Pancreatic cancer: treatment approaches and trends
Nabyla Paixão Pereira, José Raimundo Corrêa
Laboratory of Microscopy and Microanalyses, Group of Applied Chemotherapy and Fluorescent Probes, Department of Cellular Biology, Institute of Biological Science, University of Brasilia, Brasilia, DF 70910-900, Brazil.
Abstract Pancreatic cancer is one of the most challenging diseases due to its often late diagnose which results in limited therapeutic options and poor prognosis. To date, the only curative treatment is complete tumor removal surgery but only a few patients are eligible to do it. The median survival period after surgery followed by chemotherapy adjuvant treatment is about 2 years. Since its approval by the FDA, Gemcitabine has become the first-line chemotherapy agent for treatment of advanced pancreatic cancer. The FOLFIRINOX regimen is also used as a treatment scheme for pancreatic cancer; however, this regimen has resulted in small improvements in overall patient’s survival. It is appropriated to clarify that the FOLFIRINOX regimen can only be administered in patients with good performance status. Due to the absence of outstanding result after patient’s treatment with diverse chemotherapeutic agents combinations or unsuccessful administration of single-agent drugs to treat pancreatic cancer, the immunotherapy has become a new hope. A more comprehensive understanding of cancer microenvironment and the chemical communication between cancer cells and immune cells can result in new therapeutic approaches that will improve the elimination of pancreatic cancer cells,enhancing life quality for these patients and increasing the overall survival.
Keywords: Pancreatic cancer, chemotherapy, gemcitabine, immunotherapy
INTRODUCTION
In recent decades the worldwide incidence of cancer has increased substantially. It has been estimated that 609,640 Americans will die from cancer this year[1]and pancreatic cancer is ranked in the fourth position among cancer-related deaths in the United States[2-5]. This cancer type is responsible for 331,000 deaths per year[6], and according to GLOBOCAN, 2016 almost 340,000 new cases of pancreatic cancer are diagnosed each year worldwide.
Pancreatic cancer is more common in elderly persons (between 60 and 80 years) and some studies have shown an increased incidence among diabetes[7,8]or chronic pancreatitis patients[2,9,10]. Both environmental and inherited factors[11]can contribute to the development of this disease and the most common risk factors associated to this type of cancer are smoking[12,13]and overweight obesity[14].
The adenocarcinoma is the most common pancreatic cancer, representing 85% of all cases[15]. Furthermore,the pancreatic adenocarcinoma remains one of the most challenging malignancies with limited therapeutic options and poor prognosis[3]because it is usually diagnosed at an advanced stage[16]. This aspect partially can be explained by the fact that early stages of pancreatic cancer often present none or nonspecific symptoms,which can be translated in diagnosis challenges[12].
Normally, advanced pancreatic cancer patients can present symptoms like nausea, vomiting, bloating,unexplained weight loss, jaundice, abdominal pain, dyspepsia and sometimes pancreatitis[9]. Moreover, 70%of patients present diabetes mellitus, usually with a diabetes history of less than 2 years[17]. The poor prognosis is also attributed to the high incidence of metastasis, leading to an aggressive disease course combined with the limited efficacy of systemic treatments[5].
Surgery procedures are considered the most effective treatment and the only curative intervention but only 20% of patients are fit for it based on disease staging[4]and up to 80% of these patients relapse. When compared to other resected solid tumors, the poorest outcomes are observed in patients with resected pancreatic cancer.After surgery, those resected patients are selected for adjuvant therapy with chemoradiation or chemotherapy alone and they present a median survival post-surgery combined with adjuvant therapy averaging 2 years[14],with only 20% of patients reaching 5-year survival rate[18]. Regarding that, there are some studies with neoadjuvant chemotherapy administered in patients with resectable, borderline resectable or locally advanced disease aiming to increase resectability by achieving higher margin-negative resections and conversion rates[19].
According to the American Cancer Society, the 5-year relative survival of pancreatic cancer patients is 29%for localized stage at diagnose period, 11% for regional stage and only 3% for distant stage[20,21]. These statistical data indicate that there is an increased need for development of efficient and well-tolerated treatment options.This work intends to summarize the approved adjuvant chemotherapy approaches [Table 1] for advanced pancreatic cancer and some immunotherapy treatment trends for this aggressive and devastating disease.
