Microencapsulated Schwann cell transplantation inhibits P2X3 receptor expression in dorsal root ganglia and neuropathic pain
Ya-Ling Zhang, De-Jian Chen, Bao-Lin Yang, Tao-Tao Liu, Jia-Juan Li, Xiu-Qi Wang, Guo-Yong Xue, Zeng-Xu Liu,
1 Department of Anatomy, Basic Medical School, Nanchang University, Nanchang, Jiangxi Province, China
2 First Af filiated Hospital of Nanchang University, Nanchang, Jiangxi Province, China
3 Fourth Clinical Medical College of Nanchang University, Nanchang, Jiangxi Province, China
4 Second Clinical Medical College of Nanchang University, Nanchang, Jiangxi Province, China
5 Queen Mary College of Nanchang University, Nanchang, Jiangxi Province, China
Abstract Schwann cell transplantation is a promising method to promote neural repair, and can be used for peripheral nerve protection and myelination. Microcapsule technology largely mitigates immune rejection of transplanted cells. We previously showed that microencapsulated olfactory ensheathing cells can reduce neuropathic pain and we hypothesized that microencapsulated Schwann cells can also inhibit neuropathic pain. Rat Schwann cells were cultured by subculture and then microencapsulated and were tested using a rat chronic constriction injury (CCI) neuropathic pain model. CCI rats were treated with Schwann cells or microencapsulated Schwann cells and were compared with sham and CCI groups. Mechanical withdrawal threshold and thermal withdrawal latency were assessed preoperatively and at 1, 3, 5,7, 9, 11 and 14 days postoperatively. The expression of P2X3 receptors in L4–5 dorsal root ganglia of the different groups was detected by double-label immuno fluorescence on day 14 after surgery. Compared with the chronic constriction injury group, mechanical withdrawal threshold and thermal withdrawal latency were higher, but the expression of P2X3 receptors was remarkably decreased in rats treated with Schwann cells and microencapsulated Schwann cells, especially in the rats transplanted with microencapsulated Schwann cells. The above data show that microencapsulated Schwann cell transplantation inhibits P2X3 receptor expression in L4–5 dorsal root ganglia and neuropathic pain.
Key Words: nerve regeneration; neuropathic pain; peripheral nerve injury; sciatic nerve; microencapsulation; Schwann cells; P2X3 receptor;dorsal root ganglion; chronic constriction injury; cell transplantation; neural repair; neural regeneration
Introduction
Neuropathic pain is a perplexing chronic pain condition associated with tissue damage and nerve injury, and its underlying mechanism remains unknown. This important health issue constitutes a challenge for modern medicine throughout the world (Li et al., 2016; Pickering et al., 2016; Lin et al.,2017; Sagalajev et al., 2017). The purine, adenosine triphosphate, is not only an important messenger involved in the transmission of pain information (Zhu et al., 2015a, b; Kuan et al., 2016; Matthews et al., 2017; Tavakoli-Ardakani et al.,2017), but also activates sensory neurons, including those with cell bodies in the dorsal root ganglia. Nucleotides signal through the P2 family of receptors. These include both the P2X purinergic receptors and the P2Y receptors, which have a diverse agonist profile (Kaan et al., 2010; Ishchenko et al., 2017; Jung et al., 2017; Xie et al., 2017; Ying et al., 2017;Zhao et al., 2017). P2X receptors play a pivotal role in the conduction and regulation of neuropathic pain. In experi-mental pain models, selective P2X3 and P2X2/3 receptor antagonists effectively reduce neuropathic pain (McGaraughty et al., 2003; Gao et al., 2011; Li et al., 2013; Barragán-Iglesias et al., 2016; Chen et al., 2016).
