甜荞株高和茎粗的遗传分析
李英双 胡 丹 聂 蛟 黄科慧 张玉珂 张园莉 佘恒志 方小梅,2 阮仁武,2 易泽林,2,*
甜荞株高和茎粗的遗传分析
李英双1,**胡 丹3,**聂 蛟1黄科慧1张玉珂1张园莉1佘恒志1方小梅1,2阮仁武1,2易泽林1,2,*
1西南大学农学与生物科技学院, 重庆 400716;2重庆市荞麦产业体系创新团队, 重庆 400716;3甘肃省种子管理局, 甘肃兰州 730000
甜荞极易倒伏, 而株高和茎粗是影响甜荞倒伏的重要性状。以高秆健壮品种酉荞2号和矮秆纤细品种乌克兰大粒荞为亲本配制正、反交组合, P1、P2、F1、B1、B2和F2群体株高和茎粗的遗传分析表明, 株高和茎粗的最适遗传模型均为2对加性-显性-上位性主基因+加性-显性多基因模型。株高正交组合中2对主基因加性效应均为-1.39, 显性效应分别为-6.59和-7.91, B1、B2和F2群体主基因遗传率分别是45.73%、63.49%和81.12%, 多基因遗传率分别是27.41%、0.95%和0; 反交组合中2对主基因加性效应值均为-1.63, 显性效应分别为-7.03和-4.19, B1、B2和F2群体中主基因遗传率是41.51%、66.18%和81.81%, 多基因遗传率分别是11.19%、0和0。茎粗正交组合中2对主基因加性效应均为0.03, 显性效应分别为-0.50和-0.08, B1、B2和F2群体中主基因遗传率分别是37.26%、48.80%和72.10%, 多基因遗传率分别是11.18%、0和0; 反交组合中2对主基因加性效应均为-0.15, 显性效应分别为-0.30和-0.16, B1、B2和F2群体中主基因遗传率是76.22%、47.12%和82.51%, 多基因遗传率分别为0、14.53%和0。可见, 株高的主基因+多基因遗传率在80%以上, 可在低世代进行选择; 茎粗的主基因+多基因遗传率在80%以下, 采取合理的栽培措施可以提高荞麦抗倒伏能力。
甜荞; 株高; 茎粗; 数量性状; 遗传分析
株高和茎粗是评定作物抗倒伏能力的重要指标。在一定株高范围内, 植株越高, 倒伏率越高, 同时, 茎秆的节间长度越短, 基部茎秆越粗, 植株越不易倒伏[12-16]。目前主要通过调控种植密度[17]、烯效唑拌种[18]、茎秆解剖结构以及木质素合成研究甜荞倒伏[19-20], 对甜荞株高、茎粗的遗传分析尚未见相关报道。盖钧镒等[21]提出适合植物遗传分析的1对主基因+多基因和2对主基因+多基因混合模型的单世代和联合多世代的分析方法, 已经广泛应用于不同作物不同性状的遗传分析, 如水稻种子抗老化[22]、棉花高品质纤维性状[23]、玉米种子休眠[24]、小麦雄性不育[25]、大豆的根系性状[26]、花生产量[27]、油菜株高和抗倒性[28-29]、油菜花色[30]等。本文初步分析甜荞株高和茎粗的遗传效应, 为深入解析株高和茎粗的遗传机制奠定基础, 也为甜荞抗倒伏遗传改良与生产提供依据。
1 材料与方法
1.1 试验设计
亲本材料分别为高秆健壮品种酉荞2号(P1)和矮秆纤细品种乌克兰大粒荞(P2), 前者为本课题组育成的品种, 后者由重庆市荞麦产业体系提供。
试验材料种植在西南大学歇马科研基地(10°3′53″~29°39′10″N, 106°18′14″~106°56′53″E), 试验田土壤为沙壤土。2014年秋季, 在隔离区内配制两亲本的正、反交组合, 初花期鉴定长短柱头并去除异类型花, 酉荞2号群体去除短花柱植株, 留长花柱植株, 乌克兰群体去除长花柱植株, 留短花柱植株, 成熟后分别收获两品种植株上的种子, 分别为正、反交F1代。2015年春季, F1隔离种植正交F1代不同植株间杂交, 反交F1代不同植株间杂交, 分别收获两群体的全部种子, 获得正、反交F2代。正、反交F1代与双亲各自回交, 在母本上收获的种子分别为正、反交B1和B2代。
2015年秋季, 将正、反交组合的6个世代分别播种, 行长3.00 m, 行距0.33 m, 株距0.20 m。P1、P2、F1种植4行, B1、B2和F2种植10行, 周围种植3行保护行进行隔离。
成熟期在田间随机选取一定数量单株(表1), 用直尺测量茎秆基部至植株顶端的距离作为株高, 用游标卡尺测量茎秆最粗的部位作为茎粗。
1.2 遗传模型分析
根据盖钧镒等[21]提出的植物数量性状主基因+多基因混合遗传多世代联合分析方法, 对甜荞株高和茎粗进行联合分析,采用极大似然法和IECM算法估计各世代、各成分分布的参数, 通过AIC值选择最佳模型[31], 并进行一组适合性检验, 包括均匀性12、22和32检验、Kolmo-Smimov检验(W2)和Kolmo-Gorov检验(D), 根据检验结果选择最优遗传模型。最后采用最小二乘法依据最优模型的各成分分布参数估计各基因效应值、方差等遗传参数。
2 结果与分析
2.1 不同世代群体的株高和茎粗变异
两亲本株高和茎粗平均值分别为90.70 cm和5.45 mm, 两亲本株高相差30.76 cm, 茎粗相差1.30 mm。在正交组合中, 株高和茎粗变异丰富(表1)。
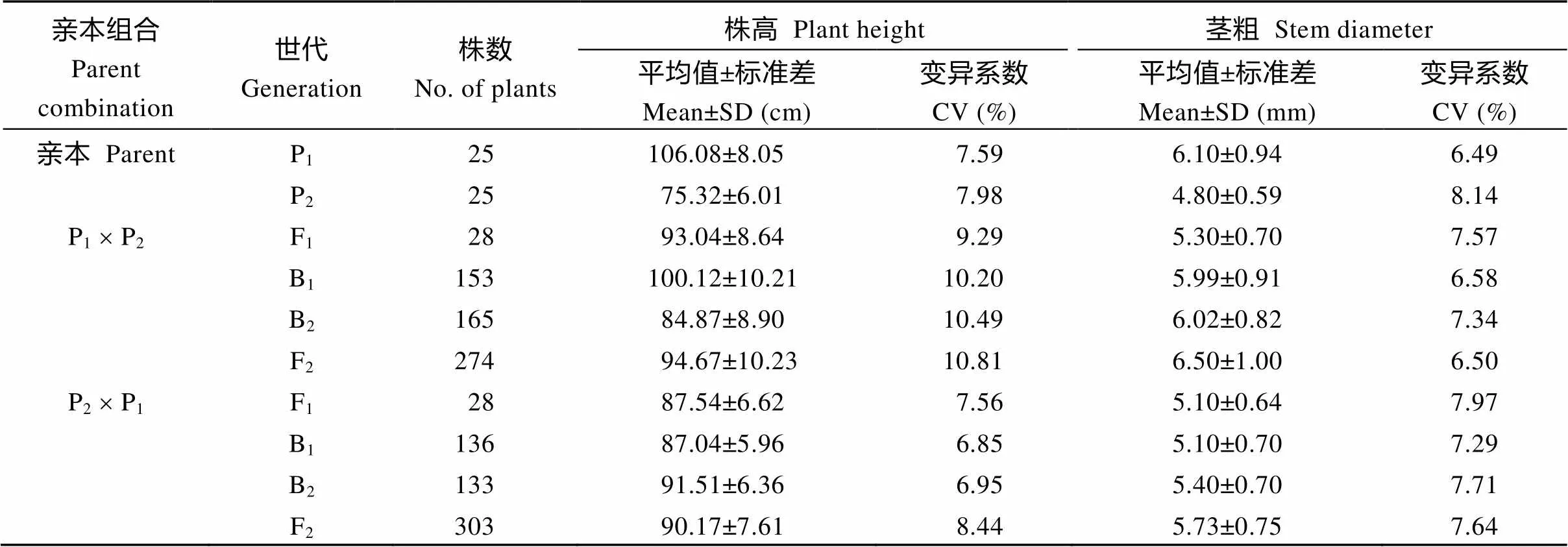
表1 正、反交组合6个世代的株高与茎粗
例如, F1株高大于双亲平均值, B1和B2代株高分别比低亲小24.80 cm和9.55 cm, F2代略偏向于高亲, B1、B2和F2群体的变异系数明显高于P1、P2和F1, 说明分离世代群体离散程度高, 遗传变异大。而在反交组合中, 株高和茎粗的变异较低(表1)。例如, F1株高小于双亲平均值, B1、B2代株高分别比低亲大11.72 cm和16.19 cm, F2略偏向于低亲, B1和B2代变异系数小于P1、P2和F1群体, 说明B1、B2群体的变异性较低, 但F2群体具有较高的遗传变异。B1、B2和F2群体茎粗的变异系数低于P1和F1群体, 说明分离世代群体的遗传多态性较低。
