Effect of acoustic stimuli in patients with disorders of consciousness: a quantitative electroencephalography study
Min Wu, Wang-Xiao Bao, Jie Zhang, Yang-Fan Hu, Jian Gao, Ben-Yan Luo,
1 Department of Neurology & Brain Medical Centre, First Af filiated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang Province,China
2 Department of Computer Science, Zhejiang University, Hangzhou, Zhejiang Province, China
3 Department of Rehabilitation, Hangzhou Hospital of Zhejiang Armed Police Corps, Hangzhou, Zhejiang Province, China
Abstract Auditory stimuli are proposed as beneficial neurorehabilitation methods in patients with disorders of consciousness. However, precise and accurate quantitative indices to estimate their potential effect remain scarce. Fourteen patients were recruited from the Neuro-Rehabilitation Unit of Hangzhou Hospital of Zhejiang Armed Police Corps of China. Altogether, there were seven cases of unresponsive wakefulness syndrome ( five males and two females, aged 45.7 ± 16.8 years) and seven cases of minimally conscious state (six males and one female,aged 42.3 ± 20.8 years). Simultaneously, fourteen healthy controls (10 males and 4 females, aged 51.7 ± 9.7 years) also participated in this case-control experiment. Brain response to music, subjects’ own name, and noise was monitored by quantitative electroencephalography(QEEG) in the resting state and with acoustic stimulation. Predictive QEEG values in various brain regions were investigated. Our results show that cerebral activation was high in subjects stimulated by their own name, especially in the temporal lobe in patients with disorders of consciousness, and the frontal lobe in the control group. Further, during resting and stimulation, QEEG index (δ + θ/α + β ratio) negatively correlated with the Coma Recovery Scale-Revised score in traumatic disorders of consciousness patients. Hence, we speculate that a subject’s own name might be an effective awakening therapy for patients with disorders of consciousness. Moreover, QEEG index in specific stimulation states may be used as a prognostic indicator for disorders of consciousness patients (sensitivity, 75%; specificity, 50%). This clinical study has been registered at ClinicalTrials.gov (identifier: NCT03385291).
Key Words: nerve regeneration; auditory stimulation; disorders of consciousness; frontal lobe; neuroplasticity; quantitative electroencephalography;Coma Recovery Scale-Revised; awakening; music; subjects’ own name; white noise; neural regeneration
Introduction
Disorders of consciousness (DOC), such as unresponsive wakefulness syndrome (UWS) and minimally conscious state (MCS), are commonly caused by severe brain damage.Proposed by Laureys, UWS is the new term for a vegetative state characterized by “wakeful unawareness” (Laureys et al., 2010). Patients in UWS show spontaneous eye opening,breathing, and occasionally meaningless limb movement,but with no evidence of awareness. Meanwhile, patients that demonstrate reproducible but fluctuating behavioral evidence of awareness of self or their environment are considered to be in MCS (Machado, 2002).
In the last decade, great efforts have been focused on identifying efficient awakening methods for DOC. Sensory stimula-tion (especially acoustic stimuli) is an experimental awakening approach that exhibits superiority over non-invasive methods and has low risk. It is based on the idea that sensory stimulation may potentially affect neural networks, accelerate brain plasticity, and avoid sensory deprivation (Herdener et al., 2010; Luo et al., 2012; Zhu et al., 2014). The “arousal and mood hypothesis”explains the enhancing effect of music and a subjects’ own name (SON). As emotionally salient content, music and SON have proven to elicit enhanced spontaneous brain responses in distributed brain networks. Moreover, they activate limbic and paralimbic structures, as well as the reward circuit (Janata,2009; Castro et al., 2015; Schnakers et al., 2016; Tacikowski and Ehrsson, 2016). Similarly, the moderate brain arousal model postulates that white noise regulates dopamine transmission and facilitates signal transmission in the brain (Soderlund et al., 2016). Dopamine is related to attention, cognition, and motivated behavior (Sikström and Söderlund, 2007). There are current studies on the effectiveness of auditory stimulation for DOC, but the results vary because of the various assessment indicators, heterogeneous patient groups, and multifarious sound characteristics of the stimulation materials used in different studies. Hence, in this study, we compared stimulation awakening effects in the same subjects, who were stimulated with three acoustic stimuli: music, SON, and white noise.