The method of cell analgesia is to transplant special cells into the host’s central nervous system, such as the subarachnoid space. These cells act by the sustained excretion of neuroactive analgesic substances, such as enkephalin and β-endorphin, resulting in sustained analgesia (Sagen et al., 1986;Hui et al., 2011; Wu et al., 2011; Jergova et al., 2016). These cells function as a bio-analgesic pump that exerts a pharmacological effect of relieving pain or increasing pain threshold(Kim et al., 2004; Warren et al., 2017). In the peripheral nervous system, Schwann cells (SCs) are principal glial cells that play an important role in recovery after peripheral nerve injury. SCs are also valuable cells for the tissue engineering of artificial neurons (Sherman et al., 2005; Gnavi et al., 2015; Mogha et al., 2016; Liu et al., 2017; Merolli et al., 2017; Wang et al., 2017; Yeh et al., 2017). They secrete nerve growth factors that counterbalance pro-in flammatory cytokines, including, interleukin-10 and erythropoietin. SCs provide favorable conditions for the regeneration of axons and guide the distal growth of axons, and have also been widely explored as promising donors for transplantation to promote axonal regeneration after nerve injury (Griffin et al., 2008; Qin et al., 2016; Clements et al., 2017; Gomez-Sanchez et al., 2017; Quintes et al., 2017). However, aberrant SC function may underlie the formation of neuropathic pain(Campana et al., 2007). The effects of SC transplantation on neuropathic pain in rats has not been reported. Immune rejection is a key factor in fluencing the outcome of tissue and cell transplantation. Microencapsulation is a widely used immunoisolation technology that can overcome this complication (Zhu et al., 2015a, b; Leong et al., 2017; Meier et al.,2017; Shimoda et al., 2017). We previously confirmed that transplantation of microencapsulated olfactory ensheathing cells can relieve pain in chronic constriction injury (CCI)rats (Zhao et al., 2015). In the present study we transplanted SCs/microencapsulated Schwann cells (MC-SCs) into the region surrounding the injured nerve in a model of sciatic CCI, investigated the effect and mechanism of P2X3 receptor-mediated neuropathic pain, and explored new ways to prevent and treat neuropathic pain.
Materials and Methods
Animals
Thirty clean Sprague-Dawley rats of both sexes weighing 150–200 g and aged 6–7 weeks were provided by the Department of Laboratory Animal Science, Traditional Chinese Medicine University, China (license No. SYXK (Gan) 2015-0001). All protocols were approved by the Animal Care and Ethics Committee, Medical School, Traditional Chinese Medicine University, Jiangxi Province, China.
Culture and identification of SCs
RSC96 cells, derived from the long-term culture of rat primary SCs, were purchased from the BaNa Culture Collection of Beijing Beina Chuanglian Biotechnology Institute(Beijing, China). RSC96 cells were cultured using subculturing methods. Dulbecco’s modified Eagle’s medium/Nutrient Mixture (Boster, Wuhan, China) containing 15% fetal bovine serum (Cellmax, Beijing, China) was replaced every 2–3 days. Cells reaching 80–90% confluency were passaged.Cells were fixed in 4% paraformaldehyde at room temperature for 20–30 minutes, and blocked with 5% bovine serum albumin (Boster) at room temperature for 30 minutes. Cells were incubated with a mouse monoclonal S-100β antibody(1:100; Abcam, Shanghai, China) at 4°C overnight, and then with FITC-labeled goat anti-mouse IgG (1:100; Boster) in the dark at room temperature for 2 hours and then in the dark with DAPI (Boster) for 5 minutes. Cells were examined using fluorescence microscopy (Olympus, Tokyo, Japan)(Chen et al., 2017).