TREATMENT OPTIONS
Treatment of pancreatic cancer is multimodal, and most patients will receive more than one type. The primary and only curative intervention is surgery. In sequence, it includes adjuvant (treatment given after primary treatment) chemotherapy and/or radiation therapy, or palliative care depending on the stage of cancer, according to the staging system developed by American Joint Committee on Cancer, which is now in the 8th edition. Based on the cancer stage the patient will be directed to a kind of treatment. This staging system takes into account the TNM status which means: T - primary tumor size; N - lymph node involvement; M - distant metastasis [Table 2][18].
As mentioned, different treatment guidelines are used for each stage. Frequently, stage II (resected lesions)is treated by surgery and adjuvant chemotherapy, sometimes including chemoradiation; Stage III (locally advanced) chemotherapy with or without chemoradiation and stage IV (metastatic) with chemotherapy[22].
SURGERY
Pancreatic cancer patients are subdivided into four groups: resectable, borderline resectable, locally advanced nonresectable, and metastatic. Cancer that is confined to the pancreas without significant involvement of nearbyblood vessels is called resectable. Cancer that is confined to the pancreas but involves nearby blood vessels or structures to a greater extent is called borderline resectable[23]. Cancer that involves nearby blood vessels or other structures to such a significant extent that it cannot be successfully removed by surgery is called locally advanced nonresectable[24]. Cancer that has spread outside the pancreas to other organs and tissues in the body is called metastatic. Patients with metastatic disease are not indicated to have surgical resection[25].
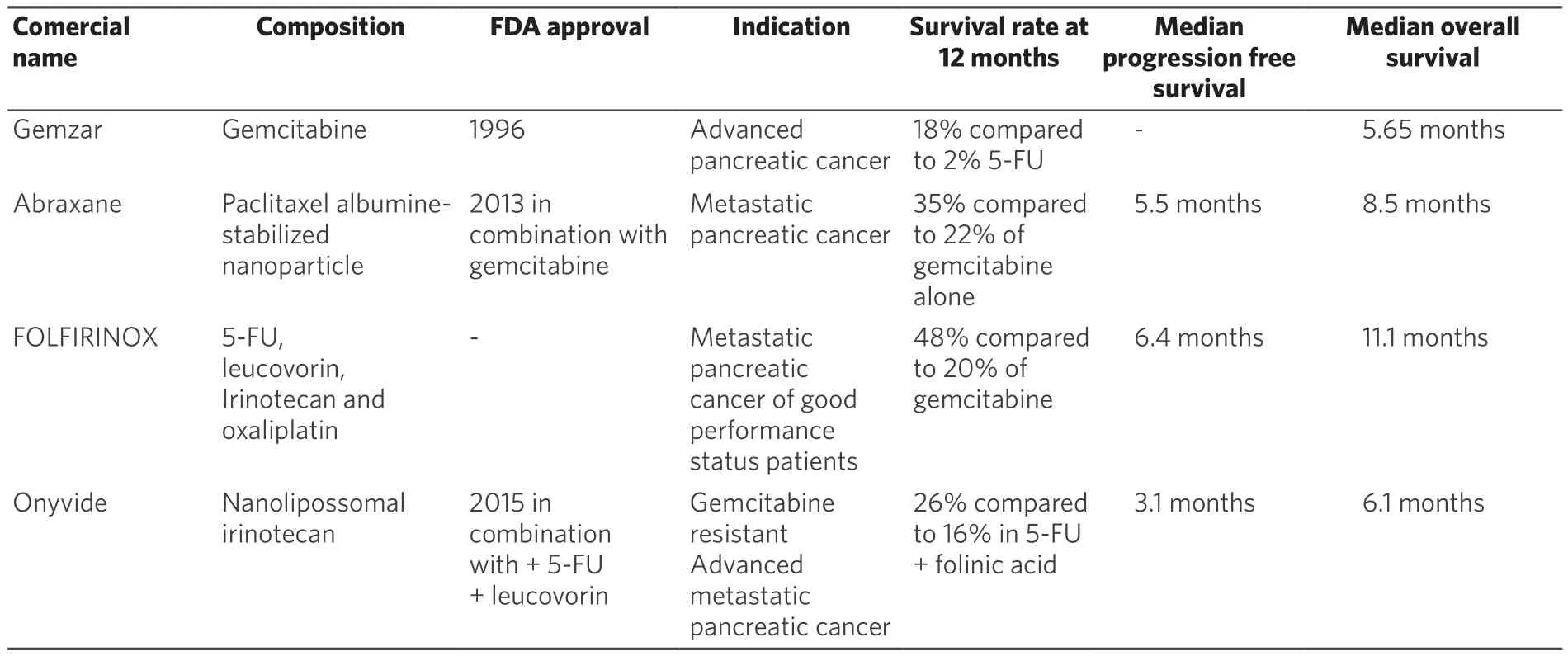
