超低酸催化纤维素一锅法制取糠醛工艺优化
徐桂转,陈炳霖,张少浩,王冬祥,李 凯
超低酸催化纤维素一锅法制取糠醛工艺优化
徐桂转1,2,陈炳霖1,2,张少浩1,2,王冬祥1,李 凯1
(1. 河南农业大学机电工程学院,郑州 450002; 2. 农业部农村可再生能源新材料与装备重点试验室,郑州 450002)
5-乙氧基甲基糠醛(5-ethoxymethylfurfural,EMF)是一种新型液体生物燃料,具有较高的能量密度和优良的燃烧性能,被视为未来潜在的燃料替代物。该文利用酸质量为乙醇质量0.1%的硫酸催化纤维素与乙醇在高压反应釜中一锅法制取EMF。首先进行单因素试验,然后利用响应面Box-Behnken模型研究不同影响因素对超低酸催化纤维素制取EMF收率的影响,对EMF制取工艺条件进行优化。结果表明:在反应时间、反应温度和底物浓度3个影响因素中,反应时间对超低酸催化纤维素一锅法制取EMF收率影响最大。根据Box-Behnken模型获得最佳反应条件:反应温度200 ℃、反应时间75 min、底物浓度30 g/L进行试验验证,EMF实际平均收率为14.93%,与理论预测值相对误差为2.03%,说明该模型具有较好的预测性。该研究首次提出以超低酸催化纤维素直接制取EMF,并优化了超低酸催化纤维素制取EMF的工艺条件,可为成本低廉、产量丰富的纤维素生物质制取EMF提供借鉴和参考。
纤维素;酸度;生物燃料;超低酸;5-乙氧基甲基糠醛;一锅法
0 引 言
生物质经酸催化水解、脱水可生成重要的平台化合物—5-乙氧基甲基糠醛(5-Ethoxymethylfurfural, EMF),被认为是极具潜力的液体燃料[1]。EMF的能量密度为 8.7 kW·h/L具有与汽油(8.8 kW·h/L)和柴油(9.7 kW·h/L)相近的能量密度,远高于乙醇的能量密度(6.1 kW·h/L),能与石化柴油以5%~25%的比例混溶,而其颗粒排放物、氮氧化物及硫氧化物排放量远远低于石化柴油,可以作为生物柴油替代石化柴油或作为燃料添加剂使用[2-3]。
目前,用于制备EMF的原料主要有:5-羟甲基糠醛(5-Hydroxymethylfurfural, HMF)等平台化合物和果糖、蔗糖、菊粉、葡萄糖等生物质糖类。以HMF为原料时,EMF收率通常高于83%[4-5],如Liu等[5]以HMF为原料,EMF产率高达91.5%。以果糖为原料时,EMF产率为60%~90%[6-8];以菊粉为原料时,EMF产率为53%~62%[9-10];以蔗糖为原料时,EMF产率为33~43%[11-12];以葡萄糖原料时,由于受葡萄糖异构化限速的影响,EMF的产率通常较低,如Lew等[13]利用复合催化剂催化葡萄糖制取EMF,其产率最高为31%。可以看出,以生物质糖类为原料时,其内部果糖小分子含量越高,则越容易发生反应,得到的EMF产率也越高,即EMF收率趋势大致为:果糖>菊粉>蔗糖>葡萄糖。以HMF和生物质糖为原料时,虽然EMF收率较高,但原料成本较高,工业化生产受到限制。
