A case of thyroid emergency with cardiac arrest supported by extracorporeal membrane oxygenation
Gan-nan Wang, Xu-feng Chen, Gang Zhang, Yong Mei, Zhe Wang, Qin Zhang, Jin-song Zhang
Department of Emergency Medicine, the First Affi liated Hospital of Nanjing Medical University, Nanjing, China
Dear editor,Thyroid emergency is a rare but potentially life-threatening condition if not recognized early and managed properly. It is usually due to a severe exacerbation of a preexisting thyrotoxicosis, which later leads to decompensation in different organ systems. The treatment of thyroid emergency remains challenging even with the armamentarium of modern intensive care technologies, especially in patients with cardiac failure and major organ dysfunction.[1–3]Herein, the authors described a case of thyroid emergency with cardiac arrest (CA) supported by extracorporeal membrane oxygenation (ECMO).
CASE
A 44-year-old woman presented with a 6-day history of chest pain, palpitation, and finger tremor.She didn't have any symptoms such as fever, nausea,vomiting, or diarrhea. She took no medications and had no particular medical history. On examination of outpatient department, she had markedly abnormal thyroid function test results: serum thyroid stimulating hormone (TSH) levels less than 0.005 mIU/L (reference range 0.27–4.20 mIU/L), free triiodothyronine (fT3)35.18 pmol/L (3.10–6.80 pmol/L) and free thyroxine(fT4) amounted to >100 pmol/L (12.00–22.00 pmol/L).Moreover, serum potassium was 4.0 mmol/L (reference range 3.5–5.1 mmol/L). The thyroid ultrasound showed the size of thyroid gland enlarged and blood flow markedly increased. In addition, an electrocardiogram(ECG) showed sinus tachycardia (110/minute).Echocardiography showed no dilatation in either the atria or ventricles (left ventricular end-diastolic dimension:48.3 mm), and left ventricular ejection fraction was approximately 70%.
Half an hour prior to onset of CA, she felt severe chest pain, palpitation, dyspnoea, hidrorrhea, dysphoric,and lost consciousness. At the time of being transferred to the emergency department, the blood pressure (BP) of the patient could not be measured. An electrocardiogram(ECG) monitor indicated a waveform of a straight line.Her body temperature was 37.3 °C and had a Glasgow Coma Scale (GCS) score of E1M1V1 (no eye opening,no verbal response, and no motor response). Physical examination revealed swelling of the thyroid gland on the anterior side of the neck; however, there were no signs of exophthalmos or peripheral edema. When CA was diagnosed, cardiopulmonary resuscitation (CPR) (i.e.,compression: ventilation was 30:2) was immediately performed and the medication was administrated(epinephrine 1 mg intravenously, every 4 minutes).During the period of resuscitation, the patient was detected ECG as ventricular fi brillation (VF). As a result,defi brillation was administered 4 times (150 J, 200 J, 200 J,200 J biphasic) using a direct current type defibrillator(Philips HeartStart MRx defibrillator, Philips Corp.,Netherlands). After 32 minutes of basic life support,the sinus rhythm was restored. An ECG revealed no signifi cant changes in ST-T or QT interval prolongation.After spontaneous circulation was returned, the patient was transferred to the emergency intensive care unit.
On admission, the reduced level of consciousness persisted (E2V1M4), mechanical ventilation and therapeutic hypothermia (rectal temperature 35–36 °C)were administered. On examination, the arterial BP was 86/59 mmHg, and the pulse 120 beats per minute. The pupils were 3 mm and reactive. In addition, the blood gas analysis revealed severe acidosis (pH 7.084, HCO3–8.8 mmol/L, BE –21 mmol/L). The patient was treated with dopamine and sodium bicarbonate. Other medications were administrated as follows: thiamazole (20 mg),propranolol (40 mg), methylprednisolone (240 mg), and immunoglobulin (20 g), etc.