Table 1. Summary of chemotherapy approaches
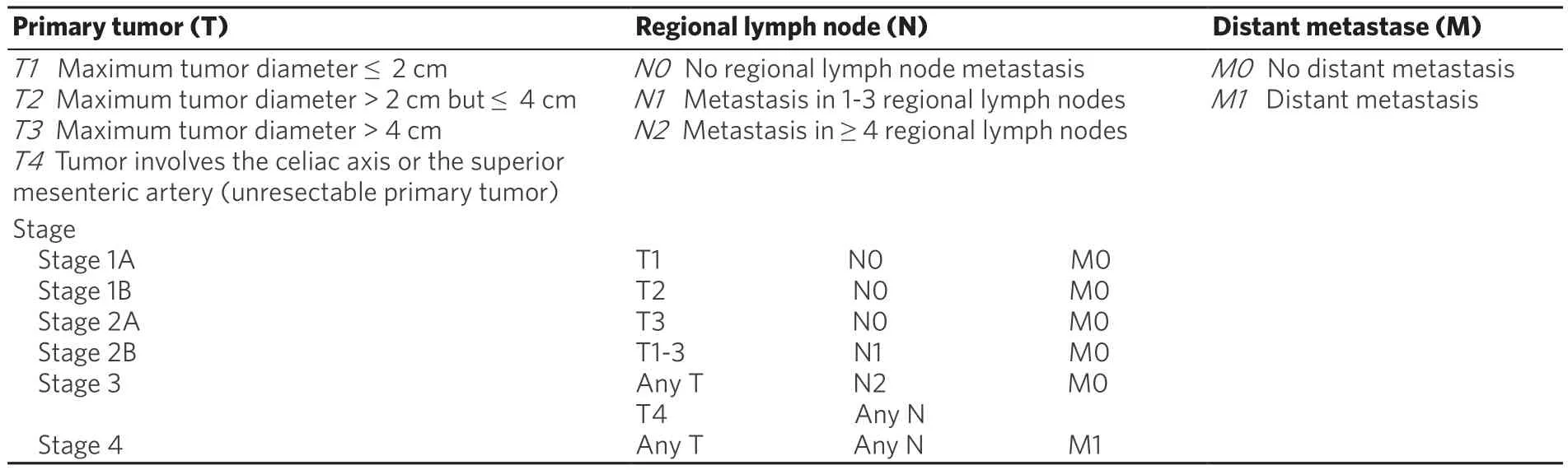
Table 2. American Joint Committee on Cancer 8th edition staging system for pancreatic cancer
All patients must undergo preoperative exams such as contrast-enhanced abdominal computed tomography or magnetic resonance imaging with cholangiopancreaticography so the surgeons can decide what kind of procedure to apply on each patient.
For those patients that are possible to undergo resection there are three types of surgery: Whipple procedure,distal pancreatectomy, and total pancreatectomy. Conventional Whipple operation or pylorus preserving,also known as pancreaticodueodenectomy, with lymphadenectomy is the choice for head or neck pancreatic cancers. Distal pancreatectomy with splenectomy is the choice for body/tail cancer. The Whipple procedure removes the head of the pancreas, the gallbladder, duodenum, part of the bile duct, and often part of the stomach. It also removes the nearest lymph nodes to biopsy. The distal pancreactectomy removes the body and tail of the pancreas, some nearby lymph nodes, and sometimes the spleen and its blood vessels. The total pancreactectomy removes the gallbladder, duodenum, part of the bile duct and stomach, nearby lymph nodes, and sometimes the spleen[26-28]. The prognosis for patients that go through resection depends on margin status. The one associated with the best outcomes is a R0 resection which means a total gross excision and negative histological margins; R1 resection is a total gross excision however with positive histological margins; and, R2 is a resection with residual gross tumor and patients that undergo R2 resection have similar prognosis of the unresectable patients treated with non-operative therapy, on account of that, surgeries that will result in R2 margins should not be consider as resectable[23,29].