CCI model
A rat CCI model was prepared (Zhu et al., 2014; Wang et al., 2017). Sprague-Dawley rats were anesthetized with 1%intraperitoneal sodium pentobarbital (40 mg/kg). Under aseptic conditions, the main trunk of the sciatic nerve was revealed in the upper third of the rat thigh. The sciatic nerve was ligated with 4-0 catgut, 1 mm apart. Ligation intensity was such that the nerve blood supply was not affected.Wounds were then sutured layer by layer and the rats were allowed to recover from anesthesia. Reduced pain threshold and walking impairments confirmed the success of the CCI model (Wang et al., 2014). The 30 rats were equally and randomly assigned into four groups (n = 6 per group): Sham,CCI, CCI + SCs and CCI + MC-SCs groups. In the sham group, the sciatic nerve was isolated but not ligated. In the CCI + SCs and CCI + MC-SCs groups, 0.5 μL suspensions of 1 × 105SCs or MC-SCs were, respectively, transplanted into the region surrounding the CCI injured nerve. Wounds were then sutured layer by layer and the rats were allowed to recover from anesthesia.
Encapsulation and implantation of MC-SCs
Cells in the SC suspension were counted under an inverted microscope (Olympus, Tokyo, Japan) and adjusted to 1 ×109/L. Cell survival rate was ensured to be > 90% using Trypan blue staining. The SC suspension was mixed with 1.5%sodium alginate solution (1:1) (Solarbio, Beijing, China) and then sprayed into 1.1% calcium chloride solution through a syringe pump. Samples were mixed gently and precipitated. The supernatant was discarded. After washing twice in 0.9% physiological saline, the encapsulated cells were coated with 0.05% poly-l-lysine (Solarbio) and then washed again with 0.9% physiological saline. Cells were then suspended in 0.15% sodium alginate for 5 minutes (Solarbio) to form the outer layer of the membrane. Microcapsules were suspended in just enough saline to cover the sedimentary microcapsules (Meier et al., 2015). In the CCI + SCs group, the SC suspension was transplanted into the injured sciatic nerve,but the rats in the CCI + MC-SCs group received the prepared Schwann cell microcapsules.
Measurement of mechanical withdrawal threshold (MWT)
The MWT was determined using Von Frey filaments (BME-404, Tianjin, China) applied through a wire mesh (1 × 1 cm2)in the bottom of a box in an area adjacent to the operated hindlimb. At 1, 3, 5, 7, 9, 11 and 14 days after surgery, rats were placed in a clean glass box that was positioned on the wire mesh for an acclimation period of at least 15–20 minutes. The Von Frey filament was applied starting with a minimum force (0.13 g) and continuing until a foot retraction occurred or the force reached the maximum (20.1 g). The inter-stimulus interval was at least 20 seconds to allow stimulus-induced responses, such as foot-licking and leg- flicking,to disappear completely. Experiments were repeated three times and the mean of the three values (MWT) was obtained(Lin et al., 2014; Wang et al., 2014).
Measurement of thermal withdrawal latency (TWL)
TWL was measured using the Thermal Paw Stimulation System (BME-410C, Tianjin) at 1, 3, 5, 7, 9, 11 and 14 days after surgery. Rats were acclimatized to the apparatus for 15–20 minutes. Radiant heat stimulation illuminated the posterior limb of the rat by passing a beam of light through a glass plate. The light beam was switched off when the rat exhibited the withdrawal reflex. The hind paws were tested alternately at 5-minute intervals. The maximum time for thermal stimulation was 30 seconds.
Ultrastructural changes of the sciatic nerve
At 14 days after surgery, injured sciatic nerves from rats in each group were fixed with 2.5% glutaraldehyde solution,embedded in resin and semi-thin slices prepared (10 μm).After Wright’s staining and fixing, the slices were stained with osmic acid. Pathological changes of the nerve were observed under transmission electron microscopy (FEI, Hillsboro, USA).