正、反交组合6个世代的株高(附图1和附图2)和茎粗(附图3和附图4)均呈连续性分布, 具有典型的数量遗传特征。株高与茎粗在B1、B2和F2世代群体具有明显多峰现象, 说明株高和茎粗主要受主基因遗传效应影响。株高反交B1世代具有明显正态分布现象, 说明B1世代也受到部分多基因遗传效应影响。
相比于λ1,λ2的取值范围宽,并且具有很强的针对性。λ1是一个定值,无论功率误差增大或减小,λ1对开关函数的放大倍数都是一样的,它无法跟随系统实时正确地改变开关函数在代价函数中的比重。λ2的优势就显得十分明显,当功率误差较小时,系统功率跟踪准确,可以适当减少开关动作次数,此时,λ2增大,开关函数在代价函数中的比重上升,开关频率下降;当功率误差较大时,急需稳定功率跟踪,此时,λ2减小,功率跟踪函数在代价函数中的比重上升,系统将快速减少功率误差。
2.2 株高和茎粗主基因+多基因遗传分析
根据AIC准则, 选取AIC值最小及与最小AIC值比较接近的3组遗传模型作为备选模型(附表1)。对正交组合, B-4、D-2、E-1为株高遗传的备选模型, B-1、E-0、E-1为茎粗遗传的备选模型; 同理, 株高与茎粗反交的3个备选模型均为C-0、D-0和E-1。
对备选模型进行一组适合性测验(均匀性检验、Smirnov检验和Kolmogorov检验的5个统计量12、22、32、2和D), 选择统计量达到显著水平个数最少的模型为最适模型(表2), 正反交株高与茎粗的最佳遗传模型均为E-1模型, 即2对加性-显性-上位性主基因+加性-显性多基因模型。
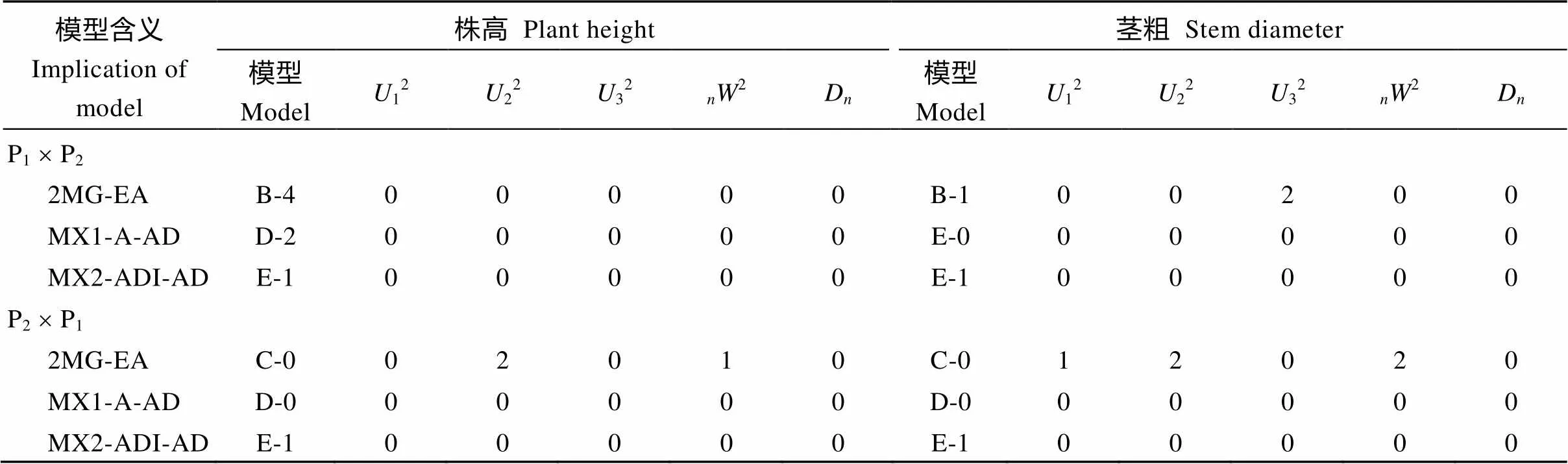
表2 正反交组合后代株高与茎粗备选遗传模型的适合性检验
MG: 主基因; MX: 主基因+多基因; A: 加性; D: 显性; I: 互作; E: 相等。例如E-1模型, MX2-ADI-AD表示2对加性-显性-上位性主基因+加性-显性多基因混合遗传模型。表中参数对应的数字指达到显著水平的统计量个数。
M G: major gene; MX: mixed major gene and polygene; A: additive; D: dominance; I: interaction; E: equal. Take model E-1 as an example, MX2-ADI-AD means mixed model with two major genes with additive-dominance-epistasis effects plus additive-dominance polygene. The numbers corresponding to the parameters in the table refer to the number of statistics that achieve significant levels.
2.3 正、反交组合株高和茎粗最适遗传模型遗传参数估算
根据选择的最佳模型E-1得出的极大似然估计值经计算得到株高的一阶(表3)、二阶遗传参数(表4), 正、反交组合中控制株高的两对主基因加性效应相等, 显性效应大于加性效应, 均为负向效应。正交组合中2对主基因显性效应均为负, 且|h|<|h|, 说明株高主基因存在杂种优势(负向), 且以第2对主基因的显性效应为主, 反交组合中|h|>|h|, 则以第1对主基因的显性效应为主。正交组合2对基因加性和显性效应之间的互作(j和j)分别为+8.44和-9.48, 表明基因互作对该正交组合株高影响较大。

表3 正、反交组合株高与茎粗一阶遗传参数估计值
: 群体均方;d: 第1对主基因的加性效应;d: 第 2 对主基因的加性效应;h: 第1对主基因的显性效应;h: 第2对主基因的显性效应;: 主基因加性×加性互作效应;j: 第1对主基因加性×第2对主基因显性互作效应;j: 第2对主基因加性×第1对主基因显性互作效应;: 主基因显性×显性互作效应; []: 多基因加性效应; []: 多基因的显性效应。
: mean of population;d: additive effect of the first major gene;d: additive effect of the second major gene;h: dominant effect of the first major gene;h: dominant effect of the second major gene;: additive × additive interaction effect of major gene;j: additive effect of the first major gene × dominant effect of the second major gene;j: additive effect of the second major gene × dominant effect of the first major gene;: dominant × dominant interaction effect of major gene; []: additive effect of polygene; []: dominant effect of polygene.