To improve survival for locally advanced patients neoadjuvant therapy has been evaluated aiming to shrink tumor, enhance resectability and also to increase rates of microscopic complete tumor resection[30].
CHEMOTHERAPY GEMZAR - GEMCITABINE
Gemcitabine is a deoxycytidine (dCTP) analogue, which is converted by nucleoside kinases into two metabolites diphosphate (dFdCDP) and triphosphate (dFdCTP). Each of these metabolites have a specific mechanism of action:(1) the diphosphate metabolite (dFdCDP) inhibits ribonuclease reductase, an enzyme known for catalyzing the reaction that generates ribonucleotides necessary for DNA synthesis; (2) the triphosphate metabolite (dFdCTP)competes with the natural dCTP for its incorporation into DNA newly synthetized strands. Once dFdCTP is incorporated, only one additional nucleotide is added to the growing DNA strands, which stops the DNA synthesis and eventually results in activation of apoptosis pathway leading the cells to death[31].
Gemcitabine, as single-agent, became the first line treatment (1996) for advanced pancreatic cancer since a randomized trial showing that 23.8% of patients had experienced a clinical benefit response compared with 4.8% of patients treated with fluorouracil (5-FU). Gemcitabine also confers a modest improvement in overall survival than those observed in patients group treated with 5-FU. The patients’ overall survival rates at 12 months were 18% for gemcitabine and 2% for patients treated with 5-FU[32].
In the following decade, gemcitabine has become the backbone of combination regimen for new experimental approaches with either other cytotoxic molecules or novel chemotherapy agents[33]. Many phase II trials have demonstrated the efficacy of gemcitabine-based combinations, which comprise other cytotoxic molecules such as capecitabine, 5-FU, cisplatin, irinotecan[34-37]or the targeted agents sorafenib and cetuximab[38-40].However, in some randomized phase III trials of gemcitabine based chemotherapy combinations, these combinations failed to show statistically significant improvement in patient’s overall survival when compared to gemcitabine used as a single-agent[41-46].
Nowadays, gemcitabine is used in combination with taxol, a paclitaxel albumin-stabilized nanoparticle formulation (nab-paclitaxel) that is commercially known as abraxane. Taxol is a microtubule dynamics inhibitor that promotes the stabilization of microtubules by preventing the catastrophe process, which induces cell cycle arrest at the G2/M phase resulting in cell death[14]. In preclinical studies, nab-paclitaxel improved the intratumoral concentration of gemcitabine. The FDA approval for this approach was obtained after a phase III study that demonstrated the efficacy and safety of this combination compared to monotherapy with gemcitabine in patients with metastatic pancreatic cancer. Von Hoff et al.[47], randomized assigned 861 patients: 431 received nab-paclitaxel plus gemcitabine and 430 gemcitabine alone. The median overall survival was 1.8 months superior in the combination group, and the survival rate was 35% in the nab-paclitaxel-gemcitabine group compared to 22% in the gemcitabine group in 1 year. Moreover, this combination approach increased the median progression-free survival in 1.8 months. However, despite those benefits rates, peripheral neuropathy and myelosuppression were increased in the group that received nabpaclitaxel-gemcitabine combination[47]. De vita et al.[48]also confirmed the effectiveness in overall survival and progression free survival from patients treated with the combination of gemcitabine plus nabpaclitaxel.
Although not yet approved by the FDA as a treatment approach for pancreatic cancer, the ESPAC-4 study developed a phase III randomized trial that could establish the gemcitabine plus capecitabine combination as the treatment of choice for adjuvant setting after resection[49]. In this study, they aimed to demonstrate the safety and efficacy of the combination for resected pancreatic cancer since a phase III randomized comparison between gemcitabine plus capecitabine and gemcitabine alone showed a significant improvement in objective response rate (P = 0.03) and progression-free survival (P = 0.004) and was associated with a trend toward improved overall survival (P = 0.08) in patients with advanced pancreatic cancer that underwent the combination approach[50]. The capecitabine is an oral prodrug of 5-FU, a fluoropyrimidine carbamate, that provides prolonged fluorouracil tumor exposure at lower peak concentration. The conversion of capecitabine in the active drug needs an enzyme named thymidine phosphorylase which is present at higher levels in tumor cells compared to other tissues which improves tolerability and intratumor drug concentration[51].