Immuno fluorescence double labeling
Rats were anesthetized with intraperitoneal 1% sodium pentobarbital (40 mg/kg,). The L4–5 dorsal root ganglia in each group were separated immediately and fixed in 4%paraformaldehyde overnight at 4°C. Afterwards, the ganglia were transferred to 10%, 20% and 30% sucrose solution for dehydration at 4°C overnight. Tissues were sectioned at 10 μm on a cryostat microtome and mounted onto anti-stripping slides. After drying, slides were washed three times with PBS and then incubated with 5% normal goat serum(Solarbio) for 30 minutes in a moist chamber at room temperature. The sections were incubated with mouse monoclonal anti-P2X3 (1:500; Santa Cruz Biotechnology, Dallas,TX, USA) and rabbit polyclonal anti-NeuN (1:500; Abcam)antibodies overnight at 4°C. After rinsing three times in PBS, the sections were incubated with fluorescent goat anti-rabbit TRITC and goat anti-mouse FITC secondary antibody (1:100; Boster) in the dark at room temperature for 1 hour. The sections were washed again in PBS and coverslips then mounted with anti-fluorescent encapsulating agent.Finally, sections were examined by fluorescence microscopy(Olympus, Tokyo, Japan). The mean optical density and the percentage of P2X3 receptor-immunoreactive cells in L4–5dorsal root ganglia were quantified with Image-Pro Plus 6.0 software (Media Cybernetics Inc.) Five fields containing approximately 50 neurons each were randomly selected, and data from each rat were averaged.
Statistical analysis
Data, expressed as the mean ± SEM, were analyzed using SPSS 17.0 software (SPSS, Chicago, IL, USA). Statistical comparisons were performed with one-way analyses of variance followed by the least significant difference post hoc test.P < 0.05 was considered statistically significant.
Results
Morphology and purity of SCs in culture
Under the inverted microscope, SCs appeared bipolar, spindle-like or had multiple processes (Figure 1A). Immunohistochemical staining revealed a purity of S-100β-positive cells of greater than 95% (Figure 1B).
Assessment of MWT and TWL
There were no differences in the MWT or TWL between groups prior to the experiment. MWT and TWL were significantly lower in the CCI group compared with the sham group (P < 0.01) and were higher in the CCI + SCs and CCI+ MC-SCs groups than in the CCI group (P < 0.01). MWT and TWL were lower in the CCI + MC-SCs group than in the CCI + SCs group (P < 0.05; Figure 2).
Ultrastructural changes of the sciatic nerve in each group
Myelin sheaths in the sham group were dense, uniform and structurally intact, with lamellar structures arranged neatly,and with no atrophy or swelling of axons. In the CCI group,the myelinated fibers of the sciatic nerve were reduced in number, exhibited vacuolar defects, axonal contraction, myelin space expansion, and even the myelinated lamellae of the nerve fibers of the sciatic nerve were completely separated. However, in the CCI + SCs and CCI + MC-SCs groups,the myelin structure of the sciatic nerve was basically intact,and the degree of demyelination was remarkably reduced,especially in the CCI + MC-SCs group. Nevertheless, there were still local defects of the myelinated lamella and vacuolar formation (Figure 3).
Co-expression of P2X3 and NeuN by double-label immuno fluorescence
Immunofluorescence showed colocalization of P2X3 and NeuN immunoreactivity in dorsal root ganglia (Figure 4A–D). The ratio of P2X3/NeuN-immunolabeled cells in the CCI group was significantly increased compared with that in the sham group (P < 0.01, Figure 4E). The ratio of P2X3/NeuN-immunolabeled cells that was increased in CCI group was reduced in the CCI + SCs and CCI + MC-SCs groups (P< 0.01). The ratio of P2X3/NeuN-immunolabeled cells in the CCI + MC-SCs group was lower than that in the CCI + SCs group (P < 0.05; Figure 4E). The percentage of immunore-active cells (%) was calculated by the number of immunoreactive cells/total number of cells × 100%.
