Molecular mechanism of panaxydol on promoting axonal growth in PC12 cells
Wei-Peng Li, Ke Ma, Xiao-Yan Jiang, Rui Yang, Pei-Hua Lu, Bao-Ming Nie, , Yang Lu,
1 Department of Nuclear Medicine, the First Af filiated Hospital of Bengbu Medical College, Bengbu, Anhui Province, China
2 Department of Pharmacy, Shanghai Jiao Tong University School of Medicine, Shanghai, China
3 Key Laboratory of Arrhythmias of Ministry of Education of China, Tongji University School of Medicine, Shanghai, China
Abstract Nerve growth factor (NGF) promotes axonal growth in PC12 cells primarily by regulating the RTK-RAS-MEK-ERK pathway. Panaxydol, a polyacetylene isolated from Panax notoginseng, can mimic the effects of NGF. Panaxydol promotes neurite outgrowth in PC12 cells, but its molecular mechanism remains unclear. Indeed, although alkynol compounds such as panaxydol can increase intracellular cyclic adenosine 3′,5′-monophosphate (cAMP) levels and the ERK inhibitor U0126 inhibits alkynol-induced axonal growth, how pathways downstream of cAMP activate ERK have not been investigated. This study observed the molecular mechanism of panaxydol-, NGF- and forskolin-induced PC12 cell axon growth using specific signaling pathway inhibitors. The results demonstrated that although the RTK inhibitor SU5416 obviously inhibited the growth-promoting effect of NGF, it could not inhibit the promoting effect of panaxydol on axonal growth of PC12 cells. The adenylate cyclase inhibitor SQ22536 and cAMP-dependent protein kinase inhibitor RpcAMPS could suppress the promoting effect of forskolin and panaxydol on axonal growth. The ERK inhibitor U0126 inhibited axonal growth induced by all three factors. However, the PKA inhibitor H89 inhibited the promoting effect of forskolin on axonal growth but could not suppress the promoting effect of panaxydol. A western blot assay was used to determine the effects of stimulating factors and inhibitors on ERK phosphorylation levels. The results revealed that NGF activates the ERK pathway through tyrosine receptors to induce axonal growth of PC12 cells. In contrast, panaxydol and forskolin increased cellular cAMP levels and were inhibited by adenylyl cyclase inhibitors. The protein kinase A inhibitor H89 completely inhibited forskolin-induced axonal outgrowth and ERK phosphorylation, but could not inhibit panaxydol-induced axonal growth and ERK phosphorylation. These results indicated that panaxydol promoted axonal growth of PC12 cells through different pathways downstream of cAMP.Considering that exchange protein directly activated by cAMP 1 (Epac1) plays an important role in mediating cAMP signaling pathways,RNA interference experiments targeting the Epac1 gene were employed. The results verified that Epac1 could mediate the axonal growth signaling pathway induced by panaxydol. These findings suggest that compared with NGF and forskolin, panaxydol elicits axonal growth through the cAMP-Epac1-Rap1-MEK-ERK-CREB pathway, which is independent of PKA.
Key Words: nerve regeneration; panax notoginseng; polyacetylene; panaxydol; nerve growth factor; PC12 cells; neurite outgrowth; cAMP;PKA; Epac1; ERK; CREB; neural regeneration
Introduction
Neurite growth is an important process in neuronal development, synaptic formation, and regeneration. Loss of neurons and their synaptic projections are common in neurological disease, such as Alzheimer’s disease. Neurite outgrowth provides the morphological basis for synaptogenesis and the foundation for learning and memory. Nerve growth factor(NGF) is very important for nervous system function and may potentially prevent the degeneration of cholinergic neurons in Alzheimer’s disease patients (Scott et al., 1995; Tuszynski et al., 2005; Williams et al., 2006). Recently, much attention has been given to the availability of NGF in Alzheimer’s disease patient brains and exploitation of naturally occurring compounds with NGF-like effects to potentially treat AD (Angelova et al., 2013, 2017; Guerzoni et al., 2017).