FOLFIRINOX REGIMEN - FLUOROURACIL, LEUCOVORIN, IRINOTECAN AND OXALIPLATIN
5-FU is a fluoropyrimidine antimetabolite drug that exerts antitumoral effects inhibiting the enzyme thymidylate synthase, impairing the synthesis of the pyrimidine thymine, which is required for genetic material synthesis. The fluoronucleotides are misincorporated into RNA and DNA strands resulting in cell death[52]. Leucovorin is a metabolite of folinic acid, known as 5-formyltetrahydrofolic acid, which is the 5-formyl derivative of tetrahydrofolic acid[53]. Leucovorin is indicated for use as rescue therapy to reduce the toxicity associated of folinic acid antagonists that inhibits de novo synthesis of purines, pyrimidines and methionine. The combination of leucovorin and 5-FU can extend the survival in the palliative treatment of patients with advanced pancreatic cancer[54,55]. Irinotecan is a derivative of camptothecin that has a cytotoxic action via a potent and specific inhibition of DNA topoisomerase I, preventing the DNA strand ligation leading to double-strand DNA breakage and cell death[56]. Oxaliplatin is a platinum-based drug that belongs to the same family of cisplatin and carboplatin. In oxaliplatin the two amine groups were replaced by cyclohexyldiamine, which increases its antitumor effect. The chlorine ligands were replaced by the oxalato bidentate derived from oxalic acid that improves its water solubility[57,58]. Oxaliplatin is converted to active derivatives via displacement of the labile oxalate ligand. Its reactive species monoaquo and diaquo diaminocyclohexane platinum binds guanine and cytosine moieties of DNA and this association produces cross-linking of DNA inhibiting the DNA synthesis and transcription[59].
A phase 1 study involving patients with advanced solid tumor was developed to determine the maximumtolerated dose and the recommended dose of the triple combination (oxaliplatin, irinotecan, leucovorin/5-FU). A fair response in patients with advanced pancreatic cancer utilizing this combining regimen was observed[60].Then, a phase 2 study of FOLFIRINOX regimen was conducted involving 46 advanced pancreatic cancer patients with good performance status. FOLFIRINOX showed a high efficacy against this malignant tumor, but it has produced severe neutropenia in half of the patients. It was prompted started the phase 2-3 trial in order to compare FOLFIRINOX regimen with gemcitabine as single antitumoral agent. In this trial, 342 patients were randomly assigned. The median overall survival and the median progressionfree survival were significantly extended for the FOLFIRINOX regimen group (48% of patients submitted to FOLFIRINOX regimen were alive after 1 year compared to 20% treated with gemcitabine). Due to its high toxicity, the group treated with FOLFIRINOX showed more intense side effects such as grade 3 or 4 neutropenia, thrombocytopenia and grade 2 alopecia. However, despite the higher incidence of intense side effects, the FOLFIRINOX treated group showed a significant increase of time period that precedes the definitive deterioration of the quality of life compared to gemcitabine group. These results lead to the conclusion that FOLFIRINOX is an effective therapeutic option but only suitable for patients with metastatic pancreatic cancer that hold a good performance status[61].
After the effectiveness of FOLFIRINOX regimen in the palliative setting has been established, Faris et al.[62]had performed a retrospective study in the Massachusetts General Hospital Cancer Center to answer two questions that remained unclear: will the benefit in response rate and overall survival in the metastatic setting translate to patients with locally advanced pancreatic cancer? And are curative-intent resections possible in patients who respond to this treatment? They found that FOLFIRINOX regimen have substantial activity in locally advanced pancreatic cancer patients and also, that the use of FOLFIRINOX regimen could induce cancer conversion to resectability in more than 20% of patients. From those patients that could resect the cancer, 3 from 5 had recurrence and 1/3 of patients had experienced significant toxicity signals that required visits to emergency department or hospitalization. The most prevalent effects were anemia grade 1 or 2, thrombocytopenia (mostly grade 1), neutropenia, diarrhea/dehydration. Due to high toxicity of FOLFIRINOX regimen, further studies were suggested to reach an optimized treatment to patients with locally advanced pancreatic cancer.
In the other hand, FOLFIRINOX has been studied as neoadjuvant option for locally advanced and borderline resectable patients[63-65]. The neoadjuvant therapy can benefit by converting a few locally advanced tumors into resectable ones and increase R0 resectability in borderline tumors[66,67]. The FOLFIRINOX combination regime was associated with an increase in R0 resection rates when administered with or without radiotherapy before surgery in borderline resectable and locally advanced patients. The most important result is the down staging of the disease in locally advanced, thus making it possible for patients to undergo surgery and increasing the median progression free survival[19,68,69]. However, phase III studies should be prompted to confirm whether preoperative neoadjuvant vs. postoperative adjuvant treatment relates to better survival for those patients that can undergo surgery[70].