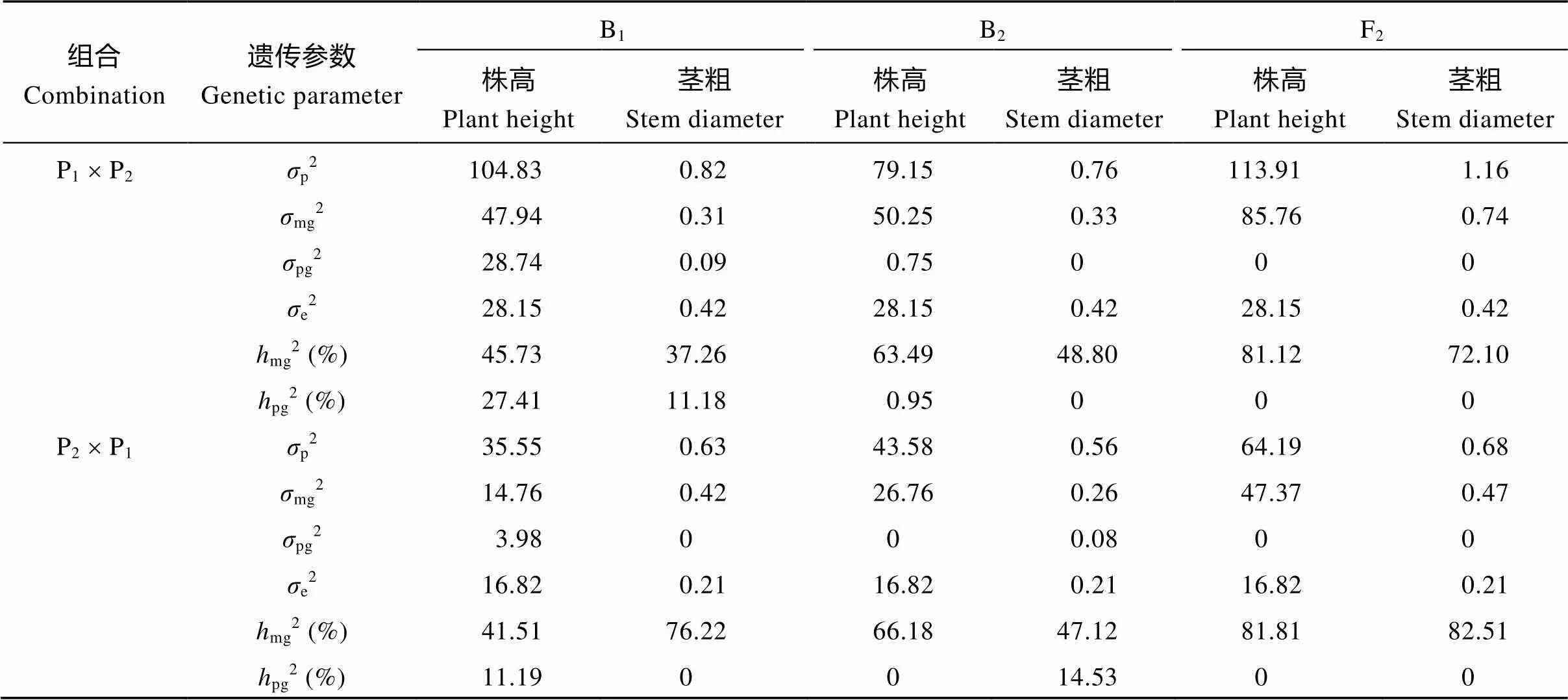
表4 正、反交组合株高与茎粗二阶遗传参数估计值
p2: 表型方差;mg2: 主基因方差;pg2: 多基因方差;e2: 环境方差;mg2: 主基因遗传力;pg2: 多基因遗传率。
p2: phenotypic variance;mg2: major gene variance;pg2: polygene variance;e2: environmental variance;mg2: heritability of major gene;pg2: heritability of polygene.
正、反交组合中, F2代株高的主基因表现出较高的遗传力, 分别达81.12%和81.81%, 具有很高的选择率。正交组合中B1、B2和F2世代的主基因+多基因效应决定株高表型变异的73.14%、64.44%和81.12%, 反交组合中这3个世代的遗传效应分别为52.70%、66.18%和81.81%, 可见, 虽然环境因素对表型变异有一定贡献, 但环境效应明显小于遗传效应, 说明环境对甜荞株高的影响较小。
正、反交组合中控制茎粗的2对主基因加性相等, 显性效应大于加性效应, 2对主基因显性效应均为负, 且|h|>|h|, 说明茎粗主基因存在杂种优势(负向), 且以第1对主基因的显性效应为主。正交组合中两对基因加性和显性互作效应(j和j)分别为+0.04和-0.41, 而反交j和j分别为-0.27和+0.86, 表明两对基因互作对茎粗影响较大。
正、反交组合F2世代茎粗的主基因表现出较高的遗传力, 分别达72.10%和82.51%, 具有较高的选择率。正交组合中, 主基因+多基因分别解释了B1、B2和F2代茎粗变异的48.44%、48.80%和72.10%, 多基因遗传率(11.18%)仅在B1代表现; 各群体环境变异占表型变异的36.21%~55.26%, 平均47.56%。反交组合中, 主基因+多基因可分别解释B1、B2和F2代茎粗变异的76.22%、61.65%和82.51%, 多基因遗传率(14.53%)只在B2代存在; 各群体环境变异占表型变异的30.88%~37.50%, 平均33.90%。可见, 环境对甜荞茎粗影响较大。
3 讨论
株高和茎粗是评定作物抗倒伏能力的重要指标, 明确其遗传规律是指导育种实践的基础, 陈桂华等[32]研究表明, 株高、节间粗度、单茎鲜重和弯曲力矩等是影响水稻抗倒伏能力的重要因素; 朱新开等[33]发现, 矮秆、基部节间短与重心低有利于小麦抗倒伏; 丰光等[34]发现玉米倒伏与株高极显著正相关。在荞麦上, 刘星贝等[35]研究发现茎秆形态特性与抗倒伏能力密切相关, 植株越矮、茎秆越粗壮、节间越密集、节间充实度高、机械组织层数多、机械组织和茎壁厚、维管束数目多且面积大会增强茎秆抗倒伏性能。
主基因+多基因混合模型联合多世代分析法自问世以来, 已在多种作物的株高和茎粗研究中得到应用, 如高淀粉玉米株高符合1对加性主基因+加性-显性多基因模型[36], 甘蓝型油菜株高受到1对加性-显性主基因+加性-显性-上位性多基因混合遗传模型控制(D-0模型)[29]。甜荞株高和茎粗相关遗传分析尚未见报道。本研究表明, 株高和茎粗的最佳遗传模型均为E-1模型, 即2对加性-显性-上位性主基因+加性-显性多基因模型, 其中显性效应大于加性效应, 表现为超显性, 这与玉米[36]和甘蓝型油菜[29]上的报道不同, 可能与不同物种的遗传机制差异有关。
从遗传率来看, 本研究正、反交组合株高和茎粗的遗传率范围分别为52.7%~81.81%和48.44%~ 82.51%, 其中主基因遗传率大于多基因遗传率。株高正交组合多基因遗传率在B1和B2世代存在, 反交组合只在B1世代存在; 茎粗正交组合多基因遗传率仅在B1世代表现, 反交组合只在B2世代存在, 正、反交组合株高和茎粗多基因遗传率在F2代都为0, 主基因遗传率在F2世代表现最高, 具有很高的选择率, 表明早期世代选择是有效的。油菜株高和茎粗主要受显性效应控制[37], 环境因素对株高的影响较小, 对茎粗有一定的影响。本研究得到相似结论, 甜荞株高各群体的环境变异占表型变异的25.46%~ 37.08%, 而遗传贡献率为62.92%~74.54%, 说明株高主要受显性效应控制; 茎粗各群体的环境变异占表型变异的33.54%~46.38%, 略低于遗传贡献率(54.62%~66.46%), 说明环境对甜荞茎粗有一定影响。无论正交还是反交, 株高和茎粗的最佳遗传模型均一致, 皆表现为超显性, 遗传率范围大致相同, 主基因遗传率大于多基因遗传率, 表明甜荞株高和茎粗不受细胞质遗传的影响。
植物数量性状的遗传模型分析方法与QTL检测主基因的数量相对一致[38], 由于分析所推论的基因只是概念上的基因, 难以做个别比较, 为了更好地解释本文中控制株高及茎粗的基因数目多少、效应值大小和是否为同一基因, 有必要利用分子标记进行QTL定位研究。因此可利用分子标记对甜荞F2群体开展株高和茎粗QTL分析, 为甜荞株型改良分子标记育种和图位克隆奠定前期研究基础。
4 结论
在高秆健壮品种酉荞2号和矮秆纤细品种乌克兰大粒荞正、反交组合衍生的P1、P2、F1、B1、B2和F2世代群体中, 株高和茎粗均呈连续分布, 且符合2对加性-显性-上位性主基因+加性-显性多基因模型。株高的主基因+多基因遗传率在80%以上, 可在早代进行株高选择, 以提高育种效率; 茎粗的主基因+多基因遗传率在80%以下, 环境因素对茎粗有一定效应, 可利用栽培措施提高甜荞抗倒伏能力。
[1] Gondola I, Papp P. Origin, geographical distribution and polygenic relationship of common buckwheat (Moench.)., 2010, 4: 17–33
[2] Alamprese C, Casiraghi E, Pagani M A. Development of gluten-free fresh egg pasta analogues containing buckwheat., 2007, 225: 205–213
[3] Awatsuhara R, Harada K, Maeda T. Antioxidative activity of the buckwheat polyphenol rutin in combination with ovalbumin., 2010, 3: 121–125
[4] Griffith J Q, Couch J F, Lindauer M A. Effect of rutin on increased capillary fragility in man., 1944, 55: 228–229
[5] Jiang P, Burczynski F, Campbell C, Pierce G, Austria J A, Briggs C J. Rutin and flavonoid contents in three buckwheat species,, andand their protective effects against lipid peroxidation., 2007, 40: 356–364
[6] Wieslander G, Fabjan N, Vogrincic M, Kreft I, Janson C, Spetz-Nystrom U. Eating buckwheat cookies is associated with the reduction in serum levels of myeloperoxidase and cholesterol: a double blind crossover study in day-care centre staffs., 2011, 225: 123–130