Panax notoginseng (Aealiaceae) is a Chinese traditional plant with anti-aging effects (Ng, 2006). Previously, we reported that panaxynol (PNN), a polyacetylene isolated from the lipophilic fractions of this plant, has neurotrophic effects in PC12 cells (Wang et al., 2006a). Later, panaxydol (PND)(Figure 1), a PNN analog, was purified from Panax notoginseng and was found to induce PC12 neurite outgrowth by our lab. Recent studies of C17-polyacetylenes including PNN and PND have indicated their potential benefits to human health for anticancer, antifungal, antibacterial, anti-in flammatory, and serotogenic effects (Dawid et al., 2015).A recent study reported that PNN and PND can improve glucose uptake in adipocytes and myotubes (El-Houri et al.,2015). Another study reported PNN can inhibit inflammatory macrophage-mediated cardiomyocyte death and hypertrophy by activating nuclear factor erythroid-2 related factor 2 (Nrf2) in macrophages (Qu et al., 2015).
PC12 cells showed a neuronal-like morphology when treated with NGF or cyclic adenosine 3′,5′-monophosphate(cAMP)-elevating agents by activating the MEK pathway (Pang et al., 1995a; Kaplan et al., 2000). Historically,cAMP-dependent protein kinase, also known as protein kinase A (PKA), was thought to be the only effector of cAMP.However, a series of studies has proven that a PKA-independent, non-canonical downstream signaling pathway of cAMP mediated by exchange protein directly activated by cAMP (Epac), plays an important role in neurite outgrowth by activating the MEK pathway (Gloerichet al., 2005; Shi et al., 2006). One well-studied Epac signaling activator is pituitary adenylate cyclase-activating polypeptide, which induces PC12 cell neurite outgrowth through Epac-mediated PKA-independent MAPK pathways (Sakai et al., 2004;Ravni et al., 2006, 2008).
This study explored the NGF-like effects of PND and mechanisms involved in neurite outgrowth in PC12 cells. By employing several inhibitors of signaling pathways during PC12 neurite outgrowth, this study compared PND-activated signaling with NGF- and forskolin-activated signaling pathways.
Materials and Methods
PND
PND was isolated and purified in our lab as previously described (Nie et al., 2008b) from the roots of P. notoginseng(Yunnan Province, China) and stored at −20°C. Its purity was confirmed to be greater than 98% by gas chromatography.
PC12 cell culture and neurite outgrowth
PC12 cells from ATCC were maintained in Dulbecco’s Modified Eagle’s Medium (Invitrogen, Carlsbad, CA) supplemented with 10% horse serum (Invitrogen) and 5% fetal calf serum (Invitrogen) on poly-L-lysine-coated dishes at 37°C in a humidified 5% CO2incubator. PC12 cells were seeded in poly-L-lysine-coated 24-well plates at a density of 1 × 104cells/well in culture medium. Before stimulation with growth factors, cells were serum-starved in medium containing 0.5% fetal bovine serum and 1% horse serum overnight, followed by media with or without PND or NGF for 24 hours.
For experiments combining PND, NGF (Sigma, St. Louis,MO, USA) or forskolin (ALEXIS, Farmingdale, NY, USA)with inhibitors, each inhibitor was added 1 hour before stimulation. Concentrations of inhibitors were as follows: 10 μM Trk inhibitor SU5416 (Sigma); 20 μM mitogen-activated protein kinase (MAPK)/Erk kinase inhibitor U0126 (Upstate, Billerica, MA, USA); 500 μM adenylate cyclase inhibitor SQ22536 (ALEXIS); 50 μM cAMP analogue Rp-cAMPS(Enzo Life Sciences, Plymouth Meeting, PA, USA); and 20 μM PKA inhibitor H89 (ALEXIS).
Numbers of differentiated cells were determined by visual examination of the field and counting cells with at least one neurite longer than the diameter of the cell body and expressed as a percentage of total cells in the field. Lengths of neurites were measured with the Image-Pro Plus 5.1 software (Mediacy, Rockville, MD, USA).
cAMP assay
Intracellular cAMP levels were detected as previously described with some modifications (Wang et al., 2006a). Brie fly,PC12 cells were plated on Petri dishes (150,000 cells/dish),pre-incubated for 30 minutes in Krebs-Ringer HEPES buffer with IBMX (500 µM) and incubated for 15 minutes in the presence of PND, forskolin, or combinations as indicated.Incubation was stopped by the addition of HClO4( final concentration is 0.5 M). Incubation medium was neutralized with KOH solution and centrifuged as required. cAMP was quantified by radioimmunoassay after acetylation as previously described (Wang et al., 2006a, b).
siRNA knockdown of Epac1
siRNAs directed against rat Epac1 and control nonspecific siRNA oligonucleotides were synthesized by RIBOBI(Guangzhou, China). Sequences of siRNA oligonucleotides for Epac1 were 5′-GGU CAA UUC UGC CGG UGA U dTdT-3′ (21 bp) and 5′-AUC ACC GGC AGA AUU GAC C dTdT-3′ (21 bp). Non-specific and Epac1 siRNAs were transfected into PC12 cells using Lipofectamine 2000 (Invitrogen) at a final concentration of 50 nM. Efficiency of Epac1 depletion was verified by reverse transcription-polymerase chain reaction (RT-PCR) after 48 hours of transfection.
After 24 hours of transfection, PC12 cells were treated with PND for 24 hours in the neurite outgrowth assay. PC12 cells were serum-starved overnight and treated with 20 μM PND for indicated time periods for the western blot assay.
RT-PCR
Total cellular RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Firststrand cDNA was synthesized using Reverse Transcription System (Promega, Madison, WI, USA). PCR was performed with rat Epac1 and β-actin primers as follows:
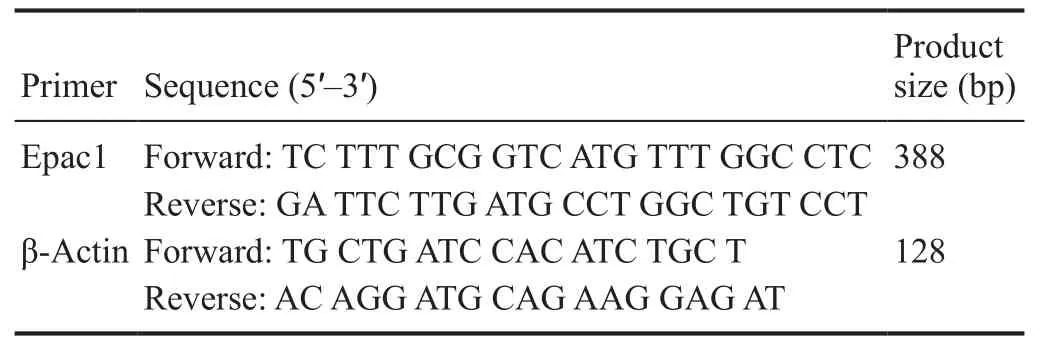
?
Additionally, each PCR product was electrophoresed onto a 2% agarose gel containing 0.5 μg/mL ethidium bromide and produced a single band of the expected size.
Western blot assay of Erk1/2 and CREB activation
Subcon fluent cells in 6-cm dishes were serum-starved and stimulated with NGF (50 ng/mL), forskolin (10 μM) or PND(20 μM) for the indicated time periods. After washing twice with ice-cold phosphate-buffered saline, cells were lysed in lysis buffer (50 mM Tris-HCl, pH 7.5; 150 mM NaCl; 1%NP-40; 0.5% sodium deoxycholate; 0.1% sodium dodecyl sulphate; 1 mM ethylenediamine tetraacetic acid; 1 mM sodium orthovanadate; 10 mM sodium fluoride; 4 μg/mL leupeptin; 1 μg/mL aprotinin and 100 μg/mL phenylmethyl sulfonyl fluoride, all from Sigma). Cell lysates were centrifuged at 10,000 × g for 10 minutes at 4°C and the resulting supernatant was saved for protein analysis and western blot assay. Total protein was determined using a commercially available kit (Pierce, Rockford, IL, USA) based on the bicinchoninic acid assay. Samples were boiled for 5 minutes and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. For detection of Erk1/2 activation, filters were probed with anti-phospho-Erk1/2 (1:1000) (#4376, Cell Signaling Technology, Beverly, MA) and anti-Erk1/2 (1:1000)(#9107, Cell Signaling Technology) for overnight at 4°C. For detection of CREB activation, filters were probed with anti-phospho-CREB (1:1000) (#9198, Cell Signaling Technology) and anti-CREB (1:1000) (#9197, Cell Signaling Technology) overnight at 4°C. Immunoreactivity was detected using a horseradish peroxidase-conjugated secondary antibody(1:10,000) (#7077, Cell Signaling Technology) incubation for 1 hour at room temperature and enhanced chemiluminescence according to the manufacturer’s instructions. Quantitative analysis was conducted by comparing band intensities normalized to Erk1/2 or CREB using SigmaGel software(Jandel Scientific, San Rafael, CA, USA).
Statistical analysis
Data are expressed as the mean ± SD using GraphPad Software (GraphPad, La Jolla, CA, USA). One-way analysis of variance followed by the Student-Newman-Keuls post hoc test was used to compare control and treated groups, or between groups as indicated, with P < 0.05 considered statistically significant.
Results
PND promoted neurite outgrowth in PC12 cells in a concentration-dependent manner
PC12 cells were maintained in medium containing 0.5% fetal bovine serum and 1% horse serum (low serum medium)overnight, followed by media with or without PND for 24 hours. The neuritogenesis effect of PND on PC12 cells was observed after treatment. In control conditions, almost all cells exhibited a round shape with very few short neurites(Figure 2A). After exposing cells to PND for 24 hours, both the percentage of neuritogenesis and length of neurites increased in a concentration-dependent manner, with a maximal effect observed at 20 μM (Figure 2B–D). 5, 10, and 20 μM PND promoted neurite outgrowth by 9.73 ± 2.83%,25.63 ± 4.88%, and 47.21 ± 3.56%, respectively (Figure 2E);and per neurite lengths were 45.29 ± 5.89, 47.46 ± 11.88, and 65.04 ± 19.50 μm (Figure 2F), respectively, after 24 hours of incubation.
PND promoted neurite outgrowth via a Trk-independent MAPK pathway
Using diverse kinase inhibitors and NGF or forskolin as a positive stimulator, we screened for signaling pathways involved in PND-induced neurite outgrowth. NGF can initiate a cascade of signaling events leading to neuronal differentiation via activation of the TrkA receptor. First, the Trk inhibitor SU5416 was used to assess if Trk signaling is involved in PND-induced differentiation. NGF (50 ng/mL) indeed induced neuroite outgrowth in 54.48 ± 13.12% of PC12 after 24 hours of incubation. As expected, 10 μM SU5416 obviously inhibited NGF-induced neurite outgrowth. However, Trk was not involved in the effects of PND, as SU5416 could not inhibit PND-induced neurite outgrowth. Second, we used the MEK/Erk inhibitor U0126 (20 μM) and found that it could effectively inhibit the neuritogenesis effect of NGF and PND,suggesting that Erk activation is essential for neurite outgrowth induced by both NGF and PND (Figure 3).
PND promoted neurite outgrowth via a cAMP-dependent but not PKA-independent MAPK pathway
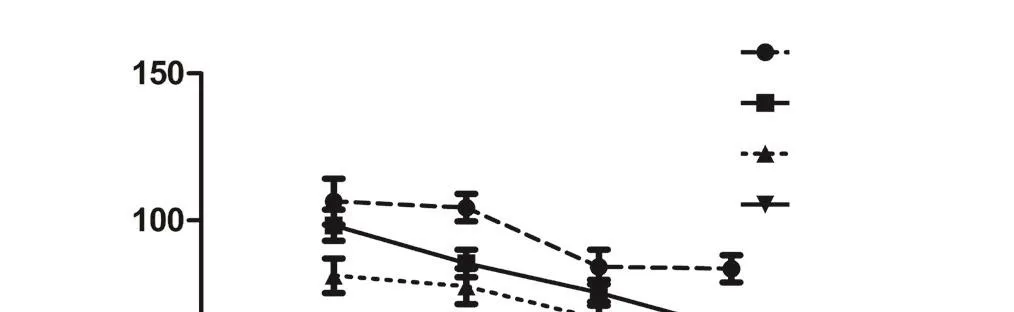
Figure 1 Chemical structure of panaxydol.
Figure 2 Representative morphology and neurite formation of PC12 cells after PND treatment.
After observing that PND may act via a different pathway than NGF to promote neurite outgrowth, we next investigated the in fluence of PND on cytoplasmic cAMP concentration, as agents that elevate cAMP levels have been shown to induce neuronal differentiation in PC12 cells (Ng et al.,2009). Previous reports show that forskolin can promote PC12 neurite outgrowth by activating adenylate cyclase and amplifying cAMP-dependent signaling (Insel and Ostrom,2003). Therefore, we used forskolin as a positive stimulator.Figure 4A showed that incubation with PND for 15 minutes could remarkably induce intracellular cAMP accumulation in PC12 cells. Forskolin also raised intracellular cAMP levels significantly. Effects of PND and forskolin on cAMP accumulation were inhibited by pretreatment with the adenylate cyclase inhibitor SQ22536 (500 μM). Moreover,both SQ22536 and the cAMP analogue Rp-cAMPS (50 μM)dramatically inhibited forskolin-induced neurite outgrowth.They also inhibited PND-induced neurite outgrowth in PC12 cells, suggesting this phenotype is mediated by cAMP signaling (Figure 4B–D).
Most cAMP actions are mediated through activation of the PKA signaling pathway. To determine whether the PKAMAPK pathway is involved in PND-mediated effects, cells were pre-treated with the PKA inhibitor H89 (20 μM) or U0126 (20 μM) for 1 hour prior to the addition of PND or forskolin. Notably, while U0126 could inhibit both forskolin- and PND-induced neurite outgrowth, H89 only inhibited the effect on forskolin and had no effect on PND (Figure 4B–D).
PND-induced Erk1/2 and CREB activation
As the MEK/Erk inhibitor U0126 could inhibit NGF-, forskolin- and PND-induced neurite outgrowth, this suggests that Erk plays a critical role in this process. The effect of PND on Erk1/2 activation was next examined by western blot assay. It was observed that 5, 10 and 20 μM PND activated Erk1/2 in a concentration-dependent manner at 5 and 15 minutes, and the Erk1/2 phosphorylation induced by PND decreased to basal levels by 30 minutes (Figure 5A). In addition, PND could induce a two-phase Erk1/2 phosphorylation for a long period. A quick Erk1/2 phosphorylation and dephosphorylation happened during the first half an hour after PND addition, but a second sustained Erk1/2 activation followed at a later stage (Figure 5B).
To confirm the involvement of cAMP-dependent but PKA-independent-MAPK pathways in PND-induced neurite outgrowth, we tested the effect of above-mentioned inhibitors on PND-stimulated Erk1/2 phosphorylation.PC12 cells were pre-treated with different kinase inhibitors for 1 hour, followed by PND, NGF or forskolin stimulation for 15 minutes. Cells were then lysed for subsequent detection of Erk1/2 phosphorylation levels. Inhibitory effects on PND-induced Erk1/2 activation showed the same tendency for PND-induced morphologic changes in PC12 cells as previously observed. SU5416 inhibited the phosphorylation of Erk1/2 induced by NGF but did not alter PND-induced Erk1/2 phosphorylation (Figure 5C). Both SQ22536 and Rp-cAMPS could dramatically inhibit either forskolin- or PND-induced Erk1/2 activation (Figure 5D, E). However,H89 could inhibit forskolin-induced Erk1/2 activation but not PND-induced Erk1/2 activation (Figure 5E), suggesting the involvement of a cAMP-dependent but PKA-independent pathway in PND-induced Erk1/2 activation.
Previous reports suggest that once MAPK/Erk is activated, it translocates from the cytosol to the nucleus to regulate transcription factors such as cAMP-responsive element binding protein (CREB) (Treisman, 1996). In response to a variety of extracellular signals, CREB binds to cAMP response elements and activates transcription, regulating the signaling pathway that enables long-term memory formation (Saura and Valero, 2011). Our results demonstrate that 20 μM PND induced CREB phosphorylation in a time-dependent manner (Figure 5F), suggesting that PND can lead to Erk1/2 and CREB phosphorylation/activation and potential affect synaptic plasticity and learning.
Epac1 was involved in PND-induced neuritogenesis
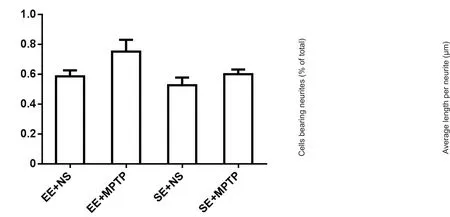
Figure 3 Effects of Trk and MEK/Erk inhibitors on PND- or NGF-induced neuritogenesis in PC12 cells.

Figure 5 Effects of certain inhibitors on phosphorylation of Erk1/2 or CREB stimulated by PND, NGF, or forskolin.
Recent studies have revealed that cAMP-activated guanine nucleotide exchange factors Epac1 and Epac2 are key mediators of PKA-independent signaling (de Rooij et al.,1998; Kawasaki et al., 1998). Meanwhile, Epac-Rap signaling triggered upon stimulation can activate Erk1/2 in PC12 cells (Kiermayer et al., 2005). The role of Epac1 in PND-induced neurite outgrowth and Erk1/2 activation was next examined by knockdown of endogenous Epac1. As shown in Figure 6A–C, Epac1 siRNA dramatically abolished the neurotrophic function of PND. After PC12 cells were transfected with 50 nM Epac1 siRNA or scramble siRNA for 24 hours, a down-regulation of Epac1 decreased PND-induced neurite-bearing cells and the length of neurites by 17.61%to 26.45 μm. Phosphorylation levels of Erk1/2 were also detected after 20 μM PND treatment for 5 and 15 minutes. The phosphorylation of Erk1/2 induced by PND was inhibited in the Epac1 siRNA group compared with the non-specific knockdown group (Figure 6D), suggesting Epac1 plays an important role in crosstalk between cAMP and Erk1/2 signaling. The efficiency of Epac1 knockdown was confirmed by RT-PCR (Figure 6E).
Discussion
A wide range of nerve cell functions, including survival,differentiation, axonal and dendritic growth, and various aspects of learning and memory are regulated by neurotrophic signaling pathways. PC12 cells are a good model system for the study of neuronal differentiation (Pang et al., 1995b;Wang et al., 2016; Zhang et al., 2016). Neurite formation involves participation of tyrosine kinase-, MAPK-, PKA-, and/or PKC-mediated signaling pathways, which are usually initialized and happen quickly at early stages of stimulation. In the present study, PND shares certain common but distinct pathways with NGF and forskolin, most likely using similar mechanisms involving regulation of neurite extension by neuropeptide pituitary adenylate cyclase-activating polypeptide in PC12 cells (Figure 7).
Inhibition of PND-stimulated neuritogenesis by the adenylate cyclase inhibitor SQ22536, cAMP-dependent protein kinase inhibitor Rp-cAMPs, and MEK/Erk kinase inhibitor U0126 suggest recruitment of these different elements in the PND-initiated signaling pathway. Moreover, the PND effect is mediated through a PKA-independent pathway, as H89 does not block PND-stimulated neuritogenesis. This PKA-independent pathway might involve direct activation of Rap1 by cAMP via Epac1, as knockdown of Epac1 abolished the effect of PND.
It is believed two parallel but connected pathways regulate the differentiation of PC12 cells: Trk-mediated NGF signaling and cAMP-mediated GPCR signaling (Vaudry et al., 2002). To investigate participation of the Trk pathway in induction of neurite outgrowth by PND, this study examined effects with the Trk inhibitor SU5416. SU5416 could only suppress NGF-induced neurite outgrowth and Erk1/2 activation, but could not inhibit those induced by PND, suggesting PND may use a different receptor to initiate neurite extension. Meanwhile, after detecting a rapid and slight,but significant, elevation of cAMP induced by PND using both the adenylate cyclase inhibitor SQ22536 and a specific competitive inhibitor of the activation of cAMP-dependent protein kinase, Rp-cAMPS, our results demonstrate that the neurotrophic effects of PND are abolished. Collectively,these data indicate that PND acts through the cAMP-mediated pathway.
Erks, members of the MAPK superfamily, play an important role in cell proliferation and differentiation. In our study, NGF-, forskolin-, or PND-induced neurite outgrowth were all inhibited by the MEK/Erk inhibitor U0126, again confirming that Erk is essential for neuronal differentiation.Previous reports showed that forskolin uses a PKA dependent C3G pathway to activate Erk1/2 in PC12 cells (Wang et al., 2006b). Regardless of the similar neurite outgrowth-promoting phenotype it shares with forskolin, in contrast, PND uses a PKA-independent pathway to activate a convergent Erk1/2 pathway. Using a specific inhibitor of PKA and siRNA knockdown technique, we found that the differentiation of PC12 cells induced by PND may be associated with activation of Epac1. After binding to and activating Epac1, cAMP could modulate the MAPK pathway seemingly through activating B-Raf and/or inhibiting the Ras-Raf pathway (Fimia and Sassone-Corsi, 2001).
The underlying mechanism remains unclear as to why different sources of cAMP can activate divergent downstream pathways. It is speculated that cAMP generation from adenylyl cyclase by forskolin and from GPCR activation may differ spatiotemporally (Gerdin and Eiden, 2007). It is possible that PND works as a Gs/Gq activator or phosphodiesterase inhibitor, but this remains to be addressed. The duration of signaling through Erk may also be a determination of the very different outcomes of different stimulation. Rapid and transient Ras- and Rap1-dependent Erk phosphorylation induced by epidermal growth factor leads to cell proliferation, whereas rapid and sustained Erk phosphorylation induced by NGF induces PC12 differentiation (York et al.,2000). Our results showed that PND can stimulate rapid and sustained two-peak Erk1/2 activation in a PKA-independent manner and the extended duration of Erk1/2 activation may convert cAMP from a proliferation to differentiation cue that promotes neurite outgrowth signaling (Kiermayer et al.,2005).
Increased cAMP levels can potentiate neurotrophin-induced survival effects for certain neurons in the central nervous system (Meyerfranke et al., 1995; Hanson et al.,1998). Bidirectional crosstalk between Erk and cAMP signaling could explain those effects. One of the downstream targets of cAMP signaling is CREB, which plays an essential role in mediating neurite outgrowth and memory formation and consolidation in the nervous system (Silva et al.,1998; Cheng et al., 2002; Gao et al., 2004). Downstream of ERK1/2, ribosomal protein S6 kinase and mitogen- and stress-activated protein kinases regulate CREB phosphorylation (Xing et al., 1996; Gupta et al., 2002; Hauge et al., 2006).Our results show that PND induces sustained CREB activation, leading to neuronal differentiation.

Figure 6 PND induced neurite outgrowth in an Epac1-dependent manner.
In summary, regardless of other potential underlying mechanisms involved, the present study strongly implicates PND as a novel natural activator of Epac1 that initializes and increases neurite elongation in PC12 cells through the cAMP-Epac1-Erk-CREB pathway. Based on neuritogenic and neuroprotective effects in combination with its lipophilic character (Hao et al., 2005; Nie et al., 2006, 2008a; Wang et al., 2006a, b; Zhu et al., 2008; He et al., 2009; Yang et al.,2010), PND may possess beneficial effects for preventing and treating certain neurodegenerative diseases, such as Alzheimer’s disease. However, the neurogenesis effect of PND needs to be further confirmed in primary neural cells and tested in vivo.
Author contributions: BMN, WPL and YL designed the study. BMN,WPL, KM, XYJ and RY performed the experiments. BMN, WPL and KM analyzed data. BMN, WPL, PHL and YL wrote the paper. All authors approved the final version of the paper.
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support: The study was supported partly by the National Natural Science Foundation of China, No. 30873057, 81171245; a grant from the Key Basic Project of Shanghai Municipal Science and Technology Commission of China, No. 08JC1413600, 11JC1406600. The funding bodies played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
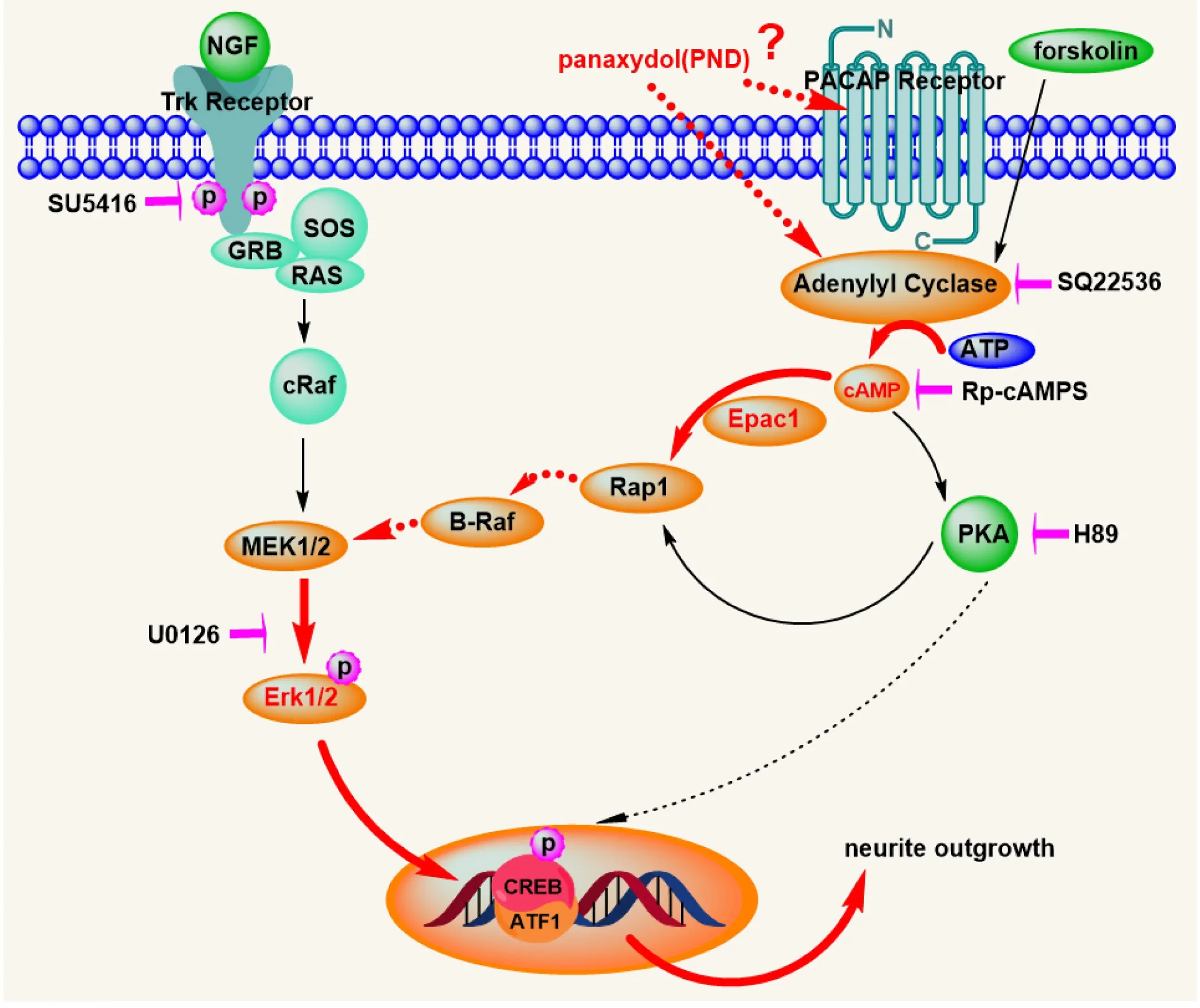
Figure 7 Schematic representation of the signal transduction pathway activated by PND in PC12 cells.
Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- The unfolded protein response signaling and retinal Müller cell metabolism
- Sequencing of high-efficacy disease-modifying therapies in multiple sclerosis: perspectives and approaches
- Targeting prion-like protein spreading in neurodegenerative diseases
- Cadmium-induced neurotoxicity: still much ado
- Analysis of the traf ficking system in blood-brain barrier models by high content screening microscopy
- Retinal remodeling following photoreceptor degeneration causes retinal ganglion cell death