ONYVIDE - NANOLIPOSOMAL IRINOTECAN, 5-FU AND FOLINIC ACID
Nanoliposomal irinotecan has potential antineoplastic activity; its liposome encapsulation promotes better delivery of drugs into the cytosol from the endosome compartment of the cell. This encapsulation platform of drug delivery reduces the premature systemic drug release but maintains its intra tumoral release,enhancing antitumor activity[71].
On October 22, 2015, the U.S. FDA has approved the onivyde (irinotecan liposome injection) in combination with 5-FU and leucovorin to treat patients with advanced metastatic pancreatic cancer who have been previously treated with gemcitabine-based chemotherapy. The approval was due to a phase III study,conducted after preceding trials showing promising activity of the nanoliposomal irinotecan in patients with metastatic pancreatic ductal adenocarcinoma previously treated with gemcitabine[72].
In the phase III trial, nanoliposomal irinotecan was tested alone or in combination with 5-FU and folinic acid, compared with a common control (5-FU and folinic acid) in patients with metastatic pancreatic cancer progression after a regimen of gemcitabine. It was a global, randomized, open-label trial in 14 countries.Their results showed that nanoliposomal irinotecan plus 5-FU and folinic acid significantly improved the overall survival. Also, the results related with progression-free survival, objective tumor response, time to treatment failure and CA19-9 tumor marker response for those patients were significantly improved in contrast to the 5-FU and folinic acid control group. Neutropenia, fatigue, diarrhea and regurgitating were the main side effects observed in patients group (14.5%, 13.7%, 12.8%, 11.1% respectively) submitted to treatment with the combination of nanoliposomal irinotecan with 5-FU and folinic acid. With a manageable safety profile, this approach represents a new treatment option for many patients with metastatic pancreatic cancer that previously received an unsuccessful gemcitabine therapy[73].
There is an ongoing trial, randomized, open-label, phase II study of onivyde vs. nab-paclitaxel + gemcitabine in patients with metastatic pancreatic adenocarcinoma (NCT02551991)[74].
IMMUNOTHERAPY
Despite all chemotherapy combinations and new trials with targeted therapies, overall survival of advanced pancreatic cancer patients remains poor. The establishments of new therapies that provide long-term benefit are urgently needed. The spotlights are now on new immunotherapy approaches, since it is an unexplored and growing landscape and has been applied successfully in other types of cancer. There are many evidences showing that pancreatic cancer generates antitumor immune responses, suggesting that immunotherapies can be a promising alternative for those patients[75]. As already known, pancreatic cancer creates an immunosuppressive tumor microenvironment with mucin overexpression. To overcome this immunosuppressive microenvironment, Banerjee et al.[76]have developed a nanovaccine using recombinant fragments of MUC4, a highly expressed mucin which contributes to cancer aggressiveness, and immunized KPC mice. When compared to control group, the immunized mice exhibited a slower tumor growth kinetics and a greater accumulation of CD8+ and CD4+ T cells. The suppression of tumor progression caused by the immunization points the MUC4 nanovaccine to be a potential immunotherapy for pancreatic cancer.
Another potential immunotherapy approach resulted from the study in which they administered AMD3100(plerixafor) in KPC mice. AMD3100 is an inhibitor of chemokine receptor CXCR4, a CXCL12 receptor. The inhibition of CXCR4 by the AMD3100 contributes to a fast T cell accumulation in regions of the tumor and acted together with the immunological checkpoint antagonist, α-programmed cell death 1 ligand 1, to reduce cancer cells[77].