[7] 何健, 张国治, 张虹, 次仁欧珠. 荞麦营养成分的检测及分析. 河南农业大学学报, 2002, 36: 302–304 He J, Zhang G Z, Zhang H, Ci-Ren-Ou-Zhu. Determination and analysis of nutrient ingredients of buckwheat., 2002, 36: 302–304 (in Chinese with English abstract)
[8] 杨海霞, 赵丽芹, 付媛, 候文娟, 张美莉. 甜荞主要贮藏蛋白的分离纯化及功能特性研究. 中国食品学报, 2009, 9(1): 72–76 Yang H X, Zhao L Q, Fu Y, Hou W J, Zhang M L. Extraction, purification and functional properties of main protein from buckwheat (Moench) seeds., 2009, 9(1): 72–76 (in Chinese with English abstract)
[9] Koyama M, Nakamura C, Nakamura K. Changes in phenols contents from buckwheat sprouts during growth stage., 2013, 50: 86–93
[10] Hagiwara M, Izusawa H, Inoue N, Matano T. Varietal differences of shoot growth characters related to lodging in Tartary buckwheat., 1999, 16: 67–72
[11] 郭志利, 孙常青. 北方旱地荞麦抗倒栽培技术研究. 杂粮作物, 2007, 27: 364–366 Guo Z L, Sun C Q. Study on resistance cultivation techniques of buckwheat in Northern Dryland., 2007, 27: 364–366 (in Chinese with English abstract)
[12] Dunn G J, Briggs K G. Variation in culm anatomy among barley cultivars differing in lodging resistance., 2011, 67: 1838–1843
[13] Yoshida S. Physiological aspects of grain yield., 2003, 23: 437–464
[14] 罗茂春, 田翠婷, 李晓娟, 林金星. 水稻茎秆形态结构特征和化学成分与抗倒伏关系综述. 西北植物学报, 2007, 27: 2346–2353 Luo M C, Tian C T, Li X J, Lin J X. Relationship between morpho-anatomical traits together with chemical components and lodging resistance of stem in rice (L.)., 2007, 27: 2346–2353 (in Chinese with English abstract)
[15] 杨惠杰, 杨仁崔, 李义珍, 姜照伟, 郑景生. 水稻茎秆性状与抗倒性的关系. 福建农业学报, 2000, 15(2): 1–7 Yang H J, Yang R C, Li Y Z, Jiang Z W, Zheng J S. Relationship between culm traits and lodging resistance of rice cultivars., 2000, 15(2): 1–7 (in Chinese with English abstract)
[16] 杨守仁, 张龙步, 王进民. 水稻理想株形育种的理论和方法初论. 中国农业科学, 1984, (3): 6–13 Yang S R, Zhang L B, Wang J M. The theory and method of ideal plant morphology in rice breeding., 1984, (3): 6–13 (in Chinese with English abstract)
[17] Wang C, Ruan R W, Yuan X H, Hu D, Yang H, Li Y, Yi Z L. Effects of nitrogen fertilizer and planting density on the lignin synthesis in the culm in relation to lodging resistance of buckwheat., 2015, 18: 218–227
[18] 刘星贝, 吴东倩, 汪灿, 胡丹, 杨浩, 佘恒志, 阮仁武, 袁晓辉, 易泽林. 喷施烯效唑对甜荞茎秆抗倒性能及产量的影响. 中国农业科学, 2015, 48: 4903–4915 Liu X B, Wu D Q, Wang C, Hu D, Yang H, She H Z, Ruan R W, Yuan X H, Yi Z L. Effects of spraying uniconazole on lodging resistance of culm and yield in common buckwheat., 2015, 48: 4903–4915 (in Chinese with English abstract)
[19] Hu D, Liu X B, She H Z, Gao Z, Ruan R W, Wu D Q, Yi Z L. The lignin synthesis related genes and lodging resistance of.,2017, 61: 138–146
[20] 汪灿, 阮仁武, 袁晓辉, 胡丹, 杨浩, 林婷婷, 何沛龙, 李燕, 易泽林. 荞麦茎秆解剖结构和木质素代谢及其与抗倒性的关系. 作物学报, 2014, 40: 1846–1856 Wang C, Ruan R W, Yuan X H, Hu D, Yang H, Lin T T, He P L, Li Y, Yi Z L. Relationship of anatomical structure and lignin metabolism with lodging resistance of culm in buckwheat., 2014, 40: 1846–1856 (in Chinese with English abstract)
[21] 盖钧镒, 章元明, 王建康. 植物数量性状遗传体系. 北京: 科学出版社, 2003 Gai J Y, Zhang Y M, Wang J K. Genetic System of Quantitative Traits in Plants. Beijing: Science Press, 2003 (in Chinese)
[22] 朱世杨, 郭媛, 洪德林. 水稻种子抗老化遗传分析. 遗传, 2008, 30: 217–224 Zhu S Y, Guo Y, Hong D L. Genetic analysis on aging-resistant in rice seed.(Beijing), 2008, 30: 217–224 (in Chinese with English abstract)
[23] 袁有禄, 张天真, 郭旺珍, John Y, Russell J K. 棉花高品质纤维性状的主基因与多基因遗传分析. 遗传学报, 2002, 29: 827–834 Yuan Y L, Zhang T Z, Guo W Z, John Y, Russell J K. Major-polygene effect analysis of super quality fiber properties in upland cotton (L.)., 2002, 29: 827–834 (in Chinese with English abstract)