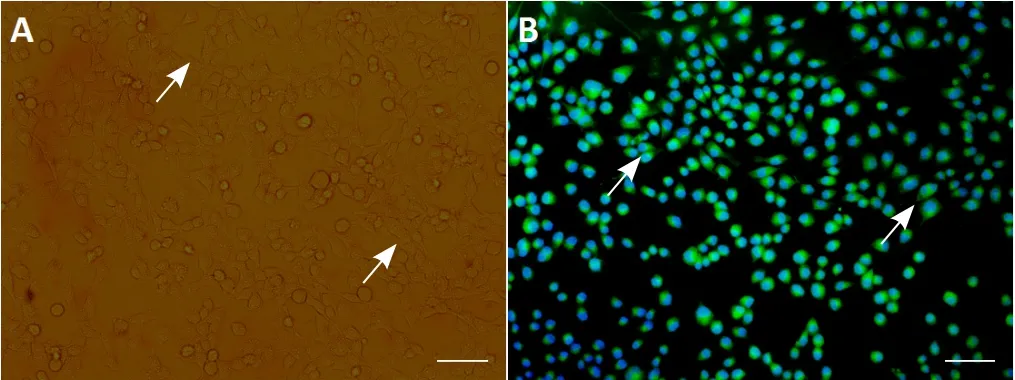
Figure 1 Morphological identification of Schwann cells.
Discussion

Figure 2 Changes in thermal withdrawal latency and mechanical withdrawal threshold after Schwann cell transplantation.
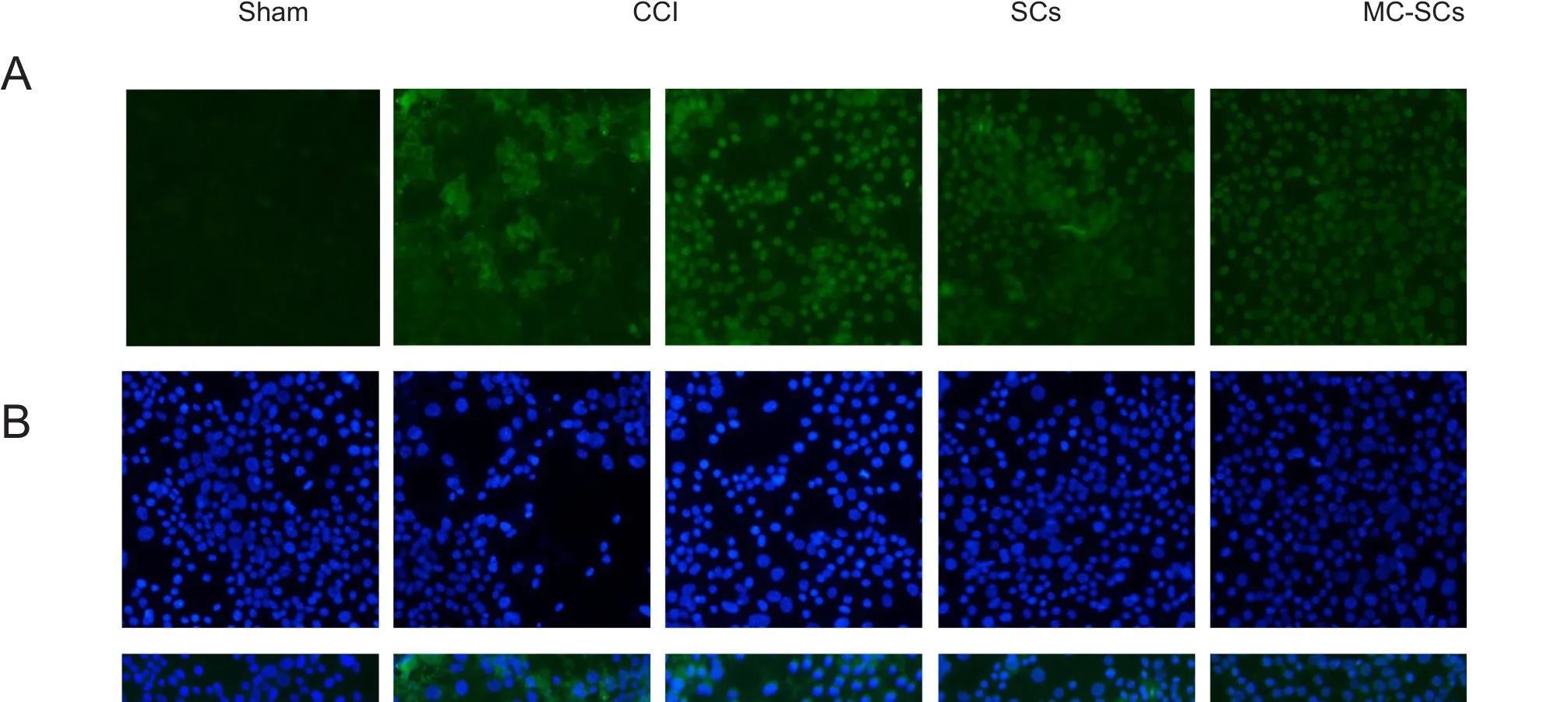
Figure 3 Ultrastructural changes of the sciatic nerve of each group at 14 days after surgery.
