to the results for studying hundreds of industrial spent S Zorb sorbents, most Ni existed as Ni metal, and neither NiO nor NixSywas detected[6,9,13]. In the present paper, the X-ray photoelectron spectroscopy(XPS) and the temperature programmed reduction-mass spectroscopy (TPR-MS) were selected to investigate typical S Zorb sorbents (spent sorbents), which were discharged from the industrial unit after use. As far as we know, it is the first time to identify the existence of NixSyand study the distribution of the Ni and NixSyphases on the industrial spent S Zorb sorbents. These results will provide an evidence for S Zorb desulfurization mechanism and update our knowledge on selectivity optimization of S Zorb sorbents and their industrial application.
2 Experimental
One spent S Zorb sorbent sample (denoted as ZH140902DS) was collected from a 1.8 Mt/a industrial unit at the SINOPEC Zhenhai Petrochemical Company on September 2, 2014. The other two spent S Zorb sorbent samples (labeled as QD150512DS and QD150516DS)were gathered from the running unit of SINOPEC Qingdao Petrochemical Company on May 12 and May 16, 2015, respectively. During the operating cycle,fresh sorbent was added to increase the efficiency for desulfurization of FCC gasoline at regular intervals.Ni2S3, NiS and ZnS (analytical grade reagents) were purchased from Alfa Aesar of USA as references.
The XPS experiments were performed using a Thermo Fisher ESCALAB 250 spectrometer equipped with a 150 W monochromatic Al Kα source. The base and analysis pressures were about 5×10-8Pa and 4×10-7Pa,respectively. To provide chemical environmental information, narrow scan was recorded for certain elemental core levels with a pass energy of 30 eV to obtain the high-resolution spectra. The obtained data were dealt with Avantage program provided by the Thermo Fisher Scientific Corporation. The charging effect was calibrated using C1s line at 284.8 eV from the deposited or adventitious carbon. The deconvolution of the spectra was made using the mixed Gaussian-Lorentzian functions with an iterative least-squares computer program.The XPS depth profiling was performed on the same apparatus. The surface erosion was accomplished by the 3-keV Ar ions from an EX05 ion source. The argon beam was rastered over an area of 3 mm×3 mm around the point of impact of the ion beam. It must be pointed out that the samples were not rotated during the sputtering.
The TPR-MS experiments were carried out by using an AutoChem II 2920 TPR apparatus coupled with a Pfeiffer Omni Star GSD200 mass spectroscope. The amount of sorbents was about 300 mg in each experiment. The sample was firstly purified in Ar gas with a flow rate of 50 mL/min at 323K for 30 min. Secondly, the reaction gas was switched to H2-Ar gas (at a volume ratio of 1:9)with a rate of 50 mL/min at 323 K for 30 min. Thirdly,the temperature was increased from 373 K to 973 K at a temperature increase rate of 10 K/min. At the same time,a mass spectrum of 34 (relative molecular mass of H2S is 34) is monitored by MS.
The XRD experiments were conducted on a Rigaku TTR-III powder diffractometer using Cu Kα radiation(λ=0.15406 nm) at a tube voltage of 40 kV and a tube current of 250 mA with a step size of 0.02° and a scan rate of 0.4(°)/min ranging from 10° to 80°. The quantitative analysis of different phases was conducted using the whole-pattern refinement technique of Rietveld method. The characteristic diffraction peaks of Ni existing in S Zorb sorbent appeared at 2θ of 44.4° and 51.8°, corresponding to (111) plane and (200) plane,respectively. The diffraction peak located at 44.4° is an independent one, while the diffraction peak located at 51.8° is overlapped with the characteristic diffraction peaks of the (103) plane of ZnS. Therefore, when the background and Kα2of the patterns is subtracted, the grain size of Ni can be calculated by the Scherrer equation using the diffraction peak located at 44.4° to avoid the problem caused by peak fitting[15].
3 Results and Discussion
Table 1 exhibits quantitative phase results of the sorbents derived from XRD analysis using the Rietveld method[6].ZnO, ZnS, ZnAl2O4, etc. are the main zinc-containing species of spent S Zorb sorbents, while Ni and ZnNi3C are the only two nickel-containing species. The powder-XRD patterns (Figure 1) show that there were no distinct diffraction peaks appearing at about 37.2°, 43.3°, 62.8°;29.9°, 34.5°, 45.6°, 53.2°; 21.7°, 31.1°, and 37.7°, which could be well indexed to NiO (JCPDS 01-089-7390), NiS(JCPDS 01-077-1624), and Ni3S2(JCPDS 01-085-1802),respectively. Therefore, the crystal phases of NiO, NiS,and Ni3S2could not be confirmed by X-ray diffraction analysis of the spent S Zorb sorbents. The grain size of Ni metal in ZH140902DS, QD150512DS, and QD150516DS calculated by the Scherrer equation was 8.1 nm, 17.0 nm,and 26.9 nm, respectively. It revealed that the existing XRD quantitative analysis methods only can provide the long-range order in phase information of nickelcontaining species.