The Coma Recovery Scale-Revised (CRS-R) is a widely used behavioral assessment scale for DOC (American Congress of Rehabilitation Medicine et al., 2010; Gerrard et al., 2014).However, high test-retest and interrater variability can in fluence the accuracy of clinical assessment (Løvstad et al., 2010).Quantitative electroencephalography (QEEG) as a non-invasive and objective assessment method may qualify as an alternative. The power spectrum is divided into four bandwidths(δ, θ, α, β). Among these, increased δ and θ activity usually reflects encephalopathy and/or structural lesions, which are interpreted as poor outcome predictors for DOC (Fingelkurts et al., 2011). Additionally, α and β power is associated with chance of recovery (Babiloni et al., 2009). A previous study found that δ+θ/α+β value was a sensitive index for brain function in DOC. Further, CRS-R score is strongly associated with spectral EEG at rest (Lechinger et al., 2013). However, there is insuf ficient evidence on validity of clinical prognosis, and few studies have explored the diagnostic and prognostic use of QEEG with stimulation settings. Functional neuroimaging demonstrates that DOC prognosis can be predicted by analysis of specific brain areas (Leon-Carrion et al., 2012). In contrast to QEEG studies on precise temporal resolution, analysis of distinct lobes is scarce, and only a few studies have investigated the predictive value of QEEG in susceptible regions.
Thus, in this study, we examined brain activation to music, SON, and white noise (as determined by QEEG in both the resting state and to acoustic stimulation) to investigate the predictive value of QEEG in various brain regions of DOC patients.
Participants and Methods
Participants
In this case-control study, 14 patients (11 men and 3 women) were recruited from the Neuro-Rehabilitation Unit of Hangzhou Hospital of Zhejiang Armed Police Corps of China. Inclusion criteria were: (1) age > 18 years; (2) diagnosis of UWS and MCS based on CRS-R (American Congress of Rehabilitation Medicine et al., 2010; Gerrard et al., 2014); (3)at least one side showing no auditory injury, as evaluated by Brainstem Auditory Evoked Potentials (Fellinger et al., 2011);(4) no history of neurological or psychiatric disease and with stable vital parameters; and (5) the guardian had provided informed consent and signed the consent form. Exclusion criteria were: (1) significant neurological history; and (2)had received centrally acting drugs, neuromuscular function blockers, or sedating drugs 24 hours prior to study (Fellinger et al., 2011). CRS-R was conducted by medical staff before the experiment, and each patient’s outcome was assessed using the Glasgow Outcome Scale (GOS). A GOS value of < 3 was considered a bad recovery, while a GOS value of ≥ 3 was considered a good recovery (Schnakers et al., 2008).
Fourteen age-matched hospital staff (10 men and 4 women, 51.7 ± 9.7 years old) also participated in the experiment as healthy controls. None of the individuals had any history of audiological or neurological disease.
The experiment was performed in agreement with the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the First Af filiated Hospital, School of Medicine, Zhejiang University, China (approval No.2015310) on 20 August 2015. The clinical study has been registered at ClinicalTrials.gov (NCT03385291).
Stimulation and procedure

Figure 1 Overview of methods.
A 5-minute baseline silence was followed by presentation of three contrasting auditory stimuli, with 2-minutes of wash-out silence separating each stimulus. The music therapy stimulus was folk music (named MOLIHUA). Non-music therapy stimuli were SON (repeatedly called by the relatives)and white noise (Fellinger et al., 2011; Lechinger et al., 2013)(Additional file 1). To control for order effects, the stimuli sequence was randomized. All auditory material was administered at 60–70 dB, which corresponds with the rhythm of the human body. A plug-type earphone was used. Each stimulus lasted for 5 minutes and the task was performed in a silent room (Figure 1).
EEG acquisition and analysis
Scalp EEG potentials were continuously recorded according to the 10–20 International System with a Brain Amp EEG amplifier (Brain Amp/Vision system; Brain Products GmbH,Gilching, Germany). The sampling rate was set at 512 Hz.An analog bandpass filter from 0.1 to 200 Hz was used. Impedances were maintained below 10 kΩ. All data were bandpass filtered between 1 and 30 Hz, and an automatic ocular correction was applied to the raw data. The power spectrum was divided according to frequency into δ (1.0–4.0 Hz), θ(4.0–8.0 Hz), α (8.0–13.0 Hz), and β (13.0–30.0 Hz) waves.
Statistical analysis
Statistical analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
In the first step, independent samples t-test and chi-square test were performed to test for group differences between factor groups (MCS vs. UWS).
Second, to determine the strength of individual stimuli,one-way repeated-measures analysis of variance with Bonferroni correction was applied. To investigate topographic distribution of QEEG, one-way repeated-measures analysis of variance was calculated for the UWS and MCS groups.
To assess potential relationships between the patients’ behavioral presentation and cortical activity, Pearson correlations were calculated between CRS-R score and δ + θ/α + β value over all scalp sites and between the four brain lobes.
A value of P < 0.05 was considered statistically significant.
Results
Comparison of general data in MCS and UWS groups
The 14 patients included in this study were diagnosed as UWS (n = 7) or MCS (n = 7) based on clinical assessment at the time of EEG monitoring. Mean age was 42.3 ± 20.8 years in the MCS group, and 45.7 ± 16.8 years in the UWS group. There was one woman in the MCS group and two women in the UWS group. All MCS patients and two UWS patients involved traumatic injury. Of the remaining five UWS patients, four had hypoxic brain injury and one had intracerebral hemorrhage. Disease duration was shorter in the MCS group compared with the UWS group (3.02 ± 1.06 months vs. 4.97 ± 3.58 months). Both groups were matched for age, sex, and disease course (P > 0.05), but etiology was statistically different (P = 0.021; Tables 1, 2).
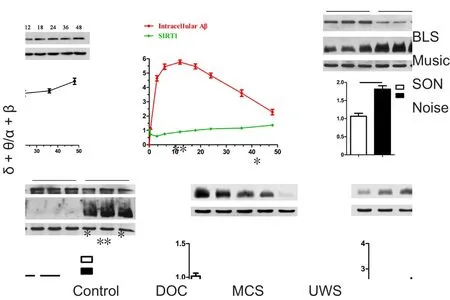
Figure 2 δ + θ/α + β value for all participants in four states.
CRS-R score and δ + θ/α + β value in MCS and UWS patients CRS-R score was higher in the MCS group than UWS group(9.43 ± 2.37 vs. 5.14 ± 1.68; P = 0.002). Nevertheless, there was obvious CRS-R overlap between groups. Regardless of resting or acoustic settings, δ + θ/α + β ratio was higher in the UWS group than MCS group (16.58 vs. 15.41; P > 0.05; Figure 2).
Comparison of QEEG with different acoustic stimulations
In the control group, δ + θ/α + β value was lower with acoustic stimulation than the resting state (P < 0.01). This was particularly apparent with SON, followed by music and white noise. Moreover, the difference between SON and music was significant (P = 0.035). No significant difference was found between music and white noise (P = 0.537). In DOC patients,SON was the most sensitive stimulus. In addition, white noise and music activated more areas of the cerebral cortex than silence. In contrast, there were no statistical differences in δ+ θ/α + β value at resting with all three stimulations in the UWS group. Meanwhile in the MCS group, brain activity was increased with SON (P = 0.013, vs. resting state), while the difference between music and resting state showed marginal significance (P = 0.058). Activation was not obvious with white noise (P = 0.139, vs. resting state; Figure 2).
Signal intensity in the four lobes
During music stimulation, δ + θ/α + β value was higher in the parietal and occipital lobes than the frontal and temporal regions in both the control and DOC groups (control: frontal, 0.578; parietal, 1.174; temporal, 0.825; occipital, 1.199;and DOC: frontal, 9.033; parietal, 11.634; temporal, 8.433;occipital, 16.520). When stimulated with SON, brain response in the frontal-temporal lobes (frontal, 1.241; temporal, 1.225) was more active than the parietal-occipital lobes of the control group (parietal, 1.627; occipital, 1.838; P > 0.05).In DOC, the temporal lobe was most activated, with a statistically significant difference between the four lobes (frontal,11.461; parietal, 11.691; temporal, 7.652; occipital, 11.273; P <0.05). Signal intensity in the four structures showed no obvious difference in DOC for white noise (P > 0.05). In contrast,δ + θ/α + β value was significantly lower in the frontal lobe than other areas of the control group (P < 0.05; Figure 3).

Table 1 Demographic and clinical data of the study sample of MCS and UWS groups

Table 2 Comparison of general data in patients with disorders of consciousness
Correlation of δ + θ/α + β ratio and CRS-R score
Absolute δ + θ/α + β value changed with music and CRS-R score, as shown by a tendency for negative association (R= −0.541, P = 0.046). However, there was no relationship between the ratio and CRS-R score (R = −0.241, P = 0.407).All patients were categorized by etiology into traumatic and non-traumatic groups. Robust negative correlation was detected between CRS-R score and δ + θ/α + β ratio in both the resting state (R = −0.785, P = 0.012) and with stimulation.Furthermore, using SON, change in δ + θ/α + β signal was associated with CRS-R score (R = −0.771, P = 0.015; Figure 4).
In addition, correlation was examined between CRS-R score and δ + θ/α + β value in specific frontal regions (the brain area most relevant to consciousness). Accordingly, δ+ θ/α + β value in the frontal lobe was not correlated with CRS-R in any subjects, but greater association was observed in the traumatic group (Figure 5).
Predictive value of QEEG
Increased cerebral response to acoustic stimuli appeared to be linked to patient outcome. Nine patients (9/14) developed a significant discriminative response to stimuli.Among the nine patients, six displayed a favorable outcome(GOS ≥ 3), while the remaining three remained in the same state or died (GOS < 3). In the other five subjects with no significant increase in brain response, two showed good recovery, while the remaining three had a bad recovery. Thus,positive predictive value of QEEG at 1 year was 66.7%, while negative predictive value was 80%. Moreover, predictive value of QEEG signal change to a good recovery was highly sensitivity (sensitivity, 75%; specificity, 50%).
Discussion
In this study, we detected cerebral responses of 14 DOC patients using acoustic stimulation and present three main findings. First, SON evoked the most salient cerebral activation compared with music and white noise. Second, the temporal lobe was markedly activated by saliently emotional stimulation in DOC, while the frontal lobe was most activated in healthy controls. Third, QEEG correlated with behavioral CRS-R assessment score in patients with traumatic injury and may be an indicator for prediction of 1-year prognosis.
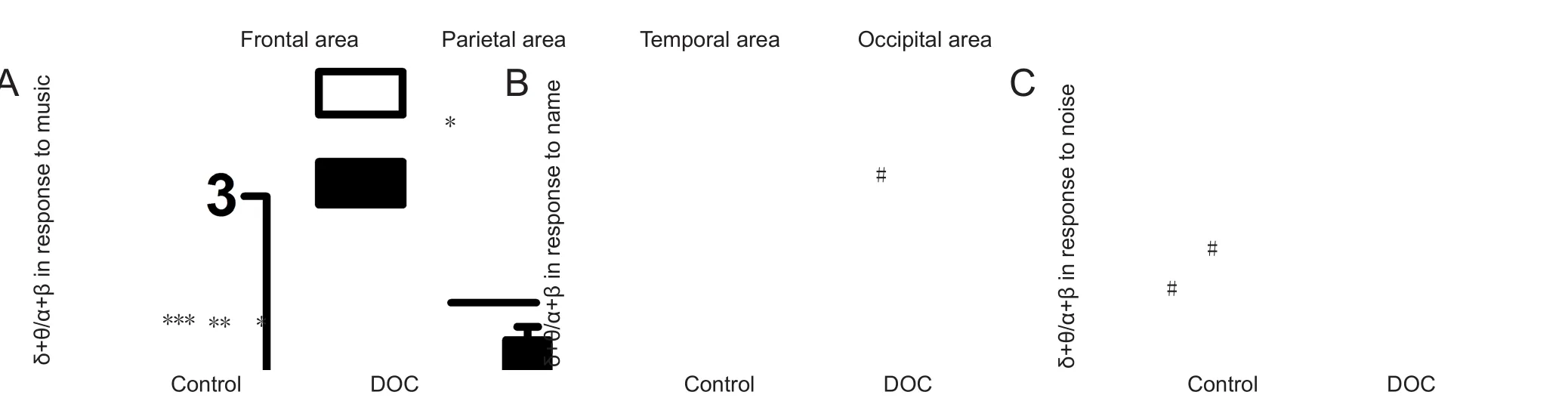
Figure 3 Effect of acoustic stimuli on different brain regions.
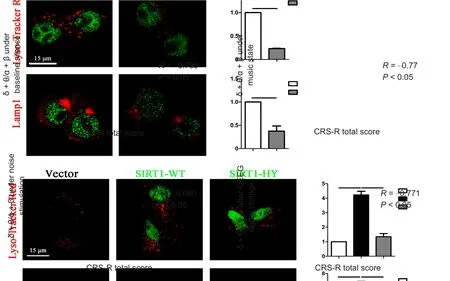
Figure 4 Correlation between CRS-R total score and δ + θ/α + β value in traumatic DOC analyzed by Pearson correlation analysis.

Figure 5 Correlation between CRS-R total score and δ + θ/α + β ratio in the frontal lobe analyzed by Pearson correlation analysis.
Reviewing real-time cerebral responses of DOC patients to three acoustic stimuli, our results are consistent with previous studies (Laureys et al., 2004; Coleman et al., 2007).Altogether, they confirm the superiority of SON and music as awakening stimuli. In addition, our study suggests that brain activation is weaker in response to music than SON.Our explanations are as follows: first, differences in cultural background and personal music preference may be involved.Further, a previous study compared the difference between two extreme stimuli, such as preferred and disliked songs(Wilkins et al., 2014). In our study, common auditory stimulation was selected, which may narrow the difference in brain response because stimulation difference was not suf ficiently significant. Regarding white noise, an aversive stimulus can activate certain cerebral structures, including limbic regions(e.g., amygdala and hippocampus) and paralimbic structures(e.g., insular and orbitofrontal cortex) (Mega et al., 1997).White noise can also regulate stimulatory neurotransmitter dopamine transmission or substitute its effects on neural communication, which plays an important role in the state of wakefulness and improves behavior, mood, language, cognition, and motor control (Oliveira and Fregni, 2011). To date,a few studies have suggested that levodopa may be effective for treating DOC, but there is no robust evidence. Our study was unable to conclude that white noise is a recommended awakening therapy for DOC. This is consistent with an event-related potential study suggesting that patients with DOC exhibit abnormal sensory gating, which inhibits their response to repetitive stimuli (Kliuchko et al., 2016).
Subsequently, we examined different levels of cerebral activation between MCS and UWS. It is notable that all MCS were traumatic in our study, while most UWS were non-traumatic. Reviewing previous data, long-term prognosis is worse in patients with anoxia or metabolic encephalopathy than in traumatic subjects (Daltrozzo et al., 2007).Recent studies have shown that traumatic brain damage is accompanied by more gray matter and diffuse axonal injury (Gennarelli et al., 1982; Newcombe et al., 2010), while non-traumatic disorders exhibit greater cerebral cortex, hippocampus, and thalamic injury (Kinney et al., 1994). Consequently, long-term prognosis is often worse in patients with anoxia and metabolic encephalopathy, partly owing to complete impairment in UWS (Adams et al., 2000; Daltrozzo et al., 2007). Furthermore, convincing evidence from Boly et al., (2011) using dynamic causal modeling suggested selective disruption of top-down processes from frontal to temporal areas in the vegetative state, with top-down processing preserved in MCS. Therefore, we propose that acoustic sound can be used with covert cognitive awareness in MCS (Boly et al., 2011). Taken together, we did not detect an obvious cerebral response in UWS with advanced cortical dysfunction in this study, while traumatic MCS showed increased brain activity. This may help retain a certain degree of cognitive function and increase the likelihood of awakening.
In summary, MCS shows an enhanced EEG response to SON, providing evidence for implementation of SON as a resuscitating therapy. Our future studies will aim to examine multiple stimuli and include a longer follow-up time to further clarify the efficacy of auditory stimulation. EEG recordings are recently increasingly used to assess the outcome of DOC. However, these studies are restricted to the resting state. To the best of our knowledge, this is the first study to examine the relationship between QEEG and CRS-R scale with acoustic stimulation, given that preferred music or stimulation of SON are more favorable for expression of residual cognitive function in DOC (Laureys and Schiff, 2012). As a result, emotional salience and autobiographical context are important for accurate evaluation of residual cognitive capacity and prognosis of traumatic DOC. Hence, we suggest that a combination of QEEG in an autobiographical context and CRS-R scale are used to predict outcome of DOC. In addition, we detected a negative correlation between CRS-R score and QEEG signal change in SON, indicating that QEEG for a specific condition may be a more sensitive indicator in prognosis assessment. Notably, we performed a 1-year follow-up of the subjects, and confirmed the effectiveness of QEEG in outcome prediction, particularly for a poorer outcome.
Based on activation differences of MCS and UWS, our study innovatively investigated cerebral response in different brain regions with QEEG and, further, examined prognosis predictive value of specific QEEG. We found that brain activity was weaker in the frontal lobe than the temporal lobe, and the latter correlated with CRS-R score. Thus, we hypothesize that frontal QEEG has a predictive value for recovery from UWS. These results are consistent with functional neuroimaging studies, showing that consciousness and cognitive function are linked to awareness networks consisting of certain brain regions (Laureys and Schiff, 2012; Demertzi et al., 2013,2015), with an intact frontal lobe being a surrogate marker for preserved consciousness (Leon-Carrion et al., 2012). Moreover, the executive control network reflects strong frontal coherence, with all cortical areas sending information to the frontal lobe to be integrated. The executive control network is activated in salient and novel situations (Seeley et al., 2007).Accordingly, the frontal lobe was most activated during music or SON stimulation in our study. In DOC patients, there is reduced connectivity within the frontal regions, especially the medial frontal areas and right middle frontal gyrus (Crone et al., 2014), with reduced EEG activity in these areas. In contrast, there is also activation of the auditory cortex in the temporal lobe (Bekinschtein et al., 2005).
To date, use of EEG as a diagnostic tool of DOC is controversial. One important study demonstrated that entropy could discriminate between UWS and MCS patients with a specificity and sensitivity of 90% in the acute setting (Sarà and Pistoia, 2010). In this study, δ + θ/α + β value was higher in the UWS group than MCS group, but the difference was not significant. Thus, we cannot consider the ratio to be a candidate tool for diagnosis. It is notable that the UWS patients included in our study had CRS-R scores between 6 and 7, close to the boundary between UWS and MCS states. Furthermore,several MCS patients had low to middle CRS-R scores in the 6–10 range. CRS-R overlap in combination with insuf ficient statistical power due to a relatively limited number of patients in each group could have blurred differences between them. As a next step, a study using a larger population and combined EEG and magnetic resonance imaging should be performed for further evaluation.
There are limitations to our study. The relatively small sample size limits reliability of the therapeutic effects of acoustic stimulation. In the context of this enormous disability, clinical trials are challenging to perform because of ethical issues linked to the severe nature of their clinical conditions and inability of pessimistic subjects’ legal guardians to provide informed consent. Additionally, patient heterogeneity may confuse the stimulation effects. Thus, further studies must take etiology into consideration for further research. As for the time course of disease, important variables (e.g., medication,therapy methods, and vital signs) are constantly changing at the early stage of disease, and inclusion of a chronic population would be a way to manage this bias as these patients are more stabilized. Nonetheless, the time course of unconsciousness in patients varied from less than 1 month to 1 year. Indeed, an early attempt to cure is of great importance, and here we have minimized the treatment differences in all patients.
Author contributions: BYL and WXB designed this study. MW and JG performed the experiments. YFH analyzed the data. MW and JZ wrote the paper. All authors approved the final version of this paper.Conflicts of interest: None declared.
Financial support: This study was supported by grants from the General Project Plan of Zhejiang Medical Technology of China, No. 2014RCA007; the Medical Science and Technology Project Co-founded by Zhejiang Province and the Ministry of Health of China, No. 2016152769. The conception, design, execution, and analysis of experiments, as well as the preparation of and decision to publish this manuscript, were made independent of any funding organization.
Institutional review board statement: The protocol has been approved by the Ethics Committee of the First Af filiated Hospital, School of Medicine,Zhejiang University, China (approval No. 2015310) and registered at ClinicalTrials.gov (identifier: NCT03385291). The study followed the relevant laws and regulations of the Declaration of Helsinki.
Declaration of participant consent: The authors certify that they have obtain participant or legal guardian consent forms. In the form, the participants or legal guardians have given consent for the particiants’ images and other clinical information to be reported in the journal. The participants or legal guandians understand that the participants’ names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement: This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Biostatistics statement: The statistical methods of this study were reviewed by the biostatistician of First Af filiated Hospital, School of Medicine, Zhejiang University in China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer: Gentian Vyshka, University of Medicine in Tirana,Albania.
Additional file:
Additional file 1: 16 stimulus materials.
- 中国神经再生研究(英文版)的其它文章
- The unfolded protein response signaling and retinal Müller cell metabolism
- Sequencing of high-efficacy disease-modifying therapies in multiple sclerosis: perspectives and approaches
- Targeting prion-like protein spreading in neurodegenerative diseases
- Cadmium-induced neurotoxicity: still much ado
- Analysis of the traf ficking system in blood-brain barrier models by high content screening microscopy
- Retinal remodeling following photoreceptor degeneration causes retinal ganglion cell death