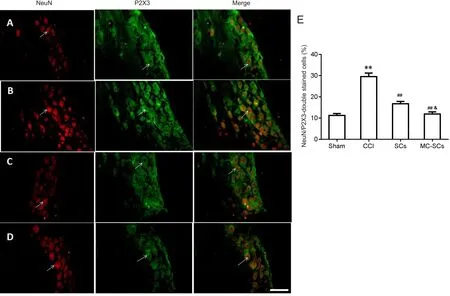
Figure 4 Changes in the number of P2X3 receptor/NeuN double-labeled cells in L4–5 dorsal root ganglia of each group.
SCs are immortal glial cells that can secrete a variety of neurotrophic factors. SCs play a very important role in nerve regeneration after peripheral nerve injury (Gnavi et al., 2015;Mogha et al., 2016; Merolli et al., 2017; Wang et al., 2017;Yeh et al., 2017). Our results have shown that transplantation of SCs or MC-SCs reduces P2X3 receptor expression in L4–5 dorsal root ganglia and increases behavioral pain threshold in rats with neuropathic pain. In a previous study,SCs were obtained from the sciatic nerve of neonatal rats, by stripping, cutting and purifying (Shakhbazau et al., 2014).However, there are many disadvantages to obtaining cells by this method: the cell content is low, and as the donor age increases, the number of cells is markedly decreases and their ability to proliferate and differentiate declines. These limitations severely limit the application of long-term transplant treatment methods to clinical practice. In this study, RSC96 cells were purchased directly, and a large number of SCs were obtained by subculturing. SCs were then microencapsulated to reduce immune rejection.