Five main categories for immunotherapy applied to pancreatic cancer have been described[78]: (1) checkpoint inhibitors/immune modulators. This strategy aims to modulate immune system through inhibitory or stimulatory signals, such as inhibition of CD28 family receptors, which controls T cell responses, modulating the immune cytotoxic response, restoring or increasing the cytotoxic antitumor activities of T cell[79]; (2)therapeutic vaccines. In these cases, occurs a patient’s active immunization with tumor specific antigen.This vaccine will trigger T cells and increase its activity against the tumor[80]; (3) adoptive T cell transfer. An adoptive T cell transfer is a kind of transfusion therapy that infuses mature T CD8+ specific cells in patients.These cells target surface proteins in tumor tissue, which are used to T CD8+ cells docking and eliminate cancer cells through granzyme and perforin secretion[81]; (4) monoclonal antibodies. This approach is a passive immunization using antibodies against the same cancer molecule epitope, created to target specific tumor antigens, which enhance the cancer cells recognition by phagocytes and T CD8+ cells improving its elimination; (5) cytokines use. The cytokines such as IL-10 and IL-17B are used to regulate tumor microenvironment, aiming to suppress the cancer cells property to express immunosuppressive cytokines that stop the immune activation against the cancer cells[78].
Even though many encouraging results have been obtained for other types of cancer[82-84], none of these treatments showed significant efficiency when applied as pancreatic cancer therapy[85,86]. Currently, although there are many ongoing trials for immunotherapy, therapeutic vaccines are the most cutting-edge clinical therapy applied as pancreatic cancer immunotherapy. Concerning to vaccines as immunotherapy category,the most advanced studies to date are those conducted with whole-cell vaccines and granulocyte-macrophage colony-stimulating factor (GM-CSF) vaccines.
THERAPEUTIC VACCINE IMMUNOTHERAPY WHOLE-CELL VACCINES
Algenpantucel - L is an irradiated, live combination of two human allogeneic pancreatic cell lines that express the murine enzyme α-1,3-galactosyl transferase. This enzyme performs the addition of α-galactosyl epitopes on surface proteins and glycolipids of such cell lines. The human cells do not express murine alphagal epitopes and these cells inoculation induce a hyperacute rejection of the vaccine pancreatic allograftcell. The hyperacute rejection results in the fast activation of antibody-dependent cell-mediate cytotoxicity.These processes will also stimulate the host immune system to eliminate endogenous pancreatic cancer cells[78,87]. Hardacre et al.[88]in 2013 performed a multi-institutional, open-label phase II trial to evaluate the use of algenpantucel-L in addition to standard adjuvant chemotherapy and chemoradiotherapy setting for resected pancreatic cancer patients (NCT00569387). In this study 70 patients were treated with gemcitabineand 5-FU based chemoradiotherapy as well as algenpantucel-L. The median follow-up was 21 months, and the one-year progression-free survival was 62% added to an 86% overall survival. Inoculation site pain and local tissue induration were the common side events; however, the allogenic cells administration was safe,and it proves to be a feasible combined approach. The results obtained from this phase II trial demonstrated that this immunotherapy component may improve survival, and due to such optimistical results a multiinstitutional phase III study is ongoing (NCT01072981).
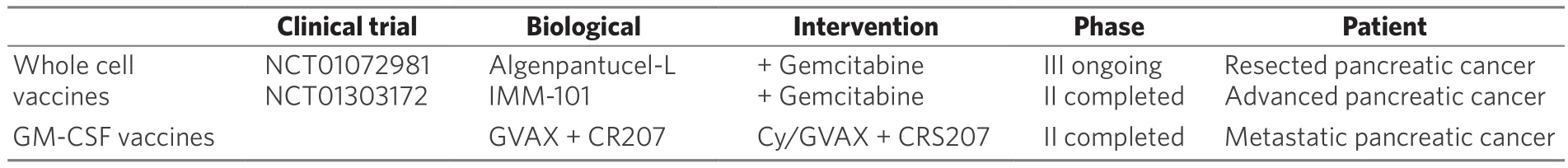
Table 3. Therapeutic vaccines immunotherapy summary
Another randomized phase II trial explored the safety and tolerability of an injectable immunomodulator from heat-killed mycobacterium obuense (IMM-101) used in combination with gemcitabine. This study showed that the administration of IMM-101 plus gemcitabine was safe and well tolerated as gemcitabine alone in patients with advanced pancreatic cancer, moreover the results from this phase II trial suggested a beneficial effect on overall survival which may support further evaluation of IMM-101 in a confirmatory study[89].