In the evening of admission, the patient’s ECG indicated pulseless electrical activity (heart rate 40 beats/minute), and arterial BP was 35/25 mmHg. CPR was subsequently conducted and medications (epinephrine and atropine) were administrated. Then, the heart rate and BP was improved. After 20 minutes, these changes happened again, and the same treatment was given.Considering that the patient had extremely unstable cardiac electrical activity, veno-arterial ECMO (VAECMO) was performed to support her cardiac function.The principal component of the ECMO circuit was a heparin-bonded surface circuit, including a centrifugal pump and hollow-fi ber oxygenator (MAQUET; Getinge Group, Germany). The circuits were primed with heparin before cannulation. ECMO cannulations were performed by the ECMO team using the venous-arterial method of surgically direct cut down of femoral artery (left) and vein (right) at the groin. Cannula of size 15 F was used for the femoral artery, whereas that of size 21 F was used for the femoral vein. The blood was drained out from the venous side via the femoral vein and pumped through the membrane lung to the arterial side via the femoral artery.The flow rate was initially set at 50–60 mL/(kg•minute) to provide adequate cardiac output. Under ECMO support and anti-thyroid drug therapy, the cardiac function started recovering (heart rate 75–90 beats/minute, mean arterial pressure 65–100 mmHg without vasoactive agents) and thyroid hormone levels were gradually improved (Figure 1).
The patient was subsequently weaned off ECMO on day 3, and extubated on day 4. After the exclusion of confounders (particularly residual sedation), evaluation of neurological outcome was conducted. The patient showed a Glasgow Motor Score of 3–5. Bilaterally pupillary and corneal reflexes existed. Bispectral index (BIS) levels ranged from 75 to 97. Furthermore,serum neuron-specific enolase (NSE) levels gradually decreased with the improvement of disease (Figure 2).On day 5 of hospitalization, she recovered consciousness(GCS score of 15). Biopsy of the thyroid gland revealed lymphocytic thyroiditis. After 10 days, she exhibited a cerebral performance category 1 status and was discharged. Subsequent anti-thyroid therapy proceeded in the clinic department of endocrinology. The followup testing revealed that fT3 and fT4 levels had returned to be normal (fT3 3.81 and fT4 18.86 pmol/L) but TSH suppression persisted (TSH <0.005 mIU/L). At the present follow-up, no sign of recurrent VF or other CA rhythms was observed.
DISCUSSION
This case suggested that thyrotoxicosis (markedly increased fT3 and fT4) can cause VF and CA even in the absence of underlying cardiovascular disease. Thyroid hormone plays a vital role in cardiac muscle, systemic circulation, and the sympathetic nervous system that affects cardiovascular hemodynamics. The action of thyroid hormone is mediated by the binding of T3 to nuclear receptors.[1]The subsequent binding of the T3-receptor complex to DNA regulates the expression of genes coding for cardiac proteins.[2]Arrhythmia due to thyrotoxicosis often manifests itself as sinus tachycardia,atrial fi brillation, or ventricular tachycardia, but rarely as a CA including VF.[3]
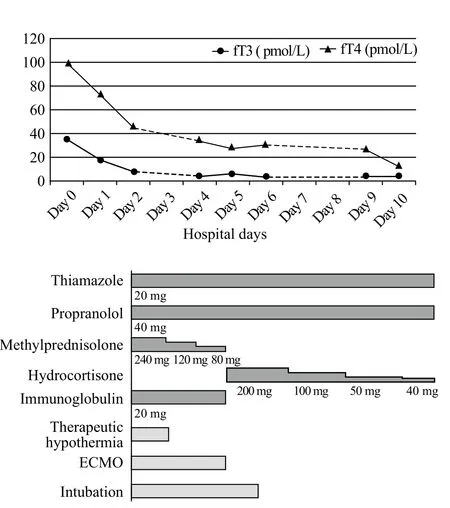
Figure 1. Clinical course after admission of the patient. fT3: free triiodothyronine; fT4: free thyroxine; ECMO: extracorporeal membrane oxygenation.
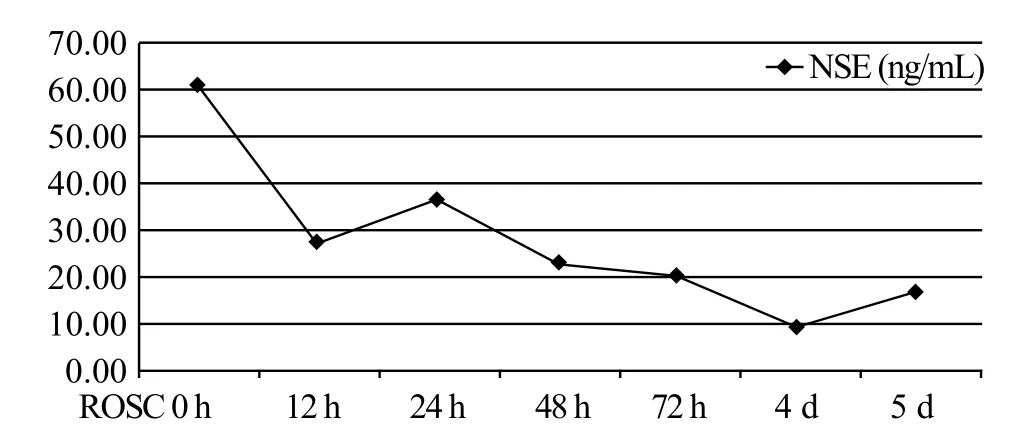
Figure 2. Sequential changes in the serum neuron-specific enolase level after return of spontaneous circulation. NSE: neuron-specific enolase; ROSC: return of spontaneous circulation.
The pathophysiological mechanism in which arrhythmia and CA occur in thyrotoxicosis is unknown. In general,excessive thyroid hormone is known to cause alteration in cardiovascular hemodynamics, including increased heart rate, ejection fraction and cardiac output.[4]It is due to the direct augmenting effect of the thyroid hormones on the β-adrenergic receptor’s sensitivity to catecholamines and increasing myocardial excitability, and on the intrinsic sinoatrial electrophysiologic function, which results in nodal blockade and the decrease of conduction time.[5]The associated increase in myocardial oxygen consumption and cardiac workload may give rise to a high output cardiac failure and can cause reversible dilated cardiomyopathy and cardiac ischemia.[6]Malignant arrhythmia even CA was arisen eventually.
Previous literatures reported possible mechanisms for VF associated with thyrotoxicosis.[7]The main mechanism was coronary vasospasm. Vascular smooth muscle cell hyperreactivity is related to several intracellular pathways that regulate vascular resistance.Thyroid hormones could affect those intracellular pathways and cause hyperreactivity and then spasms,followed by myocardial ischemia and possibly VF.Another possible mechanism of VF due to thyrotoxicosis is associated with early repolarization syndrome, which was not fully understood.
ECMO is a treatment used to temporarily replace the function of the heart and/or lungs over an extended period of time for organ recovery.[8]Several studies have reported the successful use of ECMO to rescue patients with CA in a wide spectrum of different etiologies.[9]This case presented with acute circulation failure secondary to thyroid emergency was successfully rescued by ECMO support, and fi nally had wonderful neurological outcome.Expectably, an improved awareness of the clinical value of ECMO among physicians managing patients with sudden unexplained arrests could help improve clinical outcomes.
In the present case, there was no underlying heart disease according to echocardiography. Furthermore, the effective treatment of thyrotoxicosis prevented recurrence of VF and other CA rhythms. This observation led to the suspicion of a thyroid emergency, which was then diagnosed as a possible cause of the CA. Therefore, in the event of a CA in the absence of the H’s and T’s, thyrotoxicosis should be considered as another possible treatable cause.
CONCLUSION
In the event of a CA in the absence of the H’s and T’s, thyroid emergency should be considered as another possible treatable cause. In addition, an improved awareness of the clinical value of ECMO among physicians in managing patients with sudden unexplained arrests could help improve clinical outcomes.
Funding:Commonweal Industry Fund of National Health and Family Planning Commission of China (201502019).
Ethical approval:Not needed.
Confl icts of interest:The authors declare that there is no confl ict of interest related to the content of the article.
Contributors:GW proposed the study and wrote the first draft.All authors read and approved the fi nal version of the paper.