The rats in the CCI group had no autophagy; the reduced pain threshold and walking impairments confirmed the model was successfully established (Zhu et al., 2014; Wang et al., 2017). On day 14 after surgery, the MWT and TWL in the CCI + SC group were remarkably higher than those of the CCI group. This indicates that SCs or MC-SC transplantation may alleviate pain behavior in rats with neuropathic pain. Moreover, SC transplantation reduced P2X3 receptor expression in L4–5 dorsal root ganglia. P2X3 receptor protein levels in L4–5 dorsal root ganglia were elevated in the CCI models. However, P2X3 receptor levels were diminished after SC and MC-SC transplantation. We propose the following as possible mechanisms of action: SCs alter the local microenvironment by secreting various neurotrophic factors(Grif fin et al., 2008; Qin et al., 2016; Clements et al., 2017;Gomez-Sanchez et al., 2017). SC transplantation contributes to neuronal survival and axonal regeneration (Niapour et al.,2012; Lee et al., 2017). Transplantation of SCs can reduce the secondary damage of inflammation and nerve injury, and promote the restoration of the injured sciatic nerve in rats.The expression of P2X3 receptor in L4–5 dorsal root ganglia was previously observed on days 7 and 14 in a preliminary experiment, and was especially obvious on day 14 after surgery (Zhao et al., 2015). There may be time considerations for this experiment. Noticeable improvements in behavior and immuno fluorescence were observed on day 14 in both groups that received SCs, but particularly in the CCI + MCSC group. We can deduce that because the microcapsule polymer is semipermeable, the passage of macromolecules was blocked, which led to this improvement. Therefore, an immune barrier was established between the host cells and the SCs, resulting in immunological isolation. To transplant SCs without immunosuppressant therapy, there is a need for the cells to be encapsulated in a polymer, which ensures their isolation from the host immune system. Artificial cell microencapsulation is one way to transplant cells safely into the body and to protect them from the immune system (Zhu et al., 2015a, b; Leong et al., 2017; Shimoda et al., 2017). A major obstacle to successful SCs microcapsules is an ideal microcapsule material, which will form microcapsules that can maintain cell viability and functions for a long time.Microencapsulation is durable and typically proffers a large surface to volume ratio, which is advantageous for the bidirectional diffusion of nutrients, oxygen, and bioactive materials. In the present study, due to the lack of a microcapsule generator, this experiment was conducted using a self-made jet-head droplet-forming apparatus. Hence, the size of the microcapsules may be non-homogeneous, there may be incomplete microcapsules, or cells may be able to escape from the microcapsules.
Microcapsule materials-APA membrane is currently investigated in-depth. It is an ideal material for transplantation,and is also the most widely used microcapsule material. This experiment used APA microcapsules, and confirmed that microcapsules provided immune isolation. As for many other microcapsule materials, there are still many immaturities,and a large number of experiments are required for the production methods of microcapsules. Therefore, the experiment used the APA microcapsule membrane with the most mature application and obtained the experimental results.
In conclusion, SCs or MC-SCs transplantation not only exerts a therapeutic effect on nerve damage and repair, but also diminishes immunological rejection. Microcapsules play an essential role in SCs transplantation. However, how MC-SCs specifically affect P2X3 receptor mediated neuropathic pain warrants further investigation. The relationship between the number of SCs and the axonal area, myelin sheath thickness around the injured sciatic nerve, and the survival time of SCs after transplantation still needs further investigation.
Author contributions: YLZ, TTL, JJL, XQW and GYX participated in study conception and design, data collection, analysis and interpretation of the data. YLZ, BLY and DJC had written the manuscript or provided critical revision of the manuscript for intellectual content. ZXL and YLZ participated in the statistical expertise, obtained funding, provided administrative, technical or material support, and supervision. All authors approved the final version of this paper.
Conflicts of interest: The authors declare that they have no conflicts of interest.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81760418 and 81260190; the Natural Science Foundation of Jiangxi Province, No. 20132BAB205023,20151BAB205022; a grant from Science and Technology Research Project of Jiangxi Education Department, No. GJJ13159; a grant from the Science and Technology Program of Department of Health of Jiangxi Province,No. 20173010. The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement: All experimental procedures and protocols were approved by the Experimental Animal Ethics Committee of Medical School of Nanchang University of China, No. NCDXYD-2017018. All experimental procedures were in accordance with the National Institutes of Health (NIH) guidelines for the Care and Use of Laboratory Animals.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer: Fabricio Ferreira de Oliveira, Universidade Federal de Sao Paulo, Brazil.
Additional file: Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- The unfolded protein response signaling and retinal Müller cell metabolism
- Sequencing of high-efficacy disease-modifying therapies in multiple sclerosis: perspectives and approaches
- Targeting prion-like protein spreading in neurodegenerative diseases
- Cadmium-induced neurotoxicity: still much ado
- Analysis of the traf ficking system in blood-brain barrier models by high content screening microscopy
- Retinal remodeling following photoreceptor degeneration causes retinal ganglion cell death