纤维素作为自然界中储量最为丰富的绿色可再生资源,其转化利用对社会的可持续发展意义重大[14]。然而,目前尚未有关于纤维素直接制备EMF的研究,已有的研究中[15-18],大多都是将纤维素先转化为中间产物HMF、5-氯甲基糠醛(5-Chloromethylfurfural,CMF)和5-溴甲基糠醛(5-Bromomethylfurfural,BMF)等,然后通过中间产物获得目标产物EMF,如Mascal等[16]将纤维素加入到含有5%氯化锂浓盐酸中,以二氯乙烷作为溶剂,65 ℃条件下回流、搅拌、萃取18 h后,再添加含有氯化锂的浓盐酸溶液继续萃取12 h,经过蒸馏将80%的纤维素转化为CMF,然后以乙醇为溶剂反应8 h得到EMF,收率达到95%。利用纤维素制取EMF的研究中,先转化为CMF、HMF和BMF等中间产物,再转化为EMF虽然可以获得较高收率,但依然存在以下主要问题:1)中间产物分离、提纯使制造工艺较为复杂、成本较高;2)液体催化剂使用量较大,对设备腐蚀性较强;3)反应时间过长;4)反应过程中添加的有机溶剂使后提取工艺复杂,增加了EMF生产成本[19-21]。
在制备EMF过程中,催化体系的选取是决定化学反应的重要因素,生物质醇解制备EMF的催化体系主要是由酸性催化剂组成,包括液体酸、固体酸和离子液体[4,22]等。传统液体酸容易腐蚀设备、污染环境,离子液体目前制备工艺较为复杂、成本较高,而超低液体酸对设备基本没有腐蚀,可以作为绿色催化体系看待[23],因此,超低液体酸对生物质的催化作用引起了研究者的关注[24-26]。在前期研究中,作者曾尝试利用超低酸催化生物质糖类一锅法合成乙酰丙酸乙酯(Ethyl levulinate, EL)和EMF,发现催化剂用量减少能够降低对设备腐蚀和对环境的危害[8]。受此启发,本研究尝试利用酸质量为乙醇质量0.1%的硫酸作为超低酸催化剂,在无溶剂情况下直接催化果糖和乙醇制取EMF。首先进行单因素试验,考察反应温度、反应时间和底物浓度3因素对EMF收率的影响;然后根据单因素试验结果设计响应面试验方案,对EMF反应工艺条件进行优化,以得到超低酸催化纤维素一锅法制取EMF的最佳工艺条件,为纤维素直接合成EMF提供一条绿色、经济的路径。
1 材料与方法
1.1 试验材料
微晶纤维素,Solarbi公司;浓硫酸,AR,山东双双化工有限公司;乙醇,AR,天津瑞金特化学品有限公司;纯净水,娃哈哈纯净水;乙腈,HPLC,美国VBS公司;甲醇,HPLC,湖北社文化工科技有限公司;EMF,HPLC,西格玛奥德里奇(上海)贸易有限公司;乙酸,HPLC,天津市科密欧化学试剂有限公司;滤纸和微孔滤膜等易耗品购自郑州本地经销商。
1.2 试验设备
美国安捷伦公司Agilent-1260LC高效液相色谱系统,包括G1311C四元泵、G1329B自动进样器、G1316A柱温箱和G1314F紫外检测器;JJ224BC型电子天平,常熟市双杰测试仪器厂;高压反应釜,巩义市英峪高科仪器厂;真空抽滤机,意大利VELP公司;GM-0.20型隔膜真空泵,溶剂过滤器,天津市津腾试验设备有限公司;TGL-16C型高速离心机,上海安亭科学仪器厂;SYU- 10-200DT型超声波清洗机,郑州市生元仪器有限公司。