Table 1 Quantitative phase results for zinc-containing and nickel-containing species of S Zorb sorbents identified by XRD analysis w, %

Figure 1 The powder-XRD patterns of different spent S Zorb samples
Identifying the amorphous phases of nickel and their distribution is vital to understanding their essential role played in catalysis for designing and synthesizing more active and selective catalysts, which can be verified by the analytical results obtained by using TPR-MS and XPS.The TPR-MS diagrams for the relative content of H2S(m/z = 34) in the spent S Zorb sorbents and references are illustrated in Figure 2. The TPR-MS profile of ZnS demonstrates that a small amount of H2S is produced at 873 K with the increase in temperature, and the peak temperature is much higher than 973 K, while the mass spectra for H2S (m/z=34) of Ni2S3and NiS show the obvious peaks at about 873 K and 823 K, respectively.Compared with the references, the TPR-MS curves for H2S (m/z=34) in ZH140902DS, QD150512DS, and QD150516DS display similar peak temperature as those of Ni2S3and NiS determined at below 973 K. It provided evidence that the amorphous NixSyspecies existed in spent S Zorb sorbents. It should be noted that the reduction peak temperature for H2S of spent S Zorb sorbents was different from each other, and it might occur because of the different NixSyphases, which should be further studied in the future.XPS is an effective tool which can provide qualitative,quantitative and chemical state information concerning the elements on the solid surface[11]. The relative proportions of nickel functionality concentrations were interpreted by means of the peak area ratios of the XPS spectra. The assignments of the nickel forms were based on our reference analysis and other research reports[16-18].The signal of a nickel single species was composed of two peaks representing 2p3/2and 2p1/2components, the relative intensity ratio of which is 2:1, with their energies being separated by 1.2 eV. We performed the peaksynthesis for Ni2p by mixed Gaussian and Lorentzian line shapes with full width at a half maximum of 2.60 eV for each nickel species. According to the curve resolution method, the deconvolution spectra of Ni2p2/3envelope are shown in Figure 3. For all the spent sorbents, three kinds of nickel species were detected, viz.: Ni metal, nickel sulfide, and nickel oxide. The binding energies at about 853.1 eV, 854.1 eV, and 856.7 eV are assigned to Ni2p peak from Ni metal, Ni2p peak from Ni-S of NixSy, and Ni2p peak from Ni2+of NiO, respectively. Moreover, the analysis results obtained by the deconvolution method are summarized in Table 2. The results indicate that nickel oxide is the major phase for nickel species on the surface of spent S Zorb sorbents, which can be attributed to the oxidation of nickel metal. The n(NixSy)/ n(Ni) and n(NixSy)/ n(Zn) ratios of these three spent sorbent samples range from 0.09 to 0.16, and 0.03 to 0.05, respectively. It is proved that nickel sulfide species (NixSy) really exist on the surface of spent S Zorb sorbents.
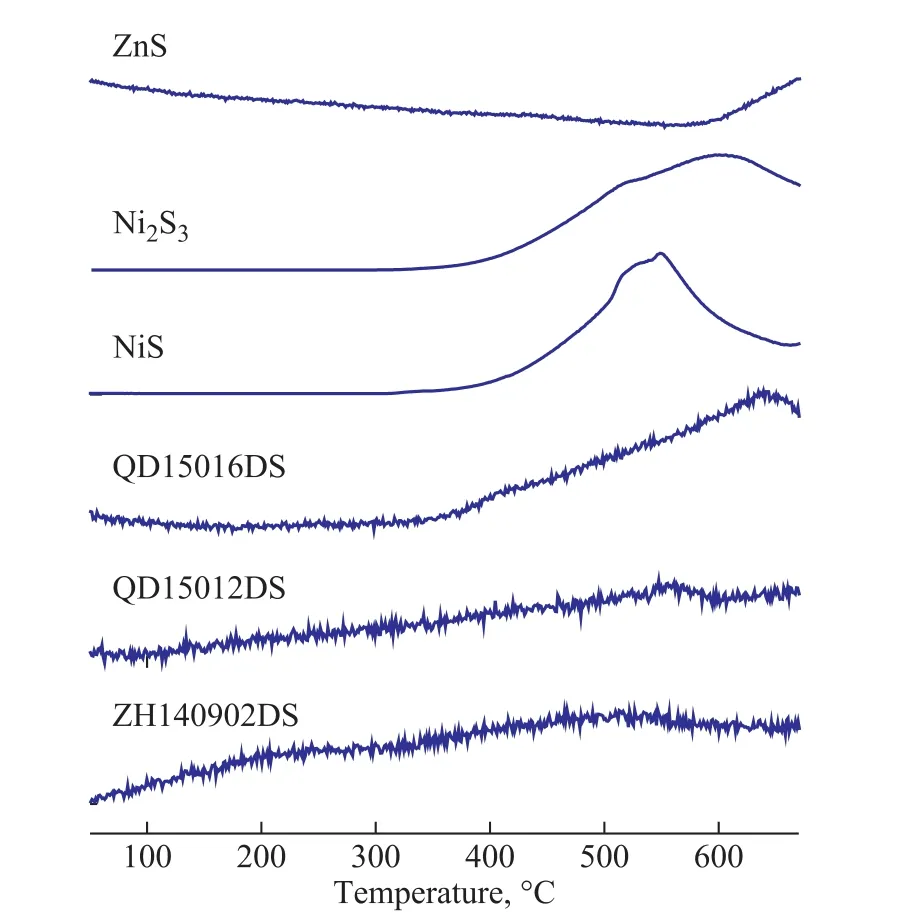
Figure 2 TPR-MS diagrams for the relative content of H2S(m/z=34) in spent S Zorb sorbents and references

Figure 3 Peak-fitting of Ni2p2/3 XPS peaks for spent S Zorb sorbents
To obtain the depthwise distribution of nickel species,the Ar+profiling XPS was preformed. Figure 4 and Table 2 present the XPS results for ZH140902DS before and after Ar+sputtering. It can be seen that the nickel metal/zinc ratio increased after Ar+sputtering, which could be attributed to both carbon deposits and adventitious carbon on the sorbent surface. It is also found that the ratio of nickel metal to nickel oxide increased along with the argon profiling, which was resulted from the oxidation of nickel metal on the surface. When the time of argon ion sputtering was longer than 420 s, the NiO content was less than 2% among total Ni, while the Ni metal content was higher than 80%, and the n(NixSy)/ n(Ni) and n(NixSy)/n(Zn) ratios were still equal to 0.18 and 0.07,respectively. When the sputtering time was longer than 1080 s, NixSycould not be detected and almost all nickel species existed as Ni metal. It has revealed that Ni metal made up the dominate species of nickel from approximate 10 nm of depth inside the surface to the core, but nickel sulfide species (NixSy) just could stably exist in the surface layer in about 0—10 nm of depth inside the surface of the spent S Zorb sorbents. These results were consistent with the study by TPR-MS mentioned therein.

Figure 4 Quantitative results of Ni2p deconvolution for ZH140902DS with different etching time
The S Zorb technology is one of the most competitive ultra-deep catalytic hydrodesulphurization processes thanks to its high selectivity. Based on our aforementioned findings and other research results[13], a mechanism for the S Zorb sorbent and the role of active components in the sorbent can be clarified. The reactions cover the following steps:

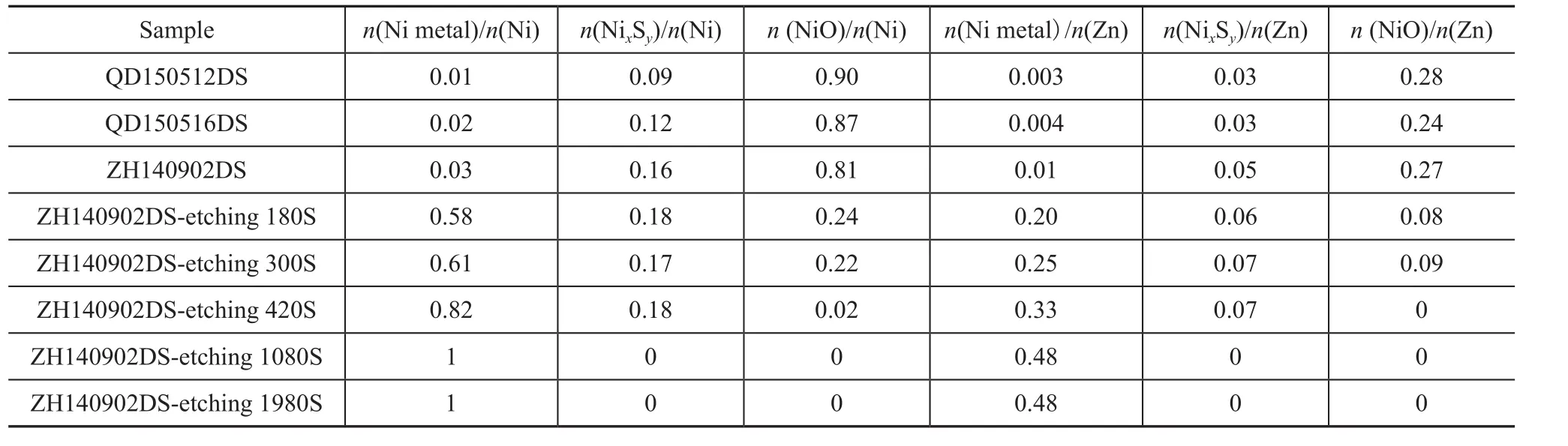
Table 2 Quantitative phase results from deconvolution results of Ni 2p2/3 XPS spectra
First of all, the fresh or regenerated sorbent was treated by H2to reduce NiO to Ni. Because of the electronegativity, S atoms in the organic sulfur-containing compounds move gradually close to the Ni atom in nickel metal. Then the S-C bonds break down and the S atoms are completely adsorbed on the Ni atoms to form NixSyin different states (such as NiS, Ni2S3,etc.), with the remaining hydrocarbons making up the oil fraction. ZnO acts as an acceptor of sulfur,which is released during regeneration of the surface nickel sulfide species. At the same time, ZnS is finally produced and the active Ni metal is regenerated. With the designed recycle, the