[24] 兰海, 余月, 王凤格, 潘光堂, 赵久然, 李新海, 荣廷昭. 玉米种子休眠性数量遗传体系的判别. 玉米科学, 2007, 15(2): 5–8 Lan H, Yu Y, Wang F G, Pan G T, Zhao J R, Li X H, Rong T Z. Detection quantitative inheritance system of seed dormancy in maize (L.)., 2007, 15(2): 5–8 (in Chinese with English abstract)
[25] 张立平, 赵昌平, 单福华, 张凤廷, 叶志杰. 小麦光温敏雄性不育系BS210育性的主基因+多基因混合遗传分析. 作物学报, 2007, 33: 1553–1557 Zhang L P, Zhao C P, Shan F H, Zhang F T, Ye Z J. The mixed genetic analysis of photoperiod-temperature sensitive male sterility of BS210 in wheat., 2007, 33: 1553–1557 (in Chinese with English abstract)
[26] 刘莹, 盖钧镒, 吕慧能, 王永军, 陈受宜. 大豆耐旱种质鉴定和相关根系性状的遗传与QTL定位. 遗传学报, 2005, 32: 855–863 Liu Y, Gai J Y, Lyu H N, Wang Y J, Chen S Y. Identification of drought tolerant germplasm and inheritance and QTL mapping of related root traits in soybean ((L.) Merr.)., 2005, 32: 855–863 (in Chinese with English abstract)
[27] Zhang X Y, Han S Y, Tang F S, Xu J, Liu H, Yan M, Dong W Z, Huang B Y, Zhu S J. Genetic analysis of yield in peanut (L.) using mixed model of major gene plus polygene., 2013, 10: 7126–7130
[28] 顾慧, 戚存扣. 甘蓝型油菜(L.)抗倒伏性状的主基因+多基因遗传分析. 作物学报, 2008, 34: 376–381 Gu H, Qi C K. Genetic analysis of lodging resistance with mixed model of major gene plus polygene inL., 2008, 34: 376–381 (in Chinese with English abstract)
[29] 周清元, 李军庆, 崔翠, 卜海东, 阴涛, 颜银华, 李加纳, 张正圣. 油菜半矮杆新品系10D130株型性状的遗传分析. 作物学报, 2013, 39: 207–215 Zhou Q Y, Li J Q, Cui C, Bu H D, Yin T, Yan Y H, Li J N, Zhang Z S. Genetic analysis of plant type in semi-dwarf new line (10D130) of rapeseed., 2013, 39: 207–215 (in Chinese with English abstract)
[30] 田露申, 牛应泽, 余青青, 郭世星, 柳丽. 甘蓝型油菜白花性状的主基因+多基因遗传分析. 中国农业科学, 2009, 42: 3987–3995 Tian L S, Niu Y Z, Yu Q Q, Guo S X, Liu L. Genetic analysis of white flower color with mixed model of major gene plus polygene inL., 2009, 42: 3987–3995 (in Chinese with English abstract)
[31] Akaike H. On entropy maximum principles. In: Krishnaiag G, ed. Applications of Statistics. Amsterdam, Netherlands: North Holland Publishing, 1977. pp 27–41
[32] 陈桂华, 邓化冰, 张桂莲, 唐文帮, 黄璜. 水稻茎秆性状与抗倒性的关系及配合力分析. 中国农业科学, 2016, 49: 407–417 Chen G H, Deng H B, Zhang G L, Tang W B, Huang H. The correlation of stem characters and lodging resistance and combining ability analysis in rice., 2016, 49: 407–417 (in Chinese with English abstract)
[33] 朱新开, 王祥菊, 郭凯泉, 郭文善, 封超年, 彭永欣. 小麦倒伏的茎秆特征及对产量与品质的影响. 麦类作物学报, 2006, 26(1): 87–92 Zhu X K, Wang X J, Guo K Q, Guo W S, Feng C N, Peng Y X. Stem characteristics of wheat with stem lodging and effects of lodging on grain yield and quality., 2006, 26(1): 87–92 (in Chinese with English abstract)
[34] 丰光, 景希强, 李妍妍, 王亮, 黄长玲. 玉米茎秆性状与倒伏性的相关和通径分析. 华北农学报, 2010, 25(增刊): 72–74 Feng G, Jing X Q, Li Y Y, Wang L, Huang C L. Correlation and path analysis of lodging resistance with maize stem characters., 2010, 25(suppl): 72–74 (in Chinese with English abstract)
[35] 刘星贝, 汪灿, 胡丹, 杨浩, 佘恒志, 阮仁武, 吴东倩, 易泽林. 烯效唑干拌种对甜荞茎秆抗倒性能的影响. 作物学报, 2016, 42: 93–103 Liu X B, Wang C, Hu D, Yang H, She H Z, Ruan R W, Wu D Q, Yi Z L.Effects of seed dressing with uniconazole powder on lodging resistance of culm in common buckwheat., 2016, 42: 93–103 (in Chinese with English abstract)
[36] 包和平, 李颖, 李春成. 高淀粉玉米“郑单958”主要农艺性状主基因+多基因遗传分析. 吉林农业大学学报, 2010, 32: 245–248 Bao H P, Li Y, Li C C. Inheritance analysis of main agronomic traits of major genes + poly-genes of high-starch corn Zhengdan 958., 2010, 32: 245–248 (in Chinese with English abstract)
[37] 张倩. 甘蓝型油菜主要株型性状的遗传分析和QTL初步定位. 西南大学硕士学位论文, 重庆, 2013 Zhang Q. Genetic Effects Analysis and QTL Mapping of Major Plant-Type Traits inL. MS Thesis of Southwest University, Chongqing, China, 2013 (in Chinese with English abstract)
[38] 王春娥, 盖钧镒, 傅三雄, 喻德跃, 陈受宜. 大豆豆腐和豆乳得率的遗传分析与QTL定位. 中国农业科学, 2008, 41: 1274–1282 Wang C E, Gai J Y, Fu S X, Yu D Y, Chen S Y. Inheritance and QTL mapping of tofu and soymilk output in soybean., 2008, 41: 1274–1282 (in Chinese with English abstract)
附表1 正反交组合株高与茎粗表型分布的极大对数似然函数值和AIC值
Supplementary table 1 Maximum log likelihood estimated value and AIC value for plant height and stem diameter in reciprocal crosses
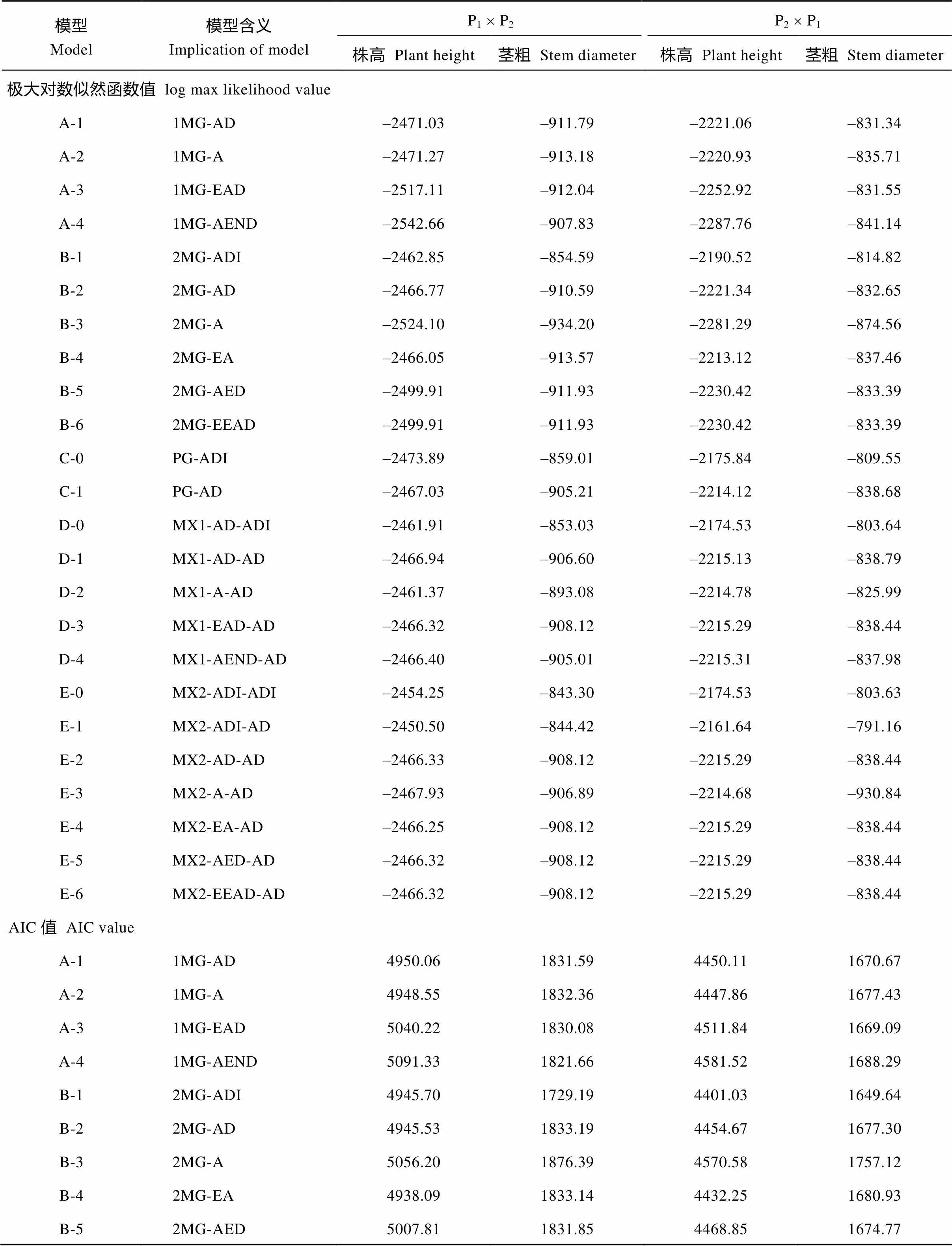
模型Model模型含义Implication of modelP1´ P2P2´ P1 株高Plant height茎粗 Stem diameter株高 Plant height茎粗 Stem diameter 极大对数似然函数值 log max likelihood value A-11MG-AD–2471.03–911.79–2221.06–831.34 A-21MG-A–2471.27–913.18–2220.93–835.71 A-31MG-EAD–2517.11–912.04–2252.92–831.55 A-41MG-AEND–2542.66–907.83–2287.76–841.14 B-12MG-ADI–2462.85–854.59–2190.52–814.82 B-22MG-AD–2466.77–910.59–2221.34–832.65 B-32MG-A–2524.10–934.20–2281.29–874.56 B-42MG-EA–2466.05–913.57–2213.12–837.46 B-52MG-AED–2499.91–911.93–2230.42–833.39 B-62MG-EEAD–2499.91–911.93–2230.42–833.39 C-0PG-ADI–2473.89–859.01–2175.84–809.55 C-1PG-AD–2467.03–905.21–2214.12–838.68 D-0MX1-AD-ADI–2461.91–853.03–2174.53–803.64 D-1MX1-AD-AD–2466.94–906.60–2215.13–838.79 D-2MX1-A-AD–2461.37–893.08–2214.78–825.99 D-3MX1-EAD-AD–2466.32–908.12–2215.29–838.44 D-4MX1-AEND-AD–2466.40–905.01–2215.31–837.98 E-0MX2-ADI-ADI–2454.25–843.30–2174.53–803.63 E-1MX2-ADI-AD–2450.50–844.42–2161.64–791.16 E-2MX2-AD-AD–2466.33–908.12–2215.29–838.44 E-3MX2-A-AD–2467.93–906.89–2214.68–930.84 E-4MX2-EA-AD–2466.25–908.12–2215.29–838.44 E-5MX2-AED-AD–2466.32–908.12–2215.29–838.44 E-6MX2-EEAD-AD–2466.32–908.12–2215.29–838.44 AIC值 AIC value A-11MG-AD4950.061831.594450.111670.67 A-21MG-A4948.551832.364447.861677.43 A-31MG-EAD5040.221830.084511.841669.09 A-41MG-AEND5091.331821.664581.521688.29 B-12MG-ADI4945.701729.194401.031649.64 B-22MG-AD4945.531833.194454.671677.30 B-32MG-A5056.201876.394570.581757.12 B-42MG-EA4938.091833.144432.251680.93 B-52MG-AED5007.811831.854468.851674.77
(续附表1)
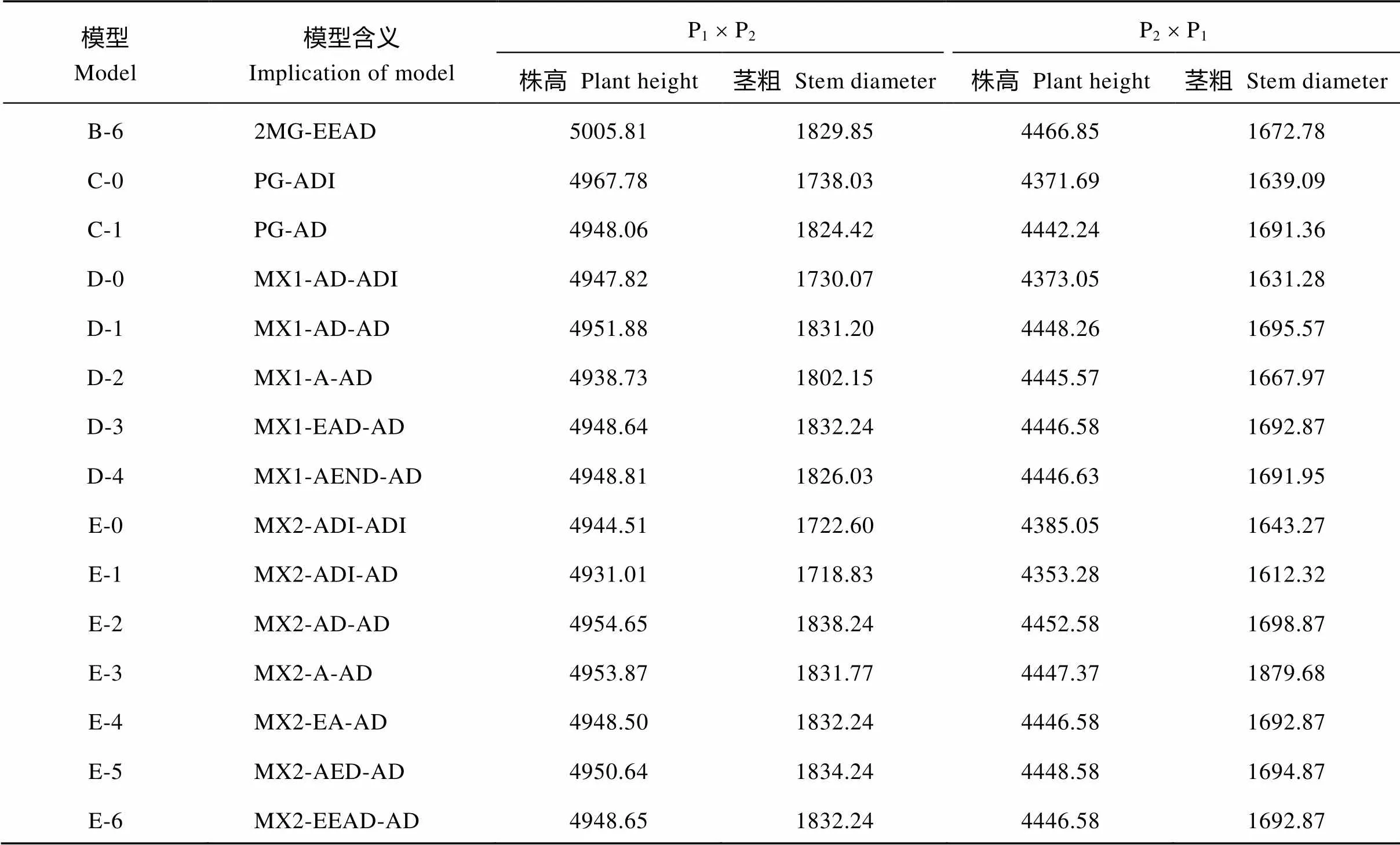
模型Model模型含义Implication of modelP1´ P2P2´ P1 株高 Plant height茎粗 Stem diameter株高 Plant height茎粗 Stem diameter B-62MG-EEAD5005.811829.854466.851672.78 C-0PG-ADI4967.781738.034371.691639.09 C-1PG-AD4948.061824.424442.241691.36 D-0MX1-AD-ADI4947.821730.074373.051631.28 D-1MX1-AD-AD4951.881831.204448.261695.57 D-2MX1-A-AD4938.731802.154445.571667.97 D-3MX1-EAD-AD4948.641832.244446.581692.87 D-4MX1-AEND-AD4948.811826.034446.631691.95 E-0MX2-ADI-ADI4944.511722.604385.051643.27 E-1MX2-ADI-AD4931.011718.834353.281612.32 E-2MX2-AD-AD4954.651838.244452.581698.87 E-3MX2-A-AD4953.871831.774447.371879.68 E-4MX2-EA-AD4948.501832.244446.581692.87 E-5MX2-AED-AD4950.641834.244448.581694.87 E-6MX2-EEAD-AD4948.651832.244446.581692.87
MG: 主基因模型; MX: 主基因+多基因混合模型; PG: 多基因遗传模型; A: 加性效应; D: 显性效应; I: 互作; N: 负向; E: 相等; 例如: E-1 模型 MX2-ADI-AD, 表示 2 对加性-显性-上位性主基因+加性-显性多基因混合遗传模型。
MG: major gene model; MX: mixed major gene and polygene model; PG: polygene model; A: additive effect; D: dominance effect; I: interaction; N: negative; E: equal; e.g. Model E-1=MX2-ADI-AD, means mixed model with two major genes of additive-dominance-epistasis effects plus additive-dominance polygene.