GM-CSF VACCINES
A recent phase II randomized multicenter study was conducted comparing cyclophosphamide (Cy)/GVAX followed by CRS-207 with Cy/GVAX alone in patients with metastatic pancreatic cancer. Cy/GVAX is composed of two irradiated GM-CSF-secreting allogeneic pancreatic cancer cell lines administered with low-dose of Cy to hinder regulatory T cells. GVAX induces T CD8+ cells activity against a tumor associated antigen named mesothelin that is over expressed in most pancreatic cancer cells. CRS-207 is a live-attenuated Listeria monocytogene-gene expressing mesothelin that induces innate and adaptative immunity response.The overall survival for the Cy-GVAX followed by CRS-207 was 6.1 months compared to 3.9 months of Cy-GVAX alone. Stable disease rate of 31% and 1-year survival rate of 24% are encouraging results. Furthermore,heterologous boost with Cy-GVAX and CRS-207 extended overall survival for pancreatic cancer patients with minimal related toxicities[90][Table 3].
Worldwide efforts should be directed to identification and selection of specific antigens in order to induce immune response against pancreatic cancer cells aiming to eliminate the immunosuppressive microenvironment that this cancer produces. Appropriate selection of target antigens and combination of treatment protocols are critical to enhance treatment efficacy, lowering related toxicities and as already demonstrated improving the overall survival[91].
Regardless of the advances in pancreatic tumor biology knowledgment, mechanisms associated with the tumor microenvironment remain poorly understood, highlighting that the distinct composition of pancreatic tumor microenvironment could be a great barrier for immunotherapy success[92]. As a consequence of newly emerging information about tumor microenvironment, there was a shift in the cancer development concept from a tumor cell-centered view to a complex tumor ecosystem, which led to the acceptance that cancer cells interact with the extracellular matrix (ECM) and stromal cells[93,94]. A major component of the extracellular matrix is hyaluronic acid (HA), a hydrophilic glycosaminoglycan that is produced in bulk by many pancreatic cancer. Accumulation of HA in tumors is associated with malignancy and poor prognosis, because HA polymers bind and trap water molecules in the ECM as a fluid gel that increases interstitial fluid pressure and creates a physical barrier that restricts antibody and immune cells access the tumor. A pegylated recombinant human hyaluronidase (PEGPH20) is an agent that degrades the hyaluronic acid and normalizes interstitial fluid pressure and has been applied to enhance the delivery of cytotoxic drugs[95]. Hingorani et al.[96]showed the results from a phase II comparison study between PEGPH20 [plus nab-paclitaxel/gemcitabine (AG)](PAG) vs. AG in patients with untreated metastatic pancreatic ductal adenocarcinoma (NCT01839487).Because of an imbalance in thromboembolic events in PAG patients 40% patients were excluded from the study. In order to conclude this trial, the enoxaparin prophylaxis was applied in both arms and the phase II study comparison was successful. This randomized phase II met both primary endpoints (progression-free survival and thromboembolic event rate), with the greater improvement in the secondary endpoint which is the progression-free survival in HA-high patients. In the subset of 80 patients whose tumors had HA-high levels, the addition of PEGPH20 to chemotherapy resulted in an increase of 4 months of stable clinic conditions before disease progression when compared to chemotherapy alone. The results of the phase II trial suggested that HA has a potential predictive biomarker for patient’s selection of PEGPH20, qualifying only patients with high levels of HA for the new phase III trial. The ongoing phase III trial (NCT02715804)intends to determine whether PEGPH20 actually increases patients’ overall survival and not just their time to disease progression.
RADIOTHERAPY
The effectiveness of radiotherapy has been continuously debated[97-99]. Recent studies have shown that the addition of radiotherapy to chemotherapy in the setting of locally advanced pancreatic cancer did not improve overall survival outcome[100,101]. A recent randomized phase III trial, LAP07 (NCT00634725)compared chemoradiotherapy in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine-based chemotherapy with chemotherapy alone. No significant difference in overall survival was found. However, an increase in progression-free survival resulted in a longer period without treatment confirming association of chemoradiotherapy with decreased local progression[102]. Other studies have proposed that chemotherapy administered before simultaneous chemoradiotherapy could enhance survival[103,104]. Therefore, the benefits of radiation therapy in the management of locally advanced pancreatic cancer remain controversial .
CONCLUSION
Although some studies had demonstrated a mild increase in survival rates, there are no available treatments to pancreatic cancer that are focused on preserving the patients’ quality of life.
Considering this deadly disease, it is time to take into account the balance between overall survival and patient's life quality. Pancreatic cancer patients desperately need more specific drugs or drugs combinations capable of eliminating cancer cells without producing so many toxic effects. The real cost for one or two more months of life, is living in pain with severe diarrhea, vomits, neutropenia and immune deficiency.
The lack of an efficient therapy against pