Pt-CeOx/CNxNT催化剂的高稳定性和高效氧还原性能
付 予 敖红亮 张伶潇 郭雨萌 梁菊梅 张丽娟 李 钒
(北京工业大学环境与能源工程学院绿色催化与分离重点实验室,北京 100124)
Over the past decades,vast resources have been devoted to developing advanced catalysts for lowtemperature fuel cells.Nonetheless,there are still some challenging problems need to be solved,including improving the sluggish oxygen reduction reaction(ORR)kinetics and enhancing the physical/chemical durability of the catalysts[1-2].
It is generally accepted that deposit Pt on support materials can reduce the cost as well as improve the catalytic activity[3-4].Due to the unique properties[5],polypyrrole (PPy)hasbeen received reasonable attention and has been used in electrocatalysts of fuel cells,especially the PPy nanotubes(PPyNTs).The unique hollow structure and the 1D morphology of PPyNTs may provide more surface area for reactions when used as support in electrocatalyst[6].For example,polypyrrole nanotubes(PPyNTs)/PtAu alloy nanoparticle(NP)hybrids,synthesized by Peng and coworkers[7],showed an enhanced catalytic activity as well as durability upon oxygen reduction.Ma′s group[8]synthesized Pt/CNxcatalyst with CNxnanofibers converted from polypyrrole nanowires as support.The catalyst has a large electrochemically active area and good electrocatalytic performance toward methanol oxidation,not only in activity but also instability.It has been proved by many researches that add oxides into catalysts can improve the performance of catalyst[9-11].Among the oxides,CeO2has attracted great concern due to its stability,high oxygen transport and storage capacities[12-14].
Herein we use CNxnanotube (CNxNT)converted from polypyrrole nanotubes as support to synthesize Pt-CeOx/CNxNT catalyst by a facile method.In this process,Pt-CeOx/CNxNT catalyst was synthesized,the stability and electrocatalytic activity towardsthe oxygen reduction reaction were examined.The result shows that the Pt,CeOxparticles are well-dispersed on the supports surface and gives an enhanced performance.
1 Experimental
1.1 Synthesis of CNxnanotube
The materials used were all analytical purity.Firstly,PPyNTs were synthesized using a self-degraded template method as reported in the literature[15](ESI).Then the PPyNTs were heated under N2atmospherein a tube furnace from room temperature to 900℃at a rate of 5 ℃·min-1and kept for 2 h,the product collected was CNxnanotube.
1.2 Synthesis of Pt-CeOx/CNxNT
Pt-CeOx/CNxNT catalyst was prepared by a facile one-pot method.Briefly,dissolve 65 mg CNxNT and 38.0 mg Ce(NO3)3·6H2O into 20 mL ethylene glycol and 5.5 mL chloroplatinic acid solution(10 g·L-1,H2PtCl6·6H2O),respectively.Then,these two solutions were sonicated for 30 min.Mix these two solutions and drop 15 mL aqueous NaOH solution (0.15 mol·L-1)into the result solution while stirring.After stirring for 1 h at room temperature,the solution was transferred into a 100 mL Teflon-lined stainless autoclave and heated at 180℃for 8 h.When solution was cooled to room temperature,the product was filtrated,washed with distilled water and ethanol in turn,then dried at 60℃for 1 day.As comparison,Pt/CNxNT was also prepared in the same way without the addition of Ce(NO3)3·6H2O.
1.3 Synthesis of Pt-CeO2/C
Pt-CeO2-15/C catalyst was prepared by a facile one-pot method.Briefly,dissolve 65 mg XC-72 and 38.0 mg Ce(NO3)3·6H2O into 20 mL ethylene glycol and 5.5 mL chloroplatinic acid solution (10 g·L-1,H2PtCl6·6H2O),respectively.Then,these two solutions were sonicated for 30 min.Mix these two solutions and drop 15 mL aqueous NaOH solution (0.15 mol·L-1)into the result solution while stirring.After stirring for 1 h at room temperature,the solution was transferred into a 100 mL Teflon-lined stainless autoclave and heated at 180℃for 8 h.When solution was cooled to room temperature,the product was filtrated,washed with distilled water and ethanol in turn,then dried at 60℃for 1 day.As comparison,Pt-CeO2-0Ce/CNxNT was also prepared in the same way without the addition of Ce(NO3)3·6H2O.
1.4 Characterization
The crystalline phases of products were characterized by X-ray diffraction (XRD)analysis(Bruker D8 Advance diffract to meter):Cu Kα (λ=0.154 nm),U=40 kV,I=40 mA,2θ=10°~80°.The morphologies of the productswere characterized by transmission electron microscopy(TEM).TEM images were obtained by using a JEOL JEM2010 transmission electron microscope operated at 200 kV.High-resolution transmission electron microscopy(HRTEM)images and the high-angleannulardark-field scanning transmission electron microscopy(HAADF-STEM)images,element analysis mapping were carriedout on a Tecnai G2 F20 field emission transmission electron microscopy(FEI)operated at 200 kV.The composition of the sample was determined by inductively coupled plasma atomic emission spectrometry(ICP-AES).The surface chemical states of the samples were characterized by XPS(Kratos Analytical Ltd,Axis Ultra).
1.5 Electrochemical characterizations
Electrochemical measurements were carried out by using a standard three-electrode glass cell.A reversible hydrogen electrode (RHE)and Pt foil were used as the counter electrode and the reference electrode,respectively.Samples for the electrochemical measurement were prepared by dispersing about 2 mg of catalyst in 3 mL ethanol,then transfer 10 μL of the suspension onto a glassy carbon substrate.After the ethanol was evaporated,the electrode was covered with 5 μL of 0.05%(w/w)Nafion solution(Aldrich),and allowed to stand for several hours.CV measurements were carried out in an N2-purged 0.1 mol·L-1HClO4solution at a scan rate of 100 mV/sunder ambient conditions.The ORR was performed by using a rotating disk electrode (RDE)in an O2-saturated aqueous solution of 0.1 mol·L-1HClO4with a scan rate of 10 mV·s-1under ambient conditions.The accelerated durability tests were performed in an N2-purged 0.1 mol·L-1HClO4solutions at a sweep rate of 100 mV·s-1for 3 000 cycles.
2 Results and discussion
To identify phase structure of the obtained Pt-CeOx/CNxNT,the sequence of X-ray diffraction(XRD)curves was shown in Fig.1.A weak and broad peak at~26°was observed in line b,demonstrating the transformation progress of PPy nanotubes to CNxnanotubes with annealing at 900℃.The feature diffraction peaks of Pt/CNxNT are consistent with cubic Pt(space group Fm3m),which are indexed to be the (111),(200),(220),(311)and(222)facets(line c and line d)[39-40].However,no peaks of CeOxare observed,which might implied that either the CeOxcontent is too low,or the CeOxexists in the amorphous state.In order to clarify this issue,the composition of different samples were determined by ICP-AES,confirming the existence of CeOx(Table 1).