1.3 分析与计算方法
EMF收率定义为产物中EMF的摩尔数与反应物(纤维素)的摩尔数之比,由公式(1)进行计算。
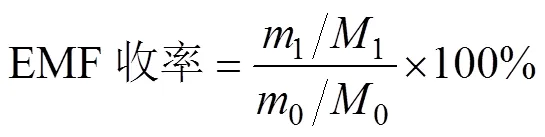
式中0和1分别为原料(纤维素)和EMF的质量,g;0(以单糖计)和1为原料和EMF的摩尔质量,分别为162和154。
1.4 试验方法
首先进行单因素试验,然后在单因素试验结果上进行响应面优化试验。单因素试验中考查反应温度、反应时间和底物浓度对超低酸催化纤维素制取EMF的影响。所有的试验均在200 mL具有机械搅拌装置的高压反应釜中进行,首先加入80 mL乙醇溶液作为反应溶剂,催化剂为0.1%(以乙醇质量为基准)的硫酸。反应釜初始搅拌速度为250 r/min,当达到设定温度时开始计时,并调节机械搅拌速度至500 r/min。当达到反应时间时立即取下反应器,将其放入冰水中冷却到室温,然后用滤纸过滤反应液,并将滤液用乙醇稀释,经10 000 r/min高速离心30 min,取上清液进行液相色谱分析。
2 结果与分析
2.1 单因素试验结果分析与讨论
2.1.1 反应温度对EMF收率的影响
反应温度在醇解过程中有非常重要的作用。在进行单因素试验时,设定反应时间为30 min,纤维素原料的底物浓度为20 g/L,乙醇用量为80 mL,催化剂为0.1%硫酸。分考察反应温度为180、190、200、210和220 ℃时对纤维素一锅法制取EMF收率的影响,结果如图1所示。
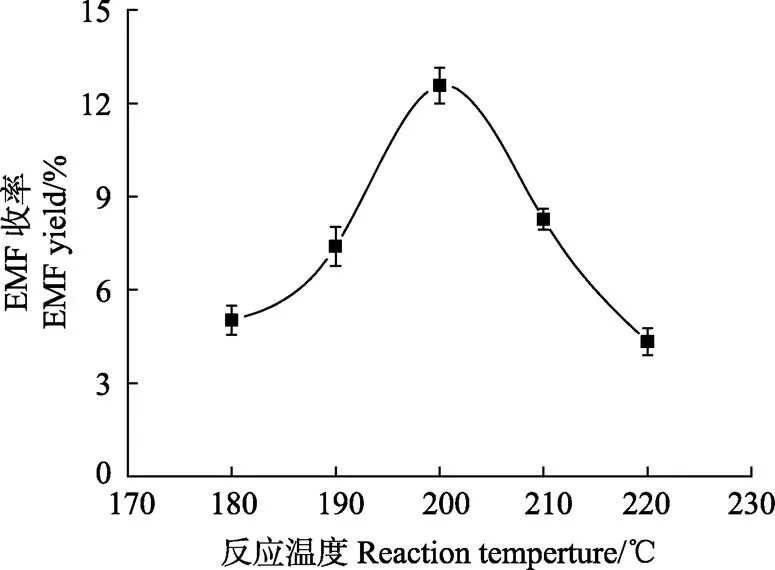
注:纤维素1.6 g,乙醇80 mL,反应时间30 min,硫酸用量0.064 g。
由图1可知,反应温度对EMF收率有显著的影响,EMF的收率随着反应温度的增加出现先增加后减少的趋势。在反应时间相同时,随着反应温度的提高,EMF的收率从反应温度为180 ℃时的5.02%提高反应温度为200 ℃时的12.57%。但当反应温度超过200 ℃时,EMF的收率出现明显的下降,这可能是因为反应温度的升高促使纤维素原料发生更多的副反应,也可能是由于目标产物在高温条件下发生降解,这些原因造成了目标产物的生成速率远远小于目标产物的降解速率,从而使EMF的收率下降[27],因此确定纤维素原料单因素试验最佳的反应温度为200 ℃。
2.1.2 反应时间对EMF收率的影响
选取反应温度200 ℃,纤维素底物浓度为20 g/L,考察反应时间分别为5、30、60、90和120 min时对纤维素生成EMF收率的影响,结果如图2所示。
1.3.1 方法 采用护理工作满意度调查问卷进行横断面调查,此次现况调查共发放问卷225份,回收220份,有效问卷回收率达97.78%。统计出患者对护理工作不满意的问题并分析原因,医院管理层领导根据原因制定落实改进措施,干预后20 d后复测患者满意度,比较干预前后病人满意度变化。
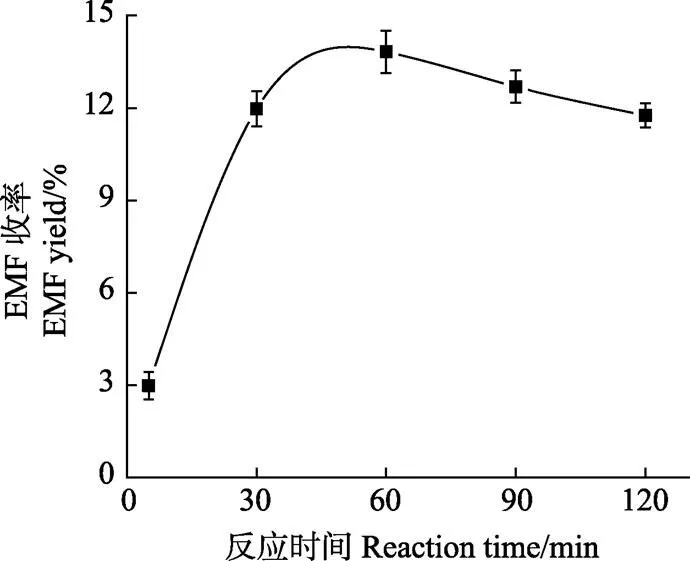
注:纤维素1.6 g,乙醇80 mL,反应温度200 ℃,硫酸用量0.064 g。