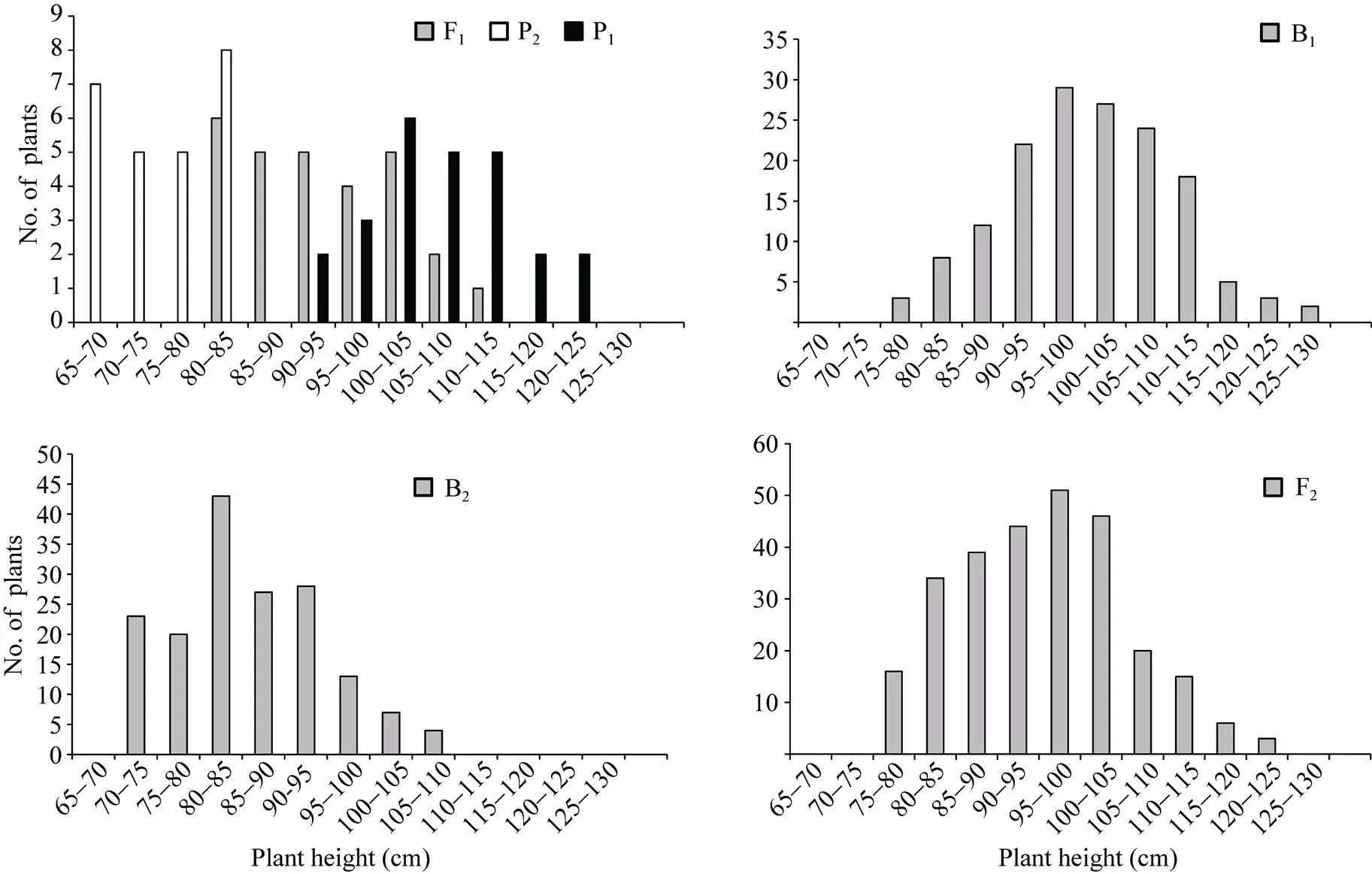
附图1 正交组合6世代株高的次数分布
Supplementary fig. 1 Frequency distributions of plant height in six generations derived from orthogonal cross
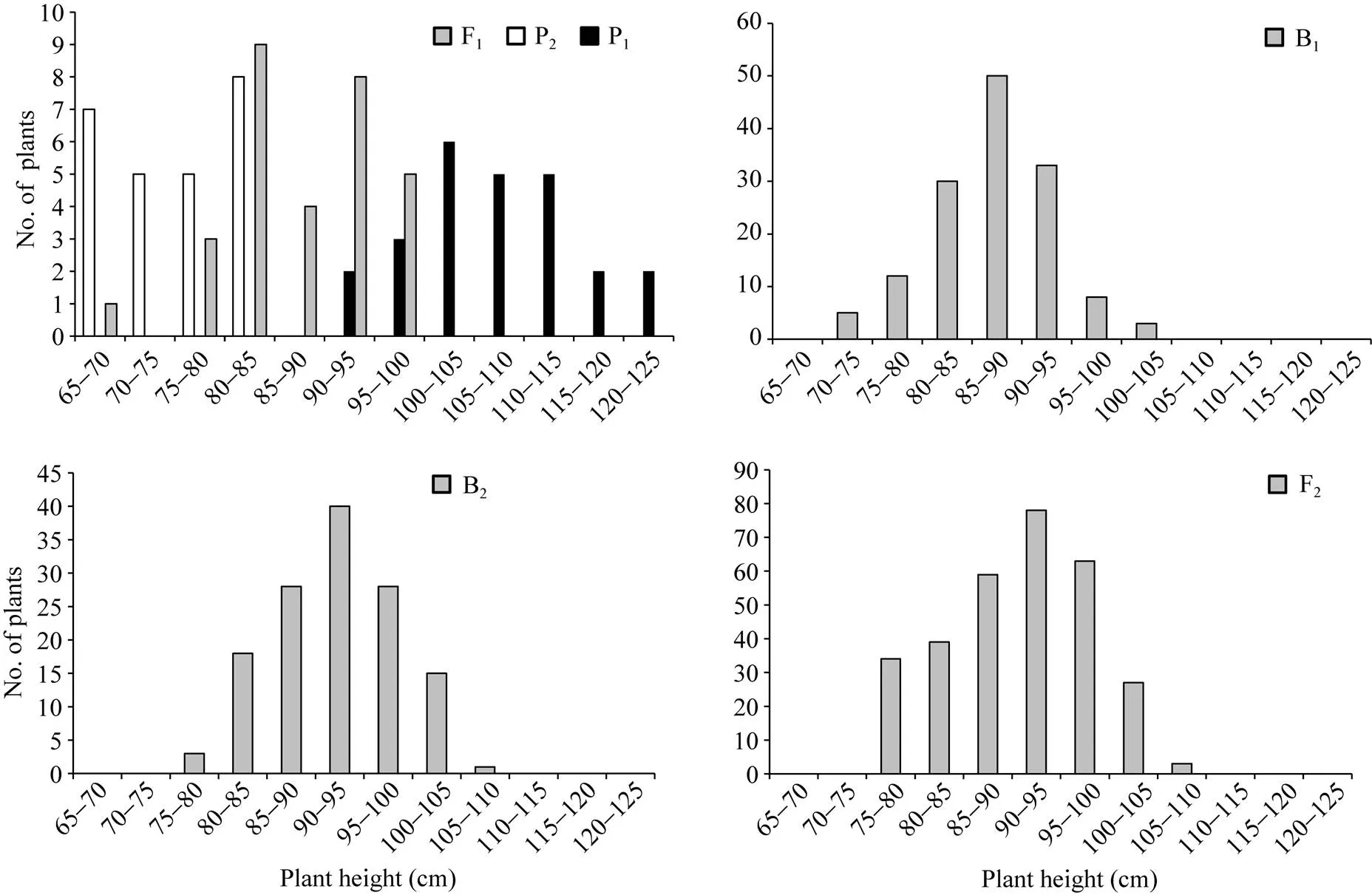
附图2 反交组合6世代株高的次数分布
Supplementary fig. 2 Frequency distributions of plant height in six generations derived from back cross
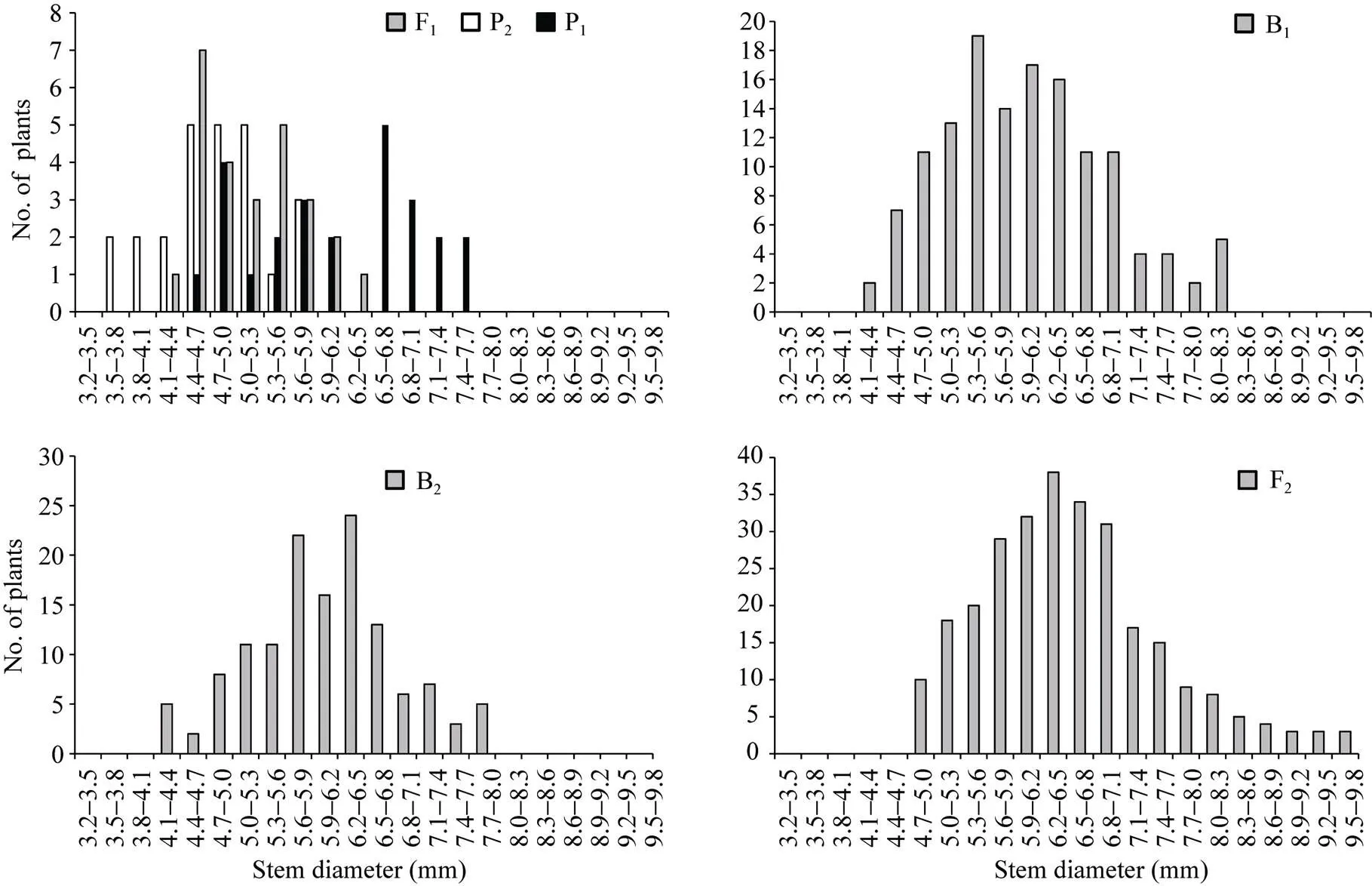
附图3 正交组合6世代茎粗的次数分布
Supplementary fig. 3 Frequency distributions of stem diameter in six generations derived from orthogonal cross
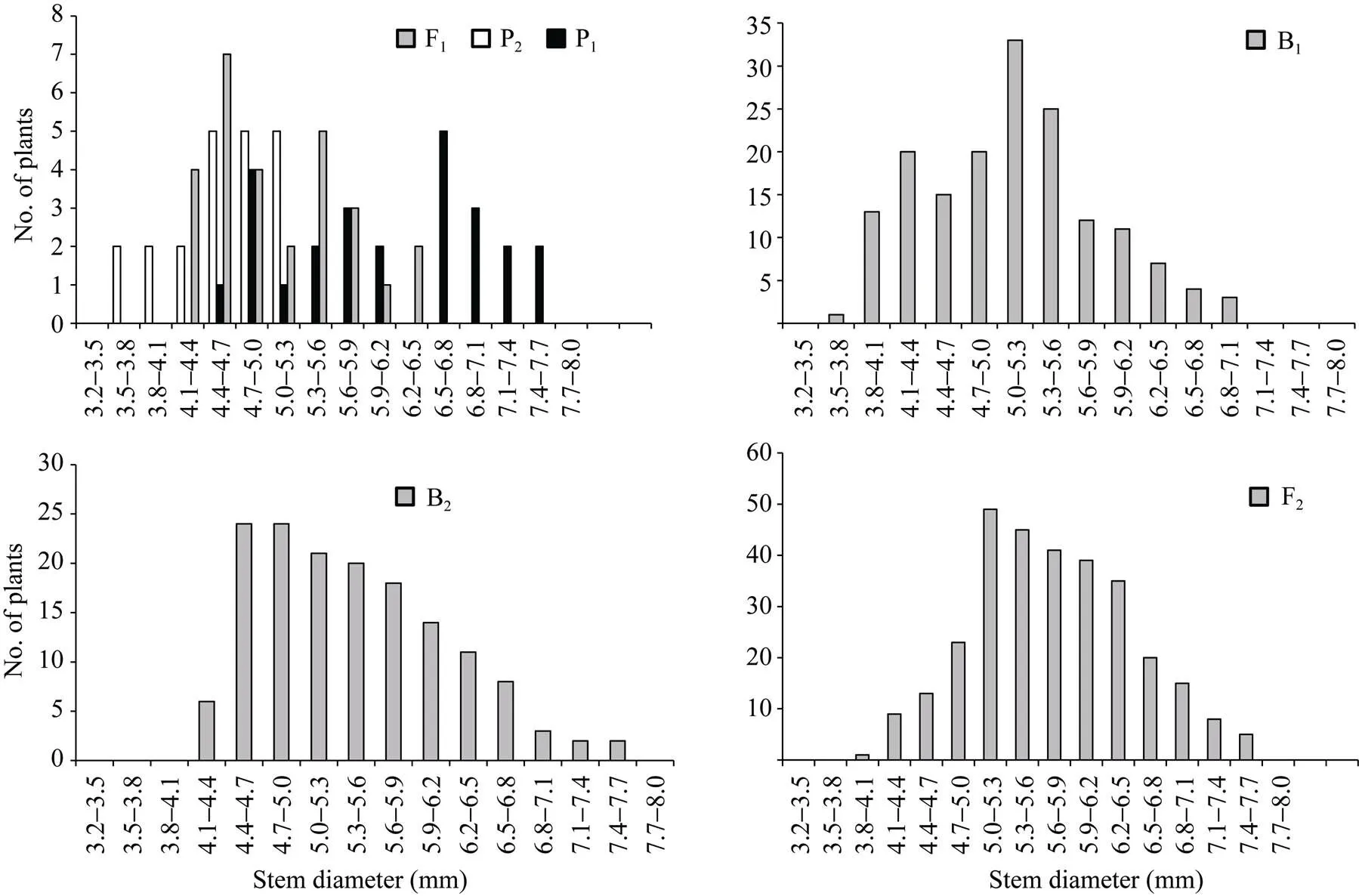
附图4 反交组合6世代茎粗的次数分布
Supplementary fig. 4 Frequency distributions of stem diameter in six generations derived from back cross
Genetic Analysis of Plant Height and Stem Diameter in Common Buckwheat
LI Ying-Shuang1,**, HU Dan3,**, NIE Jiao1, HUANG Ke-Hui1, ZHANG Yu-Ke1, ZHANG Yuan-Li1, SHE Heng-Zhi1, FANG Xiao-Mei1,2, RUAN Ren-Wu1,2, and YI Ze-Lin1,2,*
1College of Agronomy and Biotechnology, Southwest University, Chongqing 400716, China;2Innovation Team of Chongqing Buckwheat Industry System, Chongqing 400716, China;3Seed Administration Station of Gansu, Lanzhou 730000, Gansu, China
Common buckwheat (M.) is susceptible to lodging, and plant height and stem diameter are recognized as important traits for lodging resistance. In this study, we developed the Pl, P2, Fl, F2, Bl, and B2populations from the reciprocal crosses between Youqiao 2 (YQ2, lodging-resistance) and Ukraine daliqiao (UD, lodging-susceptible) and analyzed the genetic effects of plant height and stem diameter. The heredity of both traits optimally fitted to the genetic model for two major genes with additive-dominance-epistatic effects plus polygenes with additive-dominance effects. For plant height in the orthogonal combination, additive effects of both two major genes were –1.39 and the dominant effects were –6.59 and –7.91. Heritability values of the major genes in B1, B2, and F2were 45.73%, 63.49%, and 81.12%, and those of polygenes were 27.41%, 0.95%, and 0, respectively. For plant height in the back cross, additive