Fig.1 XRD patterns of the PPy nanotubes(a),CNx nanotubes(b),Pt/CNxNT catalyst(c)and Pt-CeOx/CNxNT catalyst(d)
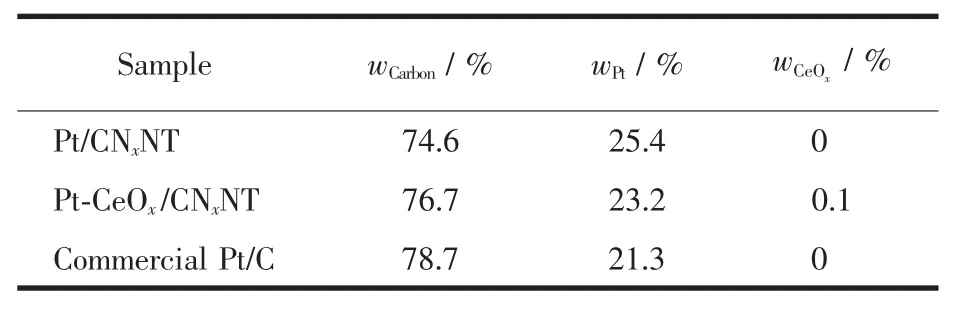
Table 1 Composition of different samples
Fig.2a,b depict the TEM images of as prepared PPy nanotubes (Fig.2a)and CNxnanotubes(Fig.2b),indicating PPy nanotubes still maintained the tubelike morphology although annealing at 900℃with an average length of several microns and an average diameter of 90 nm.Well-defined nanoparticles deposited on CNxwith uniform distribution,narrow diameter distribution and onlya few aggregated nanoparticles(Fig.2d).It was also clearly seen in Fig.3b,from the size-distribution histograms inserted in Fig.3b,the particle size distribution is mainly in the range of 3.5 to 6 nm and the mean particle diameters of Pt-CeOx/CNxNT is 4.90 nm (based on measuring of 200 particles),within the range of optimal Pt size[16].
In contrast with this observation,Pt/CNxNT was prepared in the same way,however,appearing agglomeration phenomenon seriously(Fig.2c).Therefore,it can be inferred that the intermediate product Ce(OH)3plays an key role information of well-dispersed small size Pt nanoparticles.We believe the Pt precursor would be adsorbed nearby the interface between Ce(OH)3and CNxNT,and the Ce(OH)3acted as anchoring sites to prevent Pt particles from growth and agglomeration.This may similar to the useful interfacial reaction space between Ce(OH)3and CeOxnanowire for the formation of nano sized Pt particles[17].
The crystalline structure of was further confirmed byHRTEM.Fig.3dshowsanenlargedHRTEM images of Pt-CeOx/CNxNT catalysts,within which the lattice spacing was measured to be 0.227 nm,in agreement with the(111)interplanar distance of face-centered cubic(fcc)Pt bulk.But no lattice fringes of cerium oxide were observed.
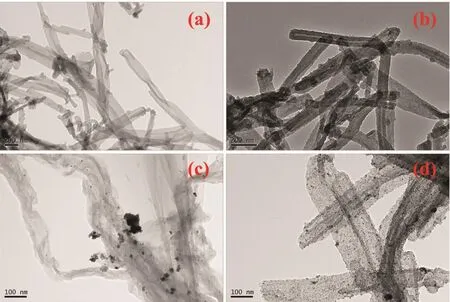
Fig.2 TEM images of PPy nanotubes(a),CNxnanotubes(b),Pt/CNxNT catalyst(c)and Pt-CeOx/CNxcatalyst(d)
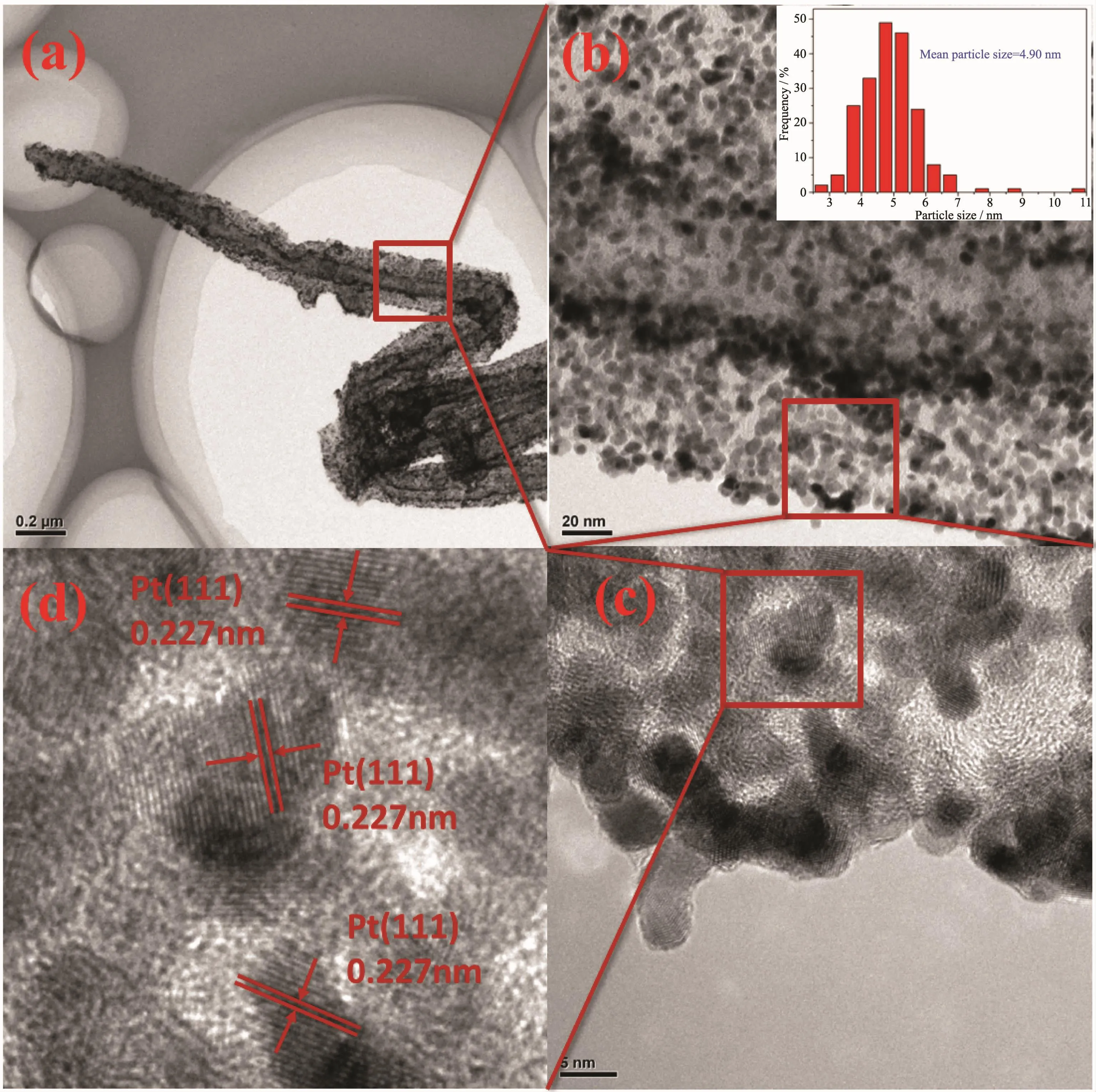
Fig.3 (a)TEM image of Pt-CeOx/CNxNT catalysts;(b)Enlarged image of a local in(a)and the size-distribution histogram(inset);(c)HRTEM image of Pt-CeOx/CNxNT catalysts of selectedregion in(b);(d)Enlarged HRTEM image of nanoparticles marked in(c)
To research the internal structure of Pt-CeOx/CNxNT in detail,HAADF-STEM and EDS mapping images results were obtained (Fig.4).Fig.4b is the HAADF image of selected region in Fig.4a,and Pt,Ce,N and O elements were detected,corresponding elements distribution of the Pt-CeOx/CNxNT shown in Fig.4c~g.The mapping shapes of all elements are similar to that of Pt-CeOx/CNxNT catalyst in a tubelike morphology,which implies the catalyst has a uniform element dispersion and composition.
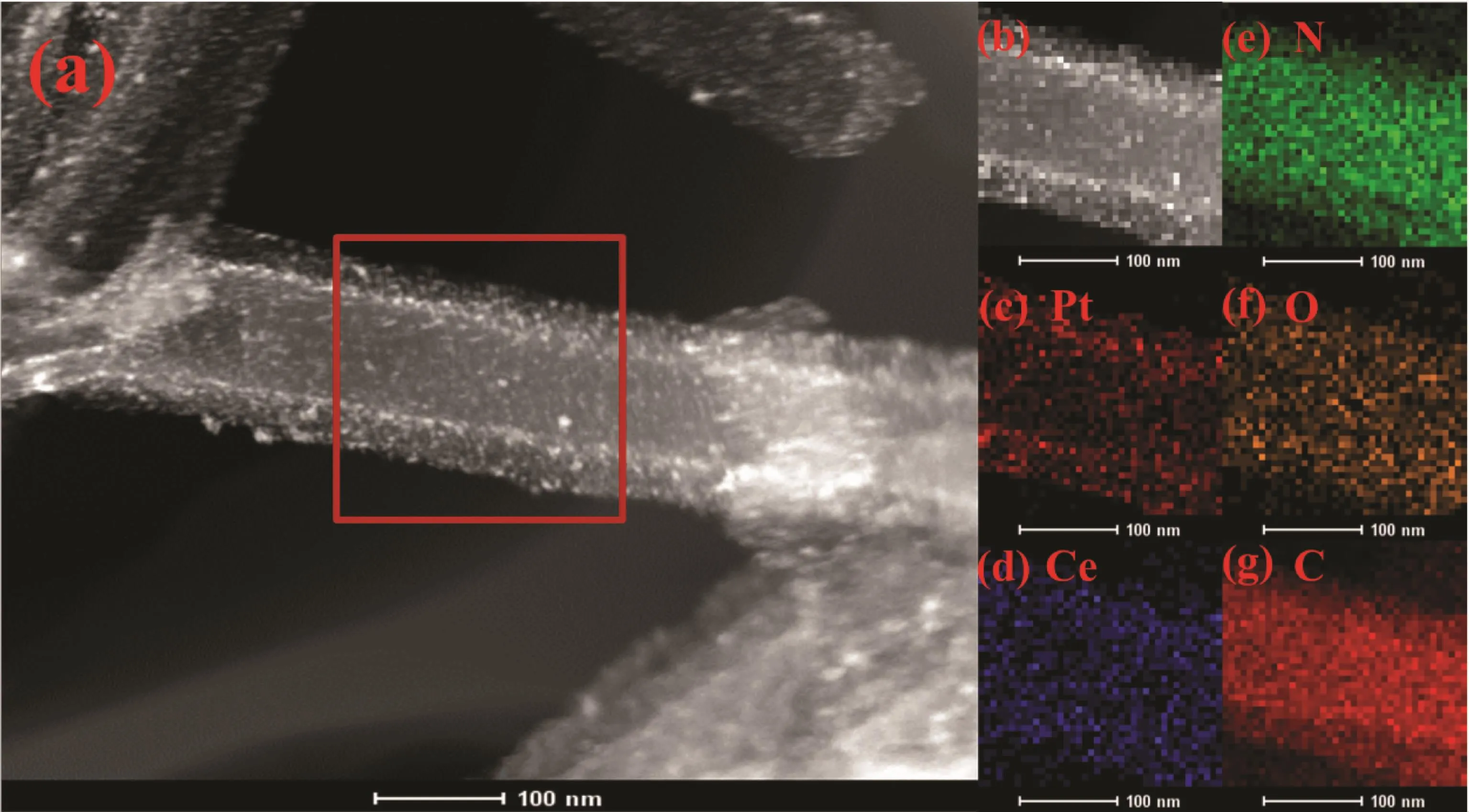
Fig.4 (a)HAADF-STEM image of Pt-CeOx/CNxNT;(b)HAADF image of selected region in(a);Mapping of Pt(c),Ce(d),N(e),O(f)and C(g)from(b)
The chemical states of the surface of Pt/CNxNT catalyst and Pt-CeOx/CNxNT catalyst were investigated by XPS.The full XPS spectra for Pt/CNxNT and Pt-CeOx/CNxNT shown in Fig.5 revealed the presence of Pt and N element.The detailed analysis of the Pt4f spectrum showed that the peak of Pt-CeOx/CNxNT(Fig.6b)occurring at 71.37 and 72.09 eV presented a positive shift than that of Pt/CNxNT(Fig.6a)at 71.14 and 71.64 eV,which positions were attributed to Pt0.It might be ascribed to this reason that the existence of CeOxenhanced the metal-support interaction between Pt nanoparticles and support.In Fig.7,the N1s spectrum can be further separated into three peaks at 398.6 eV(pyridine-like nitrogen)[19-20],401 eV(pyrrolelike nitrogen)[20-21]and 403 eV(oxidized nitrogen)[20,22],respectively.The binding energies of N1s along with their relative intensities are provided in Table 2.Compared with Pt/CNxNT,Pt-CeOx/CNxNT has higher ratio of pyridine-like nitrogen,which can be beneficial to the ORR activity[20,23].
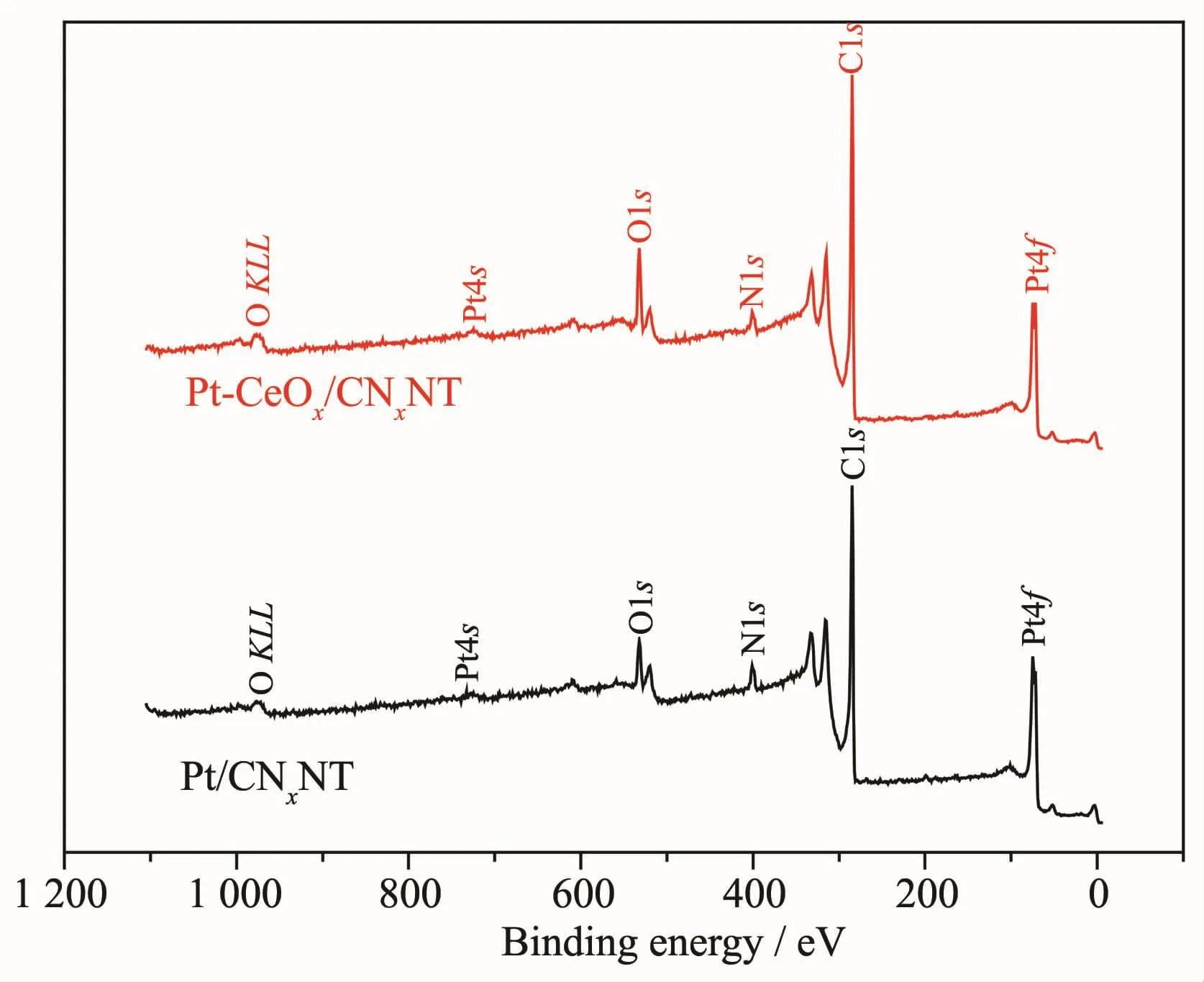
Fig.5 XPS spectra for Pt/CNxNT and Pt-CeOx/CNxNT
Because of the CeOxcontent in the Pt-CeOx/CNxNT is rather superficial.In order to get more information of the interaction between Pt and CeOx,we detect the Pt-CeO2/C′s XPS.As shown in Fig.8,Relative to pure Pt-CeOx-0/C(71.4 eV),there is a downshifted of the binding energies of Pt4f7/2(71.1 eV).According to the researcher of Adam Lewera and his partner[35],We speculate that this is because of the“strong metal-support interaction,SMSI”,and this also means that there has interaction between Pt and CeO2.In the CeO2-15′Ce3d XPS spectrum,The 882.6,888.2,901.3,907.6 and 916.9 eV are the peaks of Ceビ.And the 885.4 and 903.9 eV are the peaks of Ceバ[37].This result prove that the existence of Ceバand Ceビ.Under normal contion,Ceバ and Ceビ will both be available in the CeO2.When the metal atoms are in contact with cerium dioxide will accelerate the transform between Ce4+→Ce3+[36].The little Ce3+component in CeO2could suppress the formation of Pt oxide at high potentials by the substituted oxidation of Ce3+to Ce4+,which is bene-ficial for improving the catalytic performance of Pt for ORR[38].
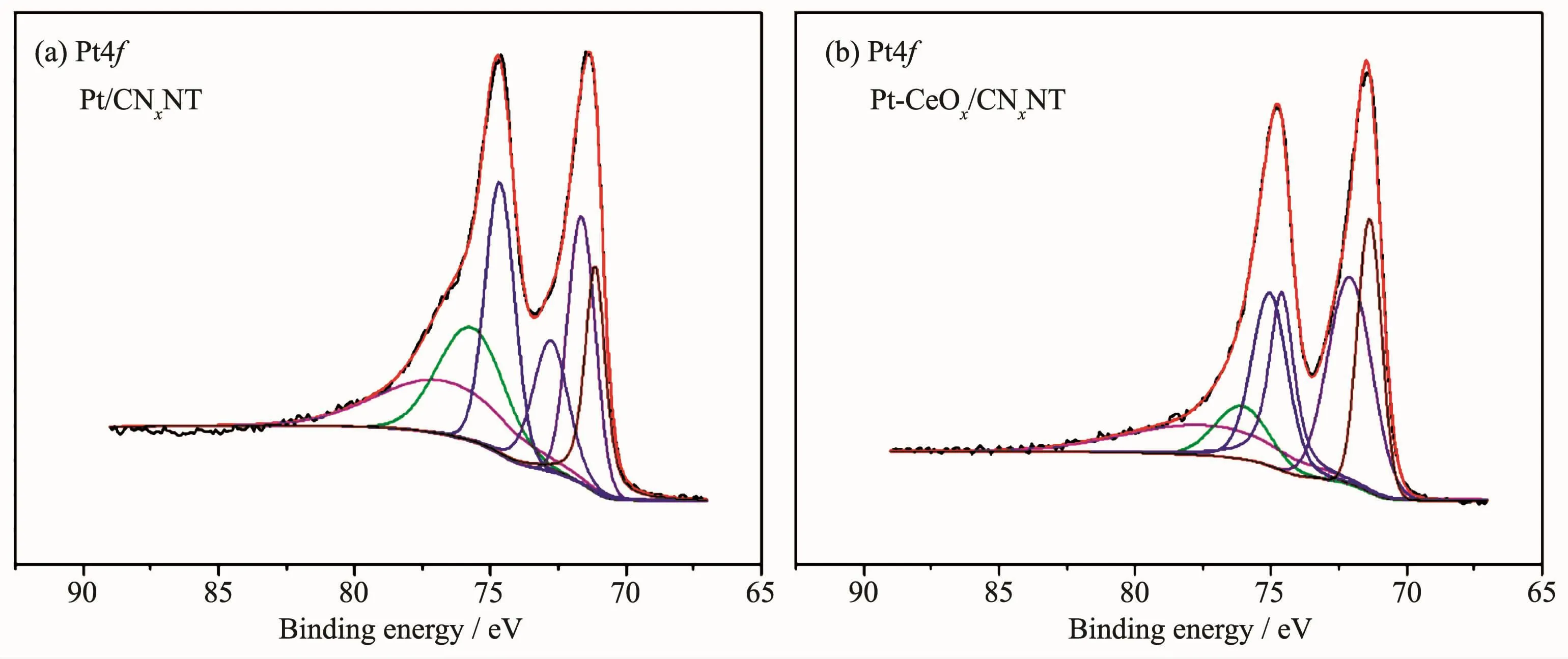
Fig.6 Pt4f XPS spectra for Pt/CNxNT(a)and Pt-CeOx/CNxNT catalyst(b)
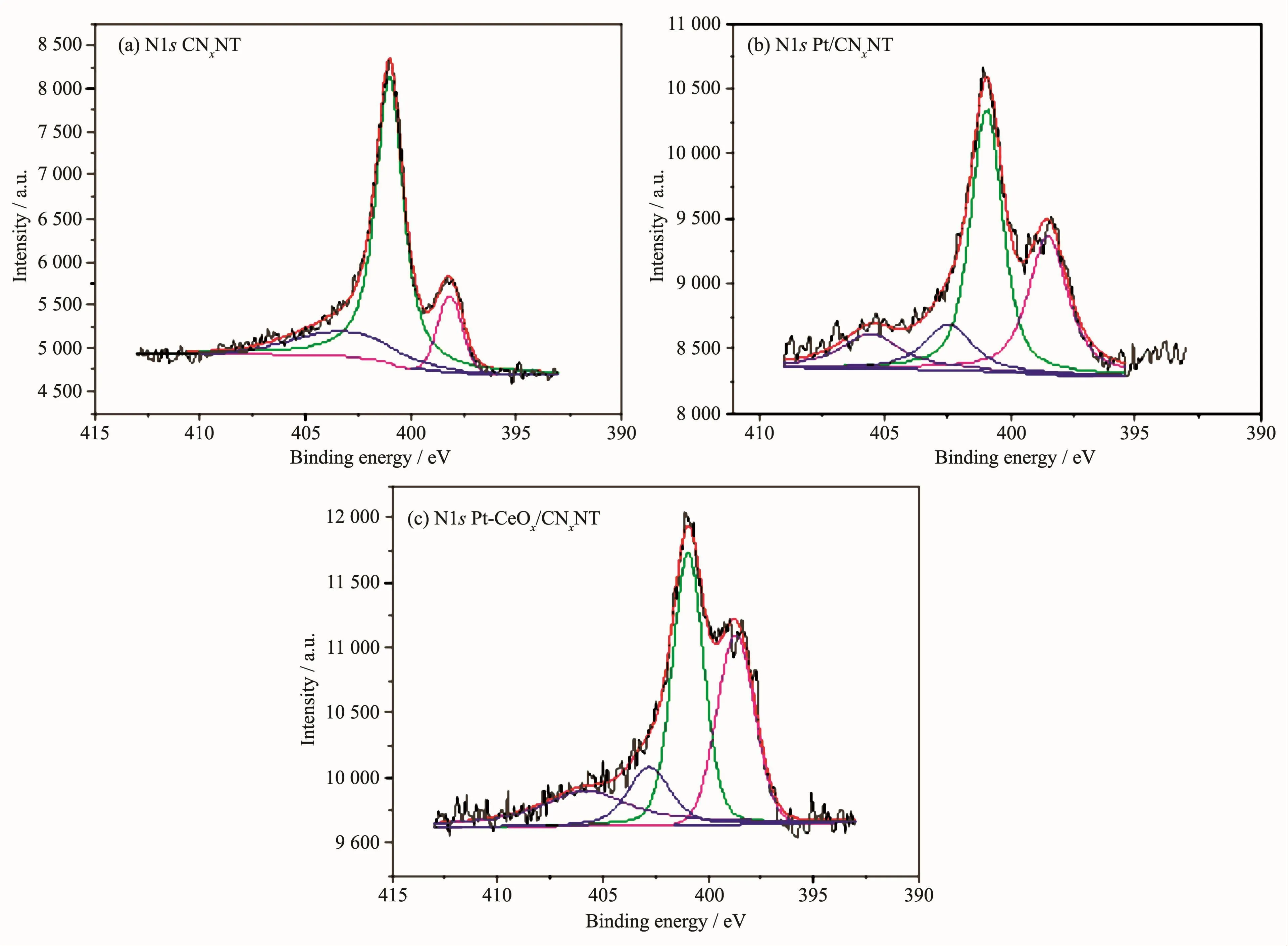
Fig.7 (a)N1s XPS spectra for CNxNT;(b)N1s XPS spectra for Pt/CNxNT;(c)N1s XPS spectra for Pt-CeOx/CNxNT
Fig.9a shows the cyclic voltammetry(CV)curves of Pt-CeOx/CNxNT,Pt/CNxNT and commercial Pt/C.Hydrogen adsorption/desorption appear in the range 0 to 3.5 V,which region was used to calculate ECSA of Pt-based catalysts.As can be seen in Fig.9b,the specific ECSA of Pt-CeOx/CNxNT was found to be 42.6 m2·gPt-1,66.7%that of commercial Pt/C catalyst(63.9 m2·gPt-1),and Pt/CNxNT exhibited the least specific ECSA(14.8 m2·gPt-1).The higher ECSA of commercial Pt/C can be ascribed to its small size(mean Pt particle size is 2.22 nm,Fig.10),and extensive agglomeration of Pt/CNxNT also needs to take into consideration.Subsequently,to further investigate the ORR activity,RDE was performed in 0.1 mol·L-1HClO4solution.Fig.9c shows the ORR polarization curves for Pt/CNxNt,Pt-CeOx/CNxNT and commercial Pt/C catalysts.The kinetic current(Jk)of Pt-CeOx/CNxNT at 0.85 V(vs RHE)was calculated from ORR polarization curves by the Koutecky-Levich equation[24].And the kinetic current(Jk)of Pt-CeOx/CNxNT is 258.6 mA·mgPt-1,1.66 and 1.78 times greater than that of commercial Pt/C catalyst(155.7 mA·mgPt-1)and Pt/CNxNT(145.1 mA·mgPt-1),respectively (Fig.9d),indicating a higher catalytic activity for the Pt-CeOx/CNxNT than the other catalysts.
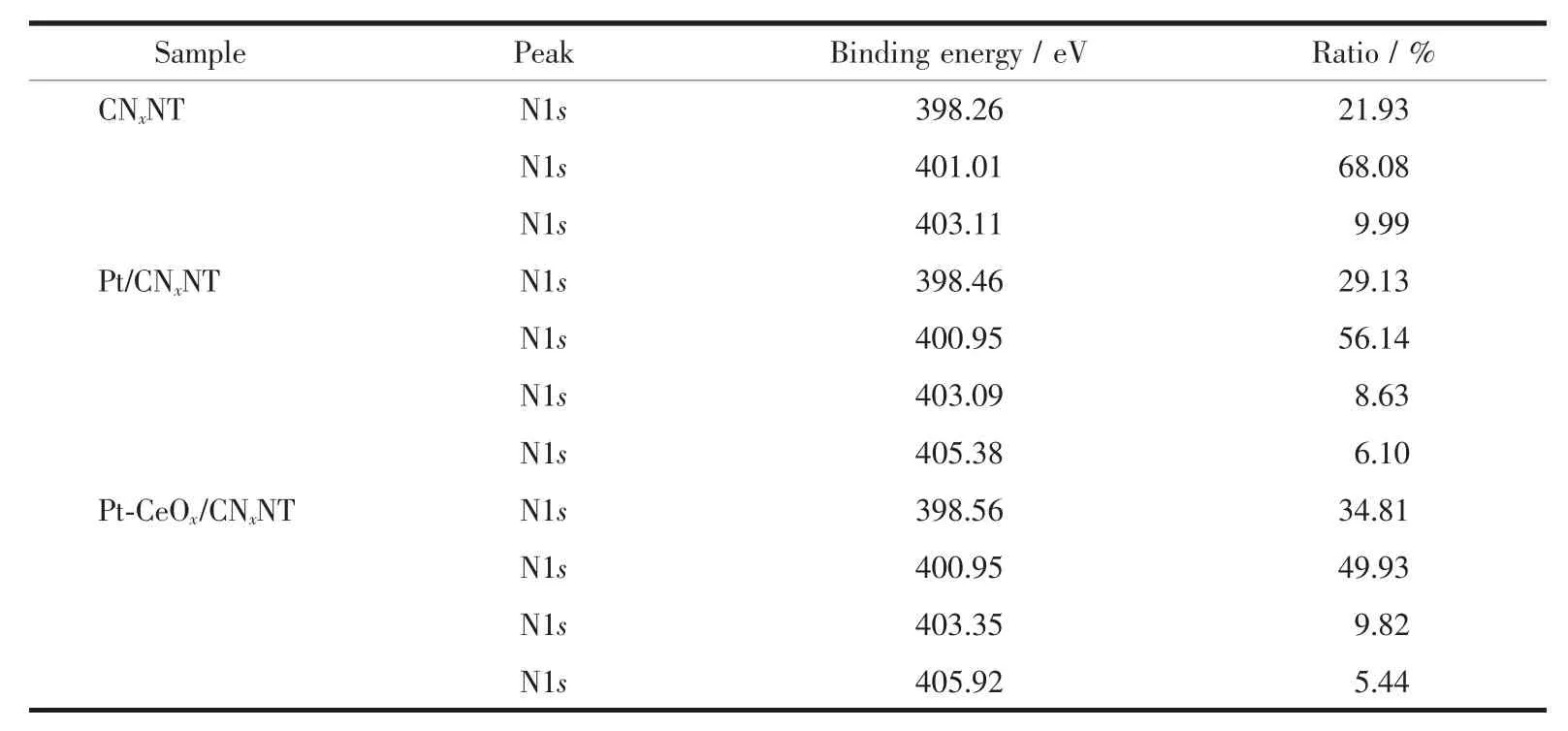
Table 2 Binding energies and surface compositions from deconvolution of XPS spectra for CNxNT,Pt/CNxNT and Pt-CeO/CNxNT
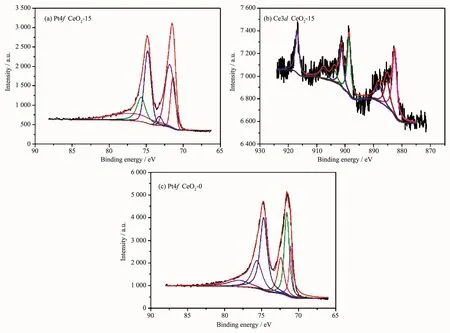
Fig.8 XPS spectra of Pt4f,Ce3d for CeO2-15(a,b)and Pt4f for CeO2-0(c)
As mentioned above,the initial specific ECSA of Pt-CeOx/CNxNTis lower than that of commercial Pt/C,but the kinetic current at 0.85 V(vs RHE)is higher.One of the reasons for improved activity of the Pt-CeOx/CNxNT is the existence of CeOx.As we know,cerium oxide is an excellent promoter for various catalytic reactions due to its unique oxygen storagecapability (Ce3+⇌Ce4+)[25].Thanks to such unique property,the transfer rate of oxygen to Pt nanoparticles is improved and the activity of CeOxcontain catalyst toward ORR is enhanced[26-28].Meanwhile,the unique oxygen storage capability of CeOxcan be further improved when interact with Pt[29].Another reason for the higher activity may attributed to the pyridine-like nitrogen in support.According to Elizabeth J.Biddinger[20],pyridinic-N appear to play a role in ORR activity,while the ratio of pyridine-like nitrogen in Pt-CeOx/CNxNT is the highest.The last possible reason is attributed to the hollow structure and 1D morphology of CNxNT support.Compared with XC-72,the stack of CNxNT is fluffier stacking,together with the hollow structure of CNxNT.This may be beneficial to the electron-electrolyte transports and decrease kinetic difficulties for oxygen transfer.
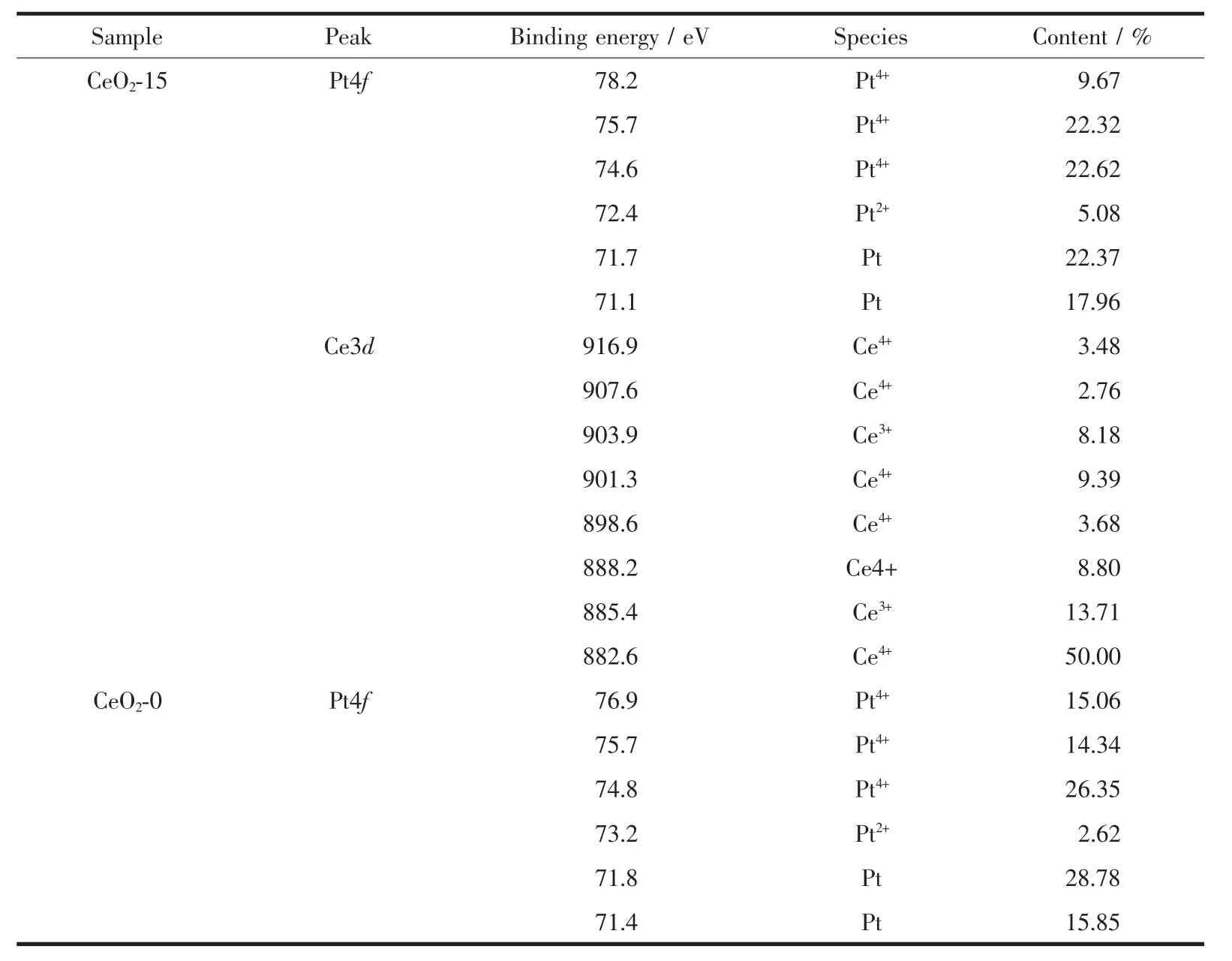
Table 3 Binding energies and surface compositions from deconvolution of XPS spectra for CeO2-15,CeO2-0 catalyst
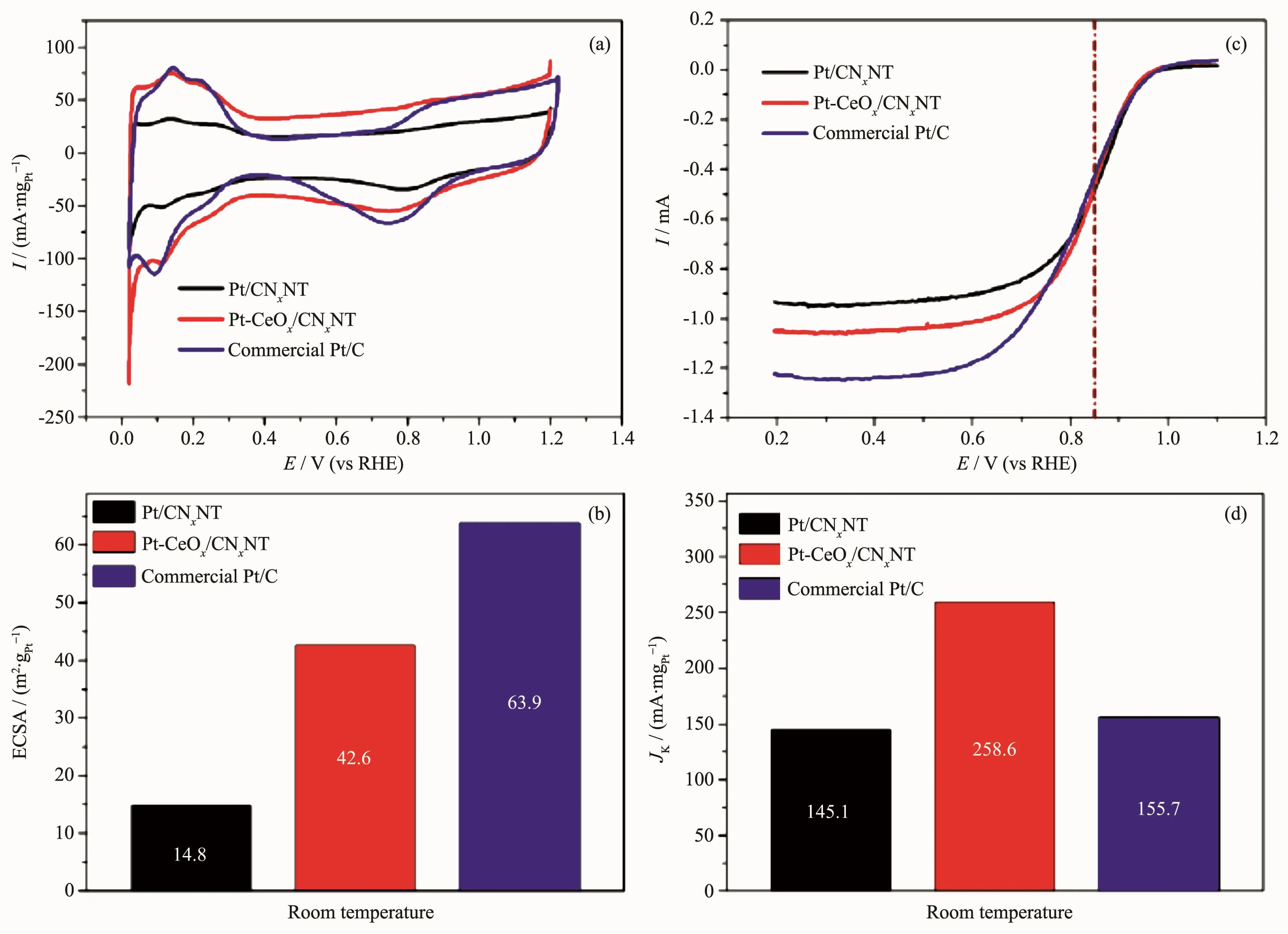
Fig.9 (a)CV curves of Pt/CNxNT,Pt-CeOx/CNxNT and commercial Pt/C catalysts recorded in N2-purged 0.1 mol·L-1HClO4 solution with a sweep rate of 100 mV·s-1;ORR polarization curves of Pt/CNxNT,Pt-CeOx/CNxNT and commercial Pt/C catalysts obtained at room temperature in O2-saturated 0.1 mol·L-1HClO4solution with a GC rotating disk electrode(1 600 r·min-1,sweep rate 10 mV·s-1);Histogram of the specific ECSA(c)and mass activity(at 0.85 V vs RHE)of the three catalysts(d)
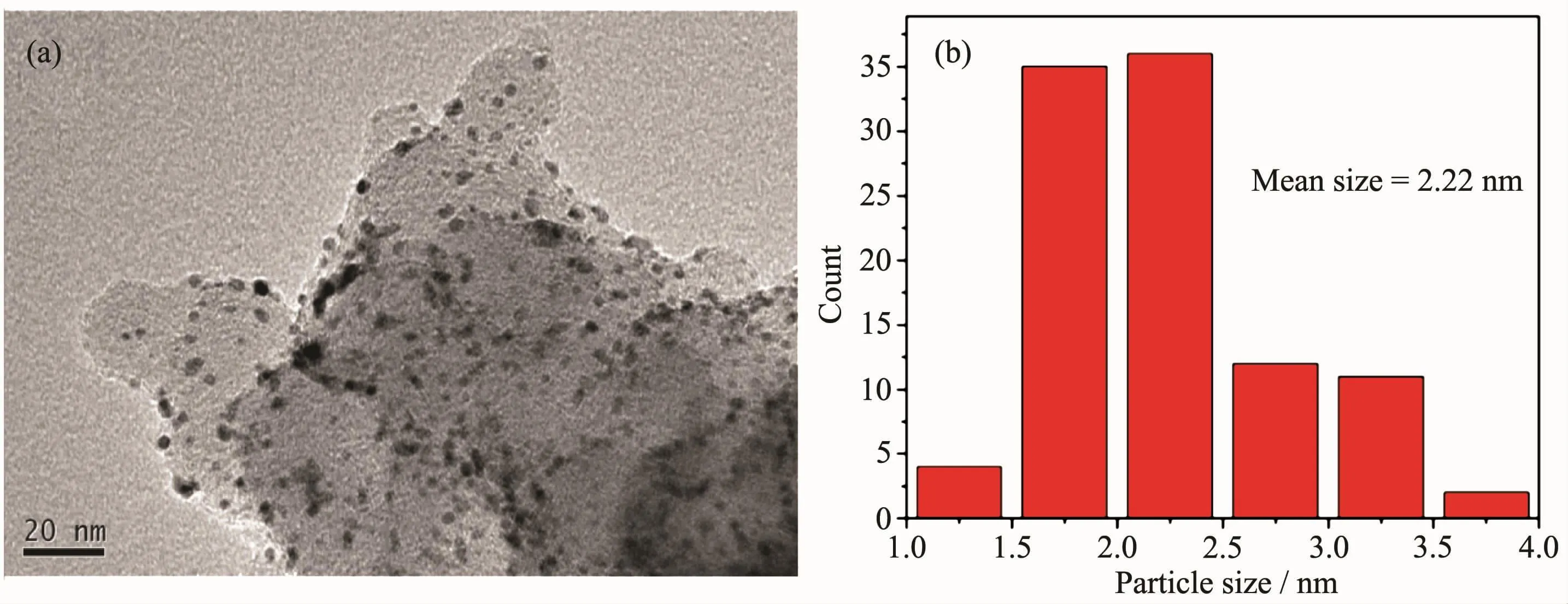
Fig.10 TEM image of commercial Pt/C(a)and the particle size distribution histogram(b)
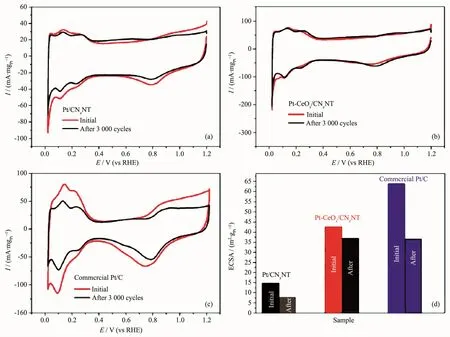
Fig.11 CV curves of Pt/CNxNT catalyst(a),Pt-CeOx/CNxNT catalyst(b),and commercial Pt/C catalyst(c)before and after 3 000 potential cycles in 0.1 mol·L-1HClO4solutions at room temperature with a scan rate of 100 mV·s-1;Histograms of the specific ECSA of different catalysts before and after 3 000 cycles(d)
The accelerated durability tests of the catalysts were conducted by applying cyclic voltammetry sweeps in N2purged 0.1 mol·L-1HClO4solutions at room temperature with a scan rate of 100 mV·s-1(Fig.11).After 3 000 cycles,only 13.1%of the initial specific ECSA of Pt-CeOx/CNxNT catalyst was lost,whereas the degradations of both Pt/CNxNT and commercial Pt/C catalysts were very severe,showing a 47.7%and 42.7%losses of the initial specific ECSA,respectively(Fig.11d).The improvement of the stability is mainly due to CeOx,because CeOxcan form strong chemical bond with late transition metal nanoparticles[30-31],which can prevent Pt migration and dissolution[32-33].Another significant cause for the performance degradation of the catalyst is the oxidation or corrosion of carbon support[32].The results show that CeOxin the catalyst can enhance the stability of carbon support[33-34].However,the CNxNT itself has no evident difference in stability Fig.10.

Fig.10 Cyclic voltammograms of CNxNT(a)and XC-72(b)before and after 3 000 potential cycles in 0.1 mol·L-1HClO4 solutions at room temperature with a scan rate of 100 mV·s-1
3 Conclusions
In this work,we have successfully synthesized Pt-CeOx/CNxNT of 4.9 nm with well dispersity by a facile hydrothermalmethod using CNxnanotube(CNxNT)as support.Compared with commercial Pt/C and Pt/CNxNT,Pt-CeOx/CNxNT catalyst exhibits excellent stability and ORR activity.The kinetic current(Jk)of Pt-CeOx/CNxNT(0.85 V vs RHE)is 258.6 mA·mgPt-1,1.66 and 1.78 times higher than that of the commercial Pt/C catalyst and the Pt/CNxNT,respectively.We believe the highly improved activity of the Pt-CeOx/CNxNT is mainly attributed to the improved unique oxygen storage capability of CeOxas well as the promotion effectofpyridine-like nitrogen in support.Another reason for the improved activity may attributed to the unique hollow structure and the fluffy stacking of CNxNT support.As for the greater electrochemical stability of Pt-CeOx/CNxNT in acidic and oxidative environments is mainly due to the unusually strong chemical bonds formed between ceria and Pt nanoparticles.