图2反映了EMF的收率随着反应时间的延长出现先增加后降低的变化趋势,在反应时间为5~30 min时,EMF收率快速增加,当反应时间达到60 min时EMF收率达到最大值,此时EMF的收率为13.32%,超过60 min后,EMF收率随着反应时间延长开始明显下降,当反应时间达到120 min时,EMF收率降到了11.76%。反应时间延长导致EMF收率下降可能是由于反应副产物附着在原料表面,使之与催化剂接触面积减小,同时有部分EMF发生分解,造成了EMF收率在反应60 min后随反应时间延长而降低。观察反应后产物溶液的颜色变化,发现随着时间的延长色泽逐渐加深,由浅黄色变化成深褐色,可能是由于反应时间的延长,产物中出现了更多不溶性腐殖质副产物而使溶液颜色变深[28]。适当的高温和较短的反应时间有利于目标产物EMF的生成。由此可知,反应时间的单因素试验最佳的反应时间为60 min。
2.1.3 底物浓度对EMF收率的影响
选取反应温度为200 ℃,反应时间为60 min,考察纤维素原料底物浓度为10、20、30、40和50 g/L时EMF的收率,结果如图3所示。
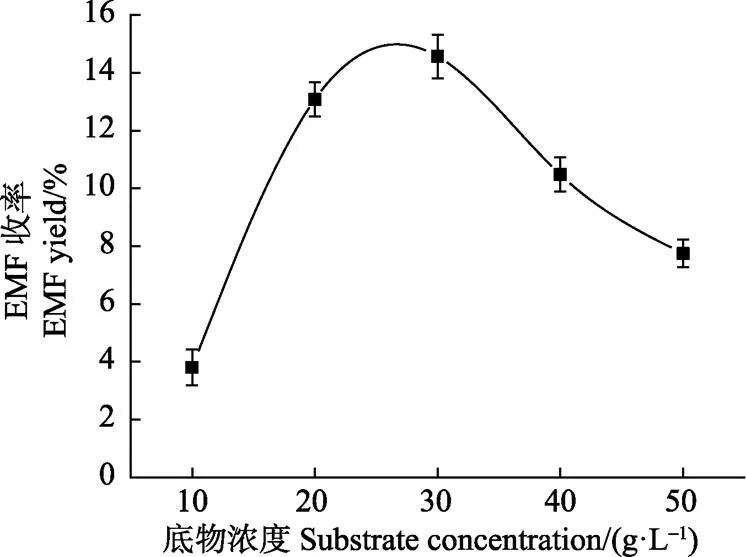
注:乙醇80 mL,反应温度200 ℃,反应时间60 min,硫酸用量0.064 g。Note: Ethanol 80 mL, reaction temperature 200 ℃, reaction time 60 min, H2SO4 0.064 g.
由图3可知,随着纤维素底物浓度增加,EMF收率出现先增加后减少的现象。在纤维素底物浓度为10 g/L到30 g/L的范围时,EMF的收率随着底物浓度的增加而快速增加,底物浓度为10 g/L时,EMF的收率为3.81%;当底物浓度增大到20 g/L时,EMF的收率增大到13.32%,此阶段EMF增长幅度最大。当纤维素浓度从20增大到30 g/L时,EMF收率增多了1.24%,达到了最大值,14.56%,此阶段EMF收率增长较为缓慢。分析原因,可能是底物浓度为10 g/L时,反应中底物含量较小,反应速率较慢,此时增加底物浓度有利于反应的进行,会增大反应速度,所以从10变化到20 g/L时,增多的底物加快了EMF的生成,使得EMF收率快速增大;但当底物浓度达到20 g/L时,底物浓度对反应的影响作用已减小,当底物浓度达到30 g/L时,体系中底物浓度已经达到最大,再增多底物反而促进了逆反应的进行。所以,从图3可以看出,当底物浓度从20变化到30 g/L时EMF收率变化较小,而从30增大到50 g/L时,EMF收率反而出现了明显的下降,可能是由于底物浓度的增大造成原料与催化剂的接触面积不够,抑制了EMF的生成,或者是由于目标产物与复杂的副产物发生结合等其他的原因,造成了目标产物EMF收率下降[29]。由此可以确定底物浓度单因素试验最佳的原料底物浓度为30 g/L。
2.2 响应面试验结果与讨论
2.2.1 响应面试验设计
选取反应温度、反应时间和反应物底物浓度3个因素为自变量,以EMF收率作为响应值,设计三因素三水平响应面试验。根据单因素试验结果,选取各因素与水平如表1所示。
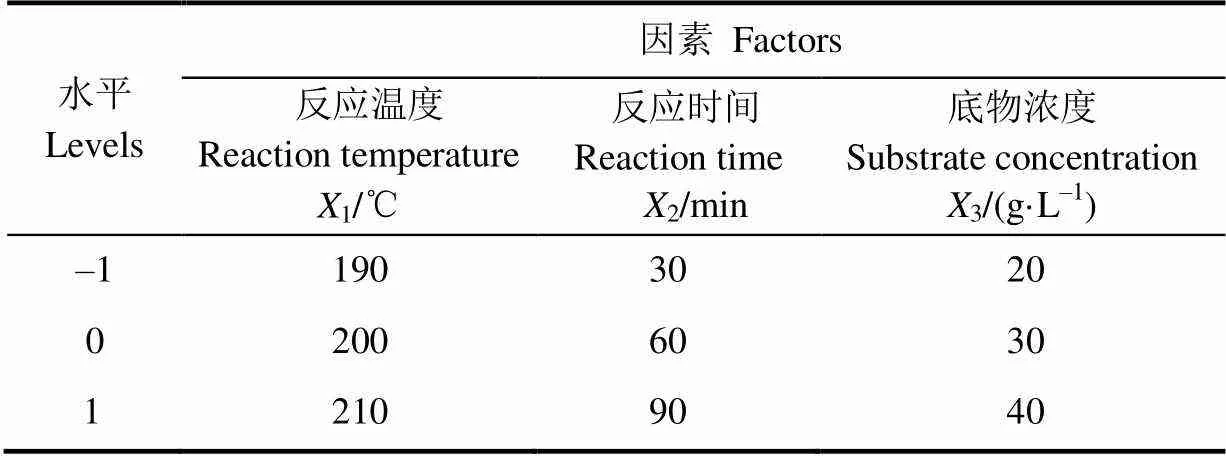
表1 响应面试验因素与水平
根据Box-Behnken设计原理,响应面试验设计方案及结果如表2所示。
2.2.2 响应面试验结果分析与讨论
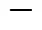
对回归方程各因素进行分析,该模型的一次项中,反应时间、反应温度和底物浓度的P值分别为0.051 5,0.114 1和0.241 3,均大于0.05,为不显著项;二次项值依次为0.000 2,0.174 3和0.020 7,其中,反应温度和底物浓度的二次项P值小于0.05,对EMF收率影响较为显著;从各因素交互的值可以发现,反应温度和反应时间以及反应时间和底物浓度的交互作用最强,值为0.064 3,其次是反应温度和底物浓度的交互作用。根据值得大小可判断各个因素对目标产物EMF收率的影响作用依次为:反应时间>反应温度>底物浓度。
表2 响应面试验设计及试验结果
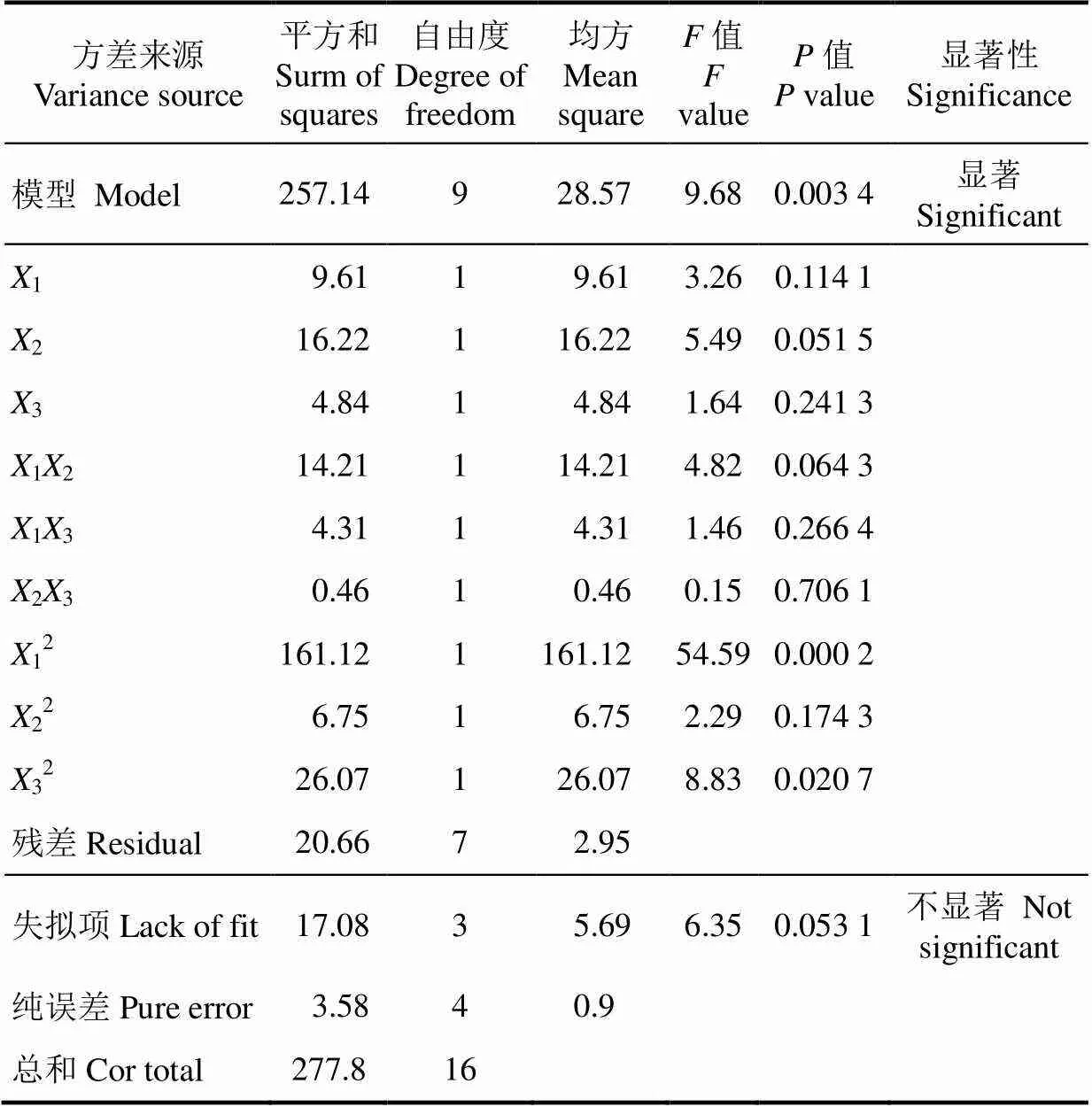
表3 回归模型方差分析
利用Design Expert 8.0.5数据统计分析软件对回归模型绘制响应面图,分析各个因素间对响应值的影响。图4为各交互项对目标产物EMF收率作用的三维曲面图及相应等高线图。从图4a可以看出,EMF收率随反应温度变化的曲面变化较为明显,而随反应时间呈现出明显的上升趋势。图4b为反应温度和底物浓度交互作用对EMF收率的影响,图中显示二维等高曲线呈椭圆形,三维曲面坡度明显,表明反应温度和底物浓度之间的交互作用对EMF收率的影响较大。在图4c中,EMF收率随着反应时间和底物浓度变化的曲面有明显的坡度,说明在该实验设计范围内,存在极值,即响应面的最高点。
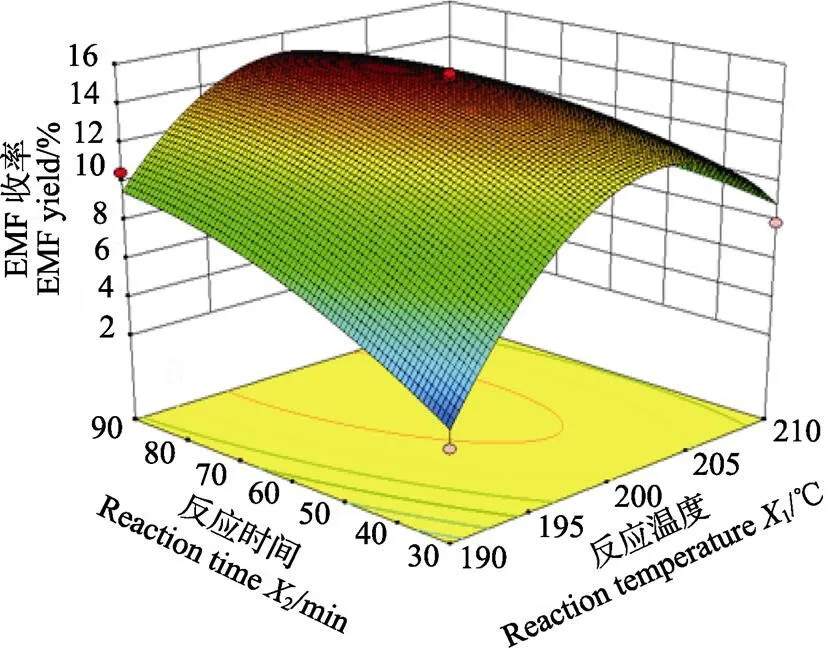
a.1和2交互作用
a. Interactions between1and2
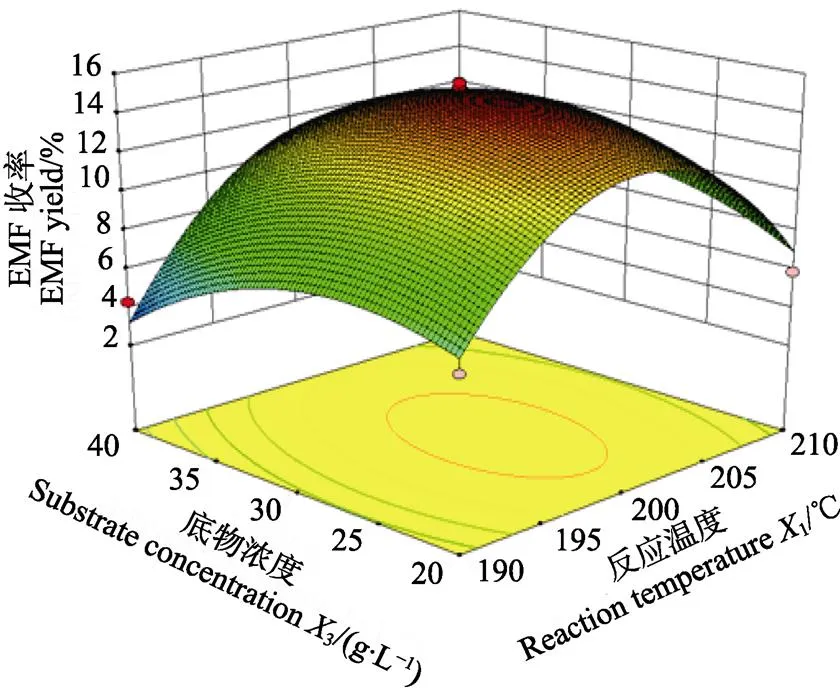
b.1和3交互作用
b. Interactions between1and3
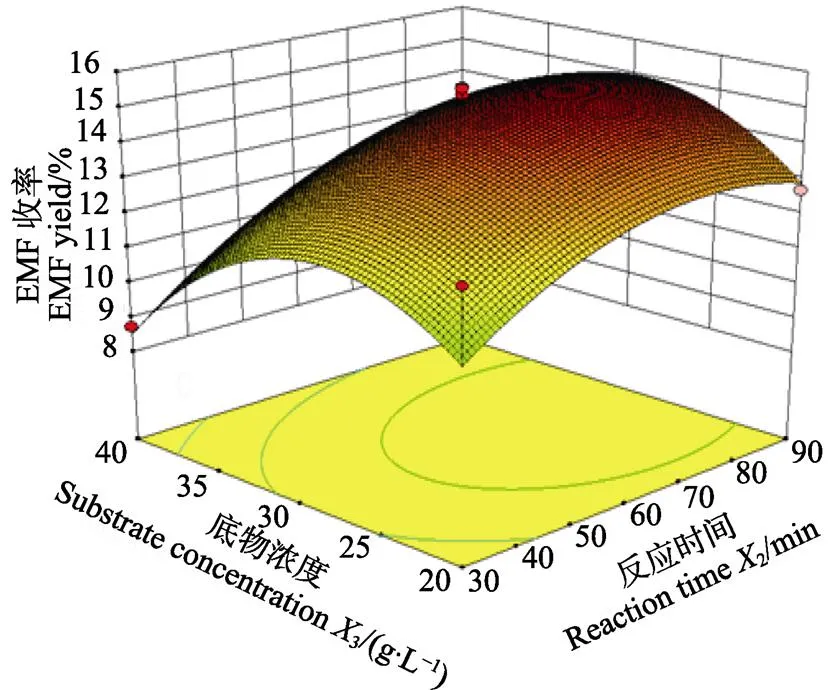
c. X2和X3交互作用
2.2.3 响应面优化工艺条件及验证
通过对回归模型的预测,超低质量浓度硫酸催化纤维素一锅法制取EMF的最佳工艺条件为:反应温度199.95 ℃、反应时间为76.50 min,底物浓度为28.79 g/L,此时预测的EMF最大收率为15.24%。考虑到工艺的可操作性,将最佳工艺条件调整为反应温度200 ℃、反应时间75 min、底物浓度30 g/L。在此反应条件下重复3次试验,EMF的平均收率为14.93%,与理论预测值的相对误差为2.03%,表明模型可靠。
2.3 纤维素醇解制备不同平台化合物的对比
将本文研究结果与文献中利用纤维素制取EMF和EL等平台化合物研究结果进行对比,结果如表4所示。Bredihhin等[15]用两步法催化纤维素制取EMF,虽然收率可以达到40%,但合成工艺较为复杂、反应时间较长,且涉及到中间产物的分离、提纯。除此之外,利用纤维素制取EMF目前还鲜有研究。本文利用一锅法催化纤维素,制备工艺较为简单、反应时间较短。由表4还可以发现,本文使用超低酸催化纤维素制取EMF的收率与文献中利用无机酸、固体酸、混合酸和离子液体催化制取EL和乙酰丙酸甲酯(methyl levulinate, ML)的收率相近,而本文中催化剂成本较低、反应时间较短,且无有机溶剂,对环境的不利影响较小。因此,本文研究成果将为纤维素生物质直接制取EMF提供借鉴和参考。
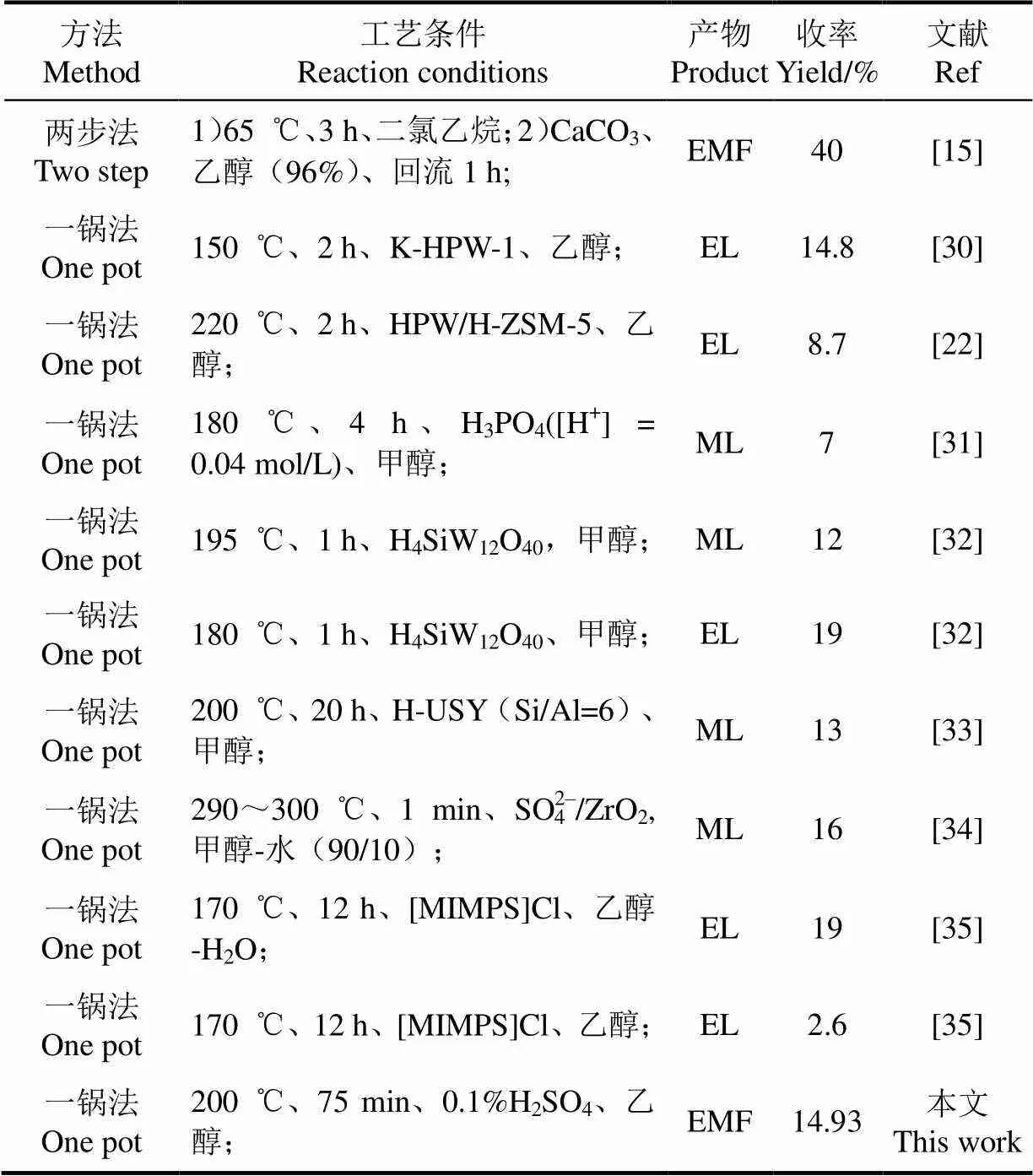
表4 纤维素制备不同平台化合物的对比
3 结 论
本研究利用单因素和响应面试验方法对超低质量浓度硫酸催化纤维素与乙醇一锅法制取EMF(5- ethoxymethylfurfural)进行了研究,考察了温度、反应时间和底物浓度3个影响因素对EMF收率的影响。结果表明,各个因素对目标产物EMF收率的影响作用依次为:反应时间>反应温度>底物浓度。根据Box- Behnken模型预测结果,选取最佳反应条件为:反应温度200 ℃、反应时间75 min和底物浓度30 g/L进行平行试验验证,得到EMF实际最大平均收率为14.93%,与理论预测值误差为2.03%。通过与其他以纤维素为原料制取EMF研究结果对比,利用超低质量浓度的硫酸催化纤维素一锅法制取EMF,具有工艺简单、反应时间短、不需有机溶剂和基本上对设备不腐蚀等优点。
本文研究结果表明,利用质量浓度超低的硫酸能有效催化纤维素一锅法制取EMF,为利用成本低廉、资源丰富的纤维素制取EMF的研究提供借鉴和参考。
[1] Zhang Zehui, Huber G W. Catalytic oxidation of carbohydrates into organic acids and furan chemicals[J]. Chemical Society Reviews, 2018, 47(4): 1351-1390.
[2] Bai Yuanyuan, Wei Lu, Yang Mengfei, et al. Three-step cascade over one single catalyst: Synthesis of 5-(ethoxymethyl)furfural from glucose over hierarchical lamellar multi-functional zeolite catalyst[J]. Journal of Materials Chemistry A, 2018, 6(17): 7693-7705.
[3] Huang Yaobing, Yang Tao, Lin Yuting, et al. Facile and high-yield synthesis of methyl levulinate from cellulose[J]. Green Chemistry, 2018, 20(6): 1323-1334.
[4] Hu Lei, Lin Lu, Wu Zhen, et al. Recent advances in catalytic transformation of biomass-derived 5-hydroxymethylfurfural into the innovative fuels and chemicals[J]. Renewable & Sustainable Energy Reviews, 2017, 74: 230-257.
[5] Liu Anqiu, Liu Bing, Wang Yimei, et al. Efficient one-pot synthesis of 5-ethoxymethylfurfural from fructose catalyzed by heteropolyacid supported on K-10 clay[J]. Fuel, 2014, 117: 68-73.
[6] Liu Bing, Zhang Zehui, Huang Kecheng. Cellulose sulfuric acid as a bio-supported and recyclable solid acid catalyst for thesynthesis of 5-hydroxymethylfurfural and 5-ethoxymethylfurfural from fructose[J]. Cellulose. 2013, 20(4): 2081-2089.
[7] Liu Anqiu, Zhang Zehui, Fang Zhongfeng, et al. Synthesis of 5-ethoxymethylfurfural from 5-hydroxymethylfurfural and fructose in ethanol catalyzed by MCM-41 supported phosphotungstic acid[J]. Journal of Industrial and Engineering Chemistry, 2014, 20: 1977-1984.
[8] Xu Guizhuan, Chen Binglin, Zheng Zhangbin, et al. One-pot ethanolysis of carbohydrates to promising biofuels: 5- ethoxymethylfurfural and ethyl levulinate: One-pot ethanolysisof carbohydrates to biofuels[J]. Asia-Pacific Journal of Chemical Engineering, 2017, 12(4): 527-535.
[9] Yuan Ziliang, Zhang Zehui, Zheng Judun, et al. Efficient synthesis of promising liquid fuels 5-ethoxymethylfurfural from carbohydrates[J]. Fuel, 2015, 150: 236-242.
[10] Chen Tao, Peng Lincai, Yu Xin, et al. Magnetically recyclable cellulose-derived carbonaceous solid acid catalyzed the biofuel 5-ethoxymethylfurfural synthesis from renewable carbohydrates[J]. Fuel, 2018, 219: 344-352.
[11] Guo Haixin, Duereh A, Hiraga Y, et al. Perfect recycle and mechanistic role of hydrogen sulfate ionic liquids as additive in ethanol for efficient conversion of carbohydrates into 5-ethoxymethylfurfural[J]. Chemical Engineering Journal, 2017, 323: 287-294.
[12] Kumari P K, Rao B S, Padmakar D, et al. Lewis acidity induced heteropoly tungustate catalysts for the synthesis of 5-ethoxymethyl furfural from fructose and 5-hydroxymethylfurfural[J]. Molecular Catalysis, 2018, 448: 108-115.
[13] Lew C M, Rajabbeigi N, Tsapatisis M. One-pot synthesis of 5-(ethoxymethylfurfural) from glucose using Sn-BEA and amberlyst catalysts[J]. Industrial & Engineering Chemistry Research, 2012, 51(14): 5364-5366.
[14] Deng Lin, Chang Chun, An Ran, et al. Metal sulfates- catalyzed butanolysis of cellulose: Butyl levulinate production and optimization[J]. Cellulose, 2017, 24(12): 1-13.
[15] Bredihhin A, Maeorg U, Vares L. Evaluation of carbohydrates and lignocellulosic biomass from different wood species as raw material for the synthesis of 5-bromomethyfurfural[J]. Carbohydrate Research, 2013, 375: 63-67.
[16] Mascal M, Nikitin E B. Direct, high-yield conversion of cellulose into biofuel[J]. Angewandte Chemie International Edition, 2008, 47(41): 7924-7926.
[17] Mascal M, Nikitin E B. High-yield conversion of plant biomass into the key value-added feedstocks 5-(hydroxymethyl) furfural, levulinic acid, and levulinic esters via 5-(chloromethyl) furfural[J]. Green Chemistry, 2010, 12(3): 370-373.
[18] Xiao Shaohua, Liu Bing, Wang Yimei, et al. Efficient conversion of cellulose into biofuel precursor 5-hydroxymethylfurfural in dimethyl sulfoxide–ionic liquid mixtures[J]. Bioresource Technology, 2014, 151: 361-366.
[19] Xu Guizhuan, Chang Chun, Zhu Weina, et al. A comparative study on direct production of ethyl levulinate from glucose in ethanol media catalysed by different acid catalysts[J]. Chemical Papers, 2013, 67(11): 1355-1363.
[20] Yang Yu, Hu Changwei, Abu-Omar M M. Conversion of glucose into furans in the presence of AlCl3in an ethanol- water solvent system[J]. Bioresource Technology, 2012, 116: 190-194.
[21] Yin Shanshan, Sun Jie, Liu Bing, et al. Magnetic material grafted cross-linked imidazolium based polyionic liquids: an efficient acid catalyst for the synthesis of promising liquid fuel 5-ethoxymethylfurfural from carbohydrates[J]. Journal of Materials Chemistry A, 2015, 3(9): 4992-4999.
[22] Zhao Shiqiang, Xu Guizhuan, Chang Junli, et al. Direct production of ethyl levulinate from carbohydrates catalyzed by H-ZSM-5 supported phosphotungstic acid.[J]. Bioresources, 2015, 10(2): 2223-2234.
[23] Peng Lincai, Lin Lu, Li Hui. Extremely low sulfuric acid catalyst system for synthesis of methyl levulinate from glucose[J]. Industrial Crops & Products, 2012, 40: 136-144.
[24] Xu Guizhuan, Chang Chun, Fang Shuqi, et al. Cellulose reactivity in ethanol at elevate temperature and the kinetics of one-pot preparation of ethyl levulinate from cellulose[J]. Renewable Energy, 2015, 78: 583-589.
[25] Chang Chun, Xu Guizhuan, Zhu Weina, et al. One-pot production of a liquid biofuel candidate—Ethyl levulinate from glucose and furfural residues using a combination of extremely low sulfuric acid and zeolite USY[J]. Fuel, 2015, 140: 365-370.
[26] Peng Lincai, Lin Lu, Li Hui, et al. Acid-catalyzed direct synthesis of methyl levulinate from paper sludge in methanol medium[J]. Bioresources, 2013, 8(4): 5895-5907.
[27] Zuo Miao, Le Kai, Feng Yunchao, et al. An effective pathwayfor converting carbohydrates to biofuel 5-ethoxymethylfurfural via 5-hydroxymethylfurfural with deep eutectic solvents (DESs)[J]. Industrial Crops & Products, 2018, 112: 18-23.
[28] Sun Yingnan, Zhang Qingqing, Zhang Panpan, et al. Nitrogen-doped carbon-based acidic ionic liquid hollow nanospheres for efficient and selective conversion of fructose to 5-ethoxymethylfurfural and ethyl levulinate[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(5): 6771-6782.
[29] Thombal R S, Jadhav V H. Application of glucose derived magnetic solid acid for etherification of 5-HMF to 5-EMF, dehydration of sorbitol to isosorbide, and esterification of fatty acids[J]. Tetrahedron Letters, 2016, 57(39): 4398-4400.
[30] Zhao Shiqiang, Xu Guizhuan, Chang Chun, et al. Direct conversion of carbohydrates into ethyl levulinate with potassium phosphotungstate as an efficient catalyst[J]. Catalysts, 2015, 5(4): 1897-1910.
[31] Wu Xiaoyu, Fu Jie, Lu Xiuyang. One-pot preparation of methyl levulinate from catalytic alcoholysis of cellulose in near-critical methanol[J]. Carbohydrate Research, 2012, 358: 37-39.
[32] Li Hu, Fang Zhen, Luo Jia, et al. Direct conversion of biomass components to the biofuel methyl levulinate catalyzedby acid-base bifunctional zirconia-zeolites[J]. Applied Catalysis B Environmental, 2017, 200: 182-191.
[33] Saravanamurugan S, Riisager A. Zeolite catalyzed transformation of carbohydrates to alkyl levulinates[J]. Chemcatchem, 2013, 5(7):1754-1757.
[34] Rataboul F, Essayem N. Cellulose reactivity in supercritical methanol in the presence of solid acid catalysts: Direct synthesis of methyl-levulinate[J]. Industrial & Engineering Chemistry Research, 2011, 50(2): 699-704.
[35] Amarasekara A S, Wiredu B. Acidic ionic liquid catalyzed one-pot conversion of cellulose to ethyl levulinate and levulinic acid in ethanol-water solvent system[J]. Bioenergy Research, 2014, 7(4): 1237-1243.
Process optimization on 5-ethoxymethylfurfural production from cellulose catalyzed by extremely low acid in one-pot reaction
Xu Guizhuan1,2, Chen Binglin1,2, Zhang Shaohao1,2, Wang Dongxiang1, Li Kai1
(1.450002,;2.450002,)
With the inevitable depletion of non-renewable petroleum resources and the rising environmental concerns, renewable biomass has been regarded as a potential resource for sustainable supply of biofuels. The development of simple, cheap and sustainable catalytic process for the production of biofuels has become a major target. Among the promising biofuels, EMF (5-ethoxymethylfurfural) has been considered as a new generation of biofuel or fuel additive due to its unique properties, such as its liquid characteristics under room temperature, non-toxicity, high lubricity, stable flashpoint and excellent flow properties under cold conditions. Recently, an increasing number of reports have been focused on the direct conversion of carbohydrates and platform chemicals such as 5-hydroxymethylfurfural (HMF), fructose, sucrose, or inulin into EMF catalyzed by different catalysts.However, from a point of view that raw materials are cheap and rich, it is more valuable to use cellulose or cellulosic biomass instead of model compounds, such as HMF and fructose, for the production of EMF. In the existing researches, cellulose was usually used as raw material to be converted into intermediate product HMF, 5-chloromethylfurfural (CMF) or 5-bromomethylfurfural (BMF), and so on, and then the target product EMF was obtained through the intermediate product. Using cheap and renewable cellulose as the raw material, and ethanol as the solvent, and combining the dehydration of cellulose to HMF, followed by the etherification of HMF to EMF in one-pot, is a more attractive reaction pathway.This one-pot reaction avoids the isolation and purification of HMF, which saves time, energy and solvent. The side reaction of process using extremely low sulfuric acid is slight, in which standard grade stainless steel facility can be used instead of high nickel alloy, which has a significant cost advantage in the equipment. In this paper, sulfuric acid with ultra low mass concentration (0.1% of the mass of ethanol) was used as catalyst to catalyze cellulose and ethanol to produce EMF in one-pot reaction. The effects of temperature, reaction time and substrate concentration on EMF yield were firstly studied and then the response surface methodology was used to design experiments to optimize the reaction conditions. The interactions of factors and the optimum reaction conditions were obtained. The results showed that the reaction temperature was the factor that mostly impacted the one-pot EMF production from cellulose and ethanol catalyzed by sulfuric acid with ultra low mass concentration. The maximum mean EMF yield of 14.93% was obtained under the optimum reaction conditions: reaction temperature of 200 ℃, reaction time of 75 min and substrate concentration of 30 g/L, with the prediction error of 2.03%, which showed that the model had a good fitting property. Compared to other studies, the study showed that EMF can be obtained directly from cellulose catalyzed by extremely low acid in one-pot reaction for the first time. The process conditions for producing EMF from cellulose catalyzed by extremely low acid in one-pot reaction had been optimized. This study can provide reference for the EMF one-pot production from much cheaper and abundant cellulosic biomass such as agricultural wastes catalyzed by sulfuric acid with extremely low concentration.
cellulose; acidity; biofuels; extremely low acid; 5-ethoxymethylfurfural; one-pot reaction
10.11975/j.issn.1002-6819.2018.19.029
S216
A
1002-6819(2018)-19-0225-07
2018-06-08
2018-09-04
河南省基础与前沿技术研究项目(162300410007)
徐桂转,教授,博士,主要从事生物质能源转换及利用技术研究。中国农业工程学会会员:徐桂转(E041200698S)。
徐桂转,陈炳霖,张少浩,王冬祥,李 凯.超低酸催化纤维素一锅法制取糠醛工艺优化[J]. 农业工程学报,2018,34(19):225-231. doi:10.11975/j.issn.1002-6819.2018.19.029 http://www.tcsae.org
Xu Guizhuan, Chen Binglin, Zhang Shaohao, Wang Dongxiang, Li Kai.Process optimization on 5-ethoxymethylfurfural production from cellulose catalyzed by extremely low acid in one-pot reaction[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2018, 34(19): 225-231. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2018.19.029 http://www.tcsae.org