integral desulfurization and regeneration process is continued and the reactive efficiency can be retained[11]. However, not all the surface nickel sulfide species are regenerated to form active Ni metal, so NixSycan exist in the surface layer of spent S Zorb sorbents. NixSyis a stable intermediate product rather than a transition state. Moreover, the amount of NixSyslightly varies with the operating time and conditions. The quantum chemical calculations made by Long[14]revealed that Ni metal in comparison with NiS exhibits high adsorption activity to thiophene,but has a relatively low adsorption activity to high octane number olefins and aromatics. So it is necessary to thoroughly study the catalytic performance for desulfurization and hydrogenation of Ni and NixSyin the S Zorb process. Selective optimization of Ni species distribution in the S Zorb sorbents is beneficial to the realization of deep desulfurization and reduction of octane loss simultaneously.
4 Conclusions
The existing XRD quantitative analysis methods only can provide the long-range order in phase information of nickel and the grain size of Ni metal. The XRD results show that there are no crystal phases of NiS and Ni3S2in spent S Zorb sorbents, while the TPR-MS and XPS characterization was developed for identifying the amorphous phases of nickel and their distribution. The results have provided evidence that amorphous NixSyspecies exist in the surface layer of spent S Zorb sorbents and their amount slightly varies with the operating time and conditions. The Ar+profiling XPS was preformed to obtain the depthwise distribution of nickel species.It reveals that Ni metal is dominant and nickel sulfide species (NixSy) accounts for about 10%—20% of total nickel in the 0—10 nm surface level. These results can contribute to clarifying the S Zorb desulfurization mechanism and offering an alternative direction for selectivity optimization of S Zorb sorbents and their industrial application.
Acknowledgment:The authors gratefully acknowledge the funding of the project by SINOPEC (No. 114138).
- 中国炼油与石油化工的其它文章
- Application of Big Data Technology in Evaluation of Operating Status of High-pressure Hydrogenation Heat Exchanger
- Removal of Basic Nitrogen Compounds from Coker Diesel by Eutectic Ionic Liquid
- Thermodynamic Analysis of Formation of Low-carbon Olefins via Coal Gasification Coupling C1 Reaction
- Conversion of Biomass to Hydrocarbon-rich Bio-oil via Microwave-assisted Catalytic Pyrolysis: A Review
- Comparison of Lubricities of Two Novel Benzotriazole Derivatives Used as Additives in Water-Glycol Hydraulic Fluid
- Structural Characterization of Petroleum Molecules by CID FT-ICR MS with Narrow Isolation Window