effects of both two major genes were –1.63 and the dominant effects were –7.03 and –4.19, respectively. Heritability values of the major genes in B1, B2, and F2were 41.51%, 66.18%, and 81.81%, and those of polygenes were 11.19%, 0, and 0, respectively. For stem diameter in the orthogonal combination, the two major genes had 0.03 and 0.03 of additive effect and –0.50 and –0.08 of dominant effect. Heritability values of the major genes in B1, B2and F2were 37.26%, 48.80%, and 72.10%, and those of polygenes were 11.18%, 0, and 0, respectively. For stem diameter in the back cross, the two major genes possessed-0.15 and-0.15 of additive effect and-0.30 and-0.16 of dominant effect. The estimated heritability values in B1, B2, and F2were 76.22%, 47.12%, and 82.51%, respectively, for the major genes and 0, 14.53%, and 0, respectively, for the polygenes. These results suggest that plant height can be selected in early generations because the heritability of major genes plus polygenes was larger than 80%, whereas proper cultivation practice may enhance lodging resistance of buckwheat because the heritability of major genes plus polygenes was lower than 80%.
common buckwheat; plant height; stem diameter; quantitative trait; genetic analysis
2018-04-11;
2018-06-11.
易泽林, E-mail: yzlin1969@126.com**同等贡献(Contributed equally to this work)
李英双, E-mail: 775903522@qq.com
2017-11-09;
10.3724/SP.J.1006.2018.01185
本研究由中央高校基本业务费专项(XDJK2017D071), 重庆市荞麦产业体系创新团队建设项目(CQCYT2017001), 荞麦抗倒伏栽培技术集成与示范推广项目(cstc2017shms-xdny80024), 中国博士后科学基金项目(2017M622944)和重庆市博士后科研项目(Xm2017176)资助。
This study was supported by the Fundamental Research Funds for the Central Universities (XDJK2017D071), Chongqing Buckwheat Industry System Innovation Team (CQCYT2017001), Integration and Demonstration Promotion for the Buckwheat Lodging-resistant Cultivation Technique (cstc2017shms-xdny80024), Chinese Postdoctoral Science Foundation (2017M622944), and Chongqing Postdoctoral Science Foundation (Xm2017176).
URL: http://kns.cnki.net/kcms/detail/11.1809.S.20180608.1320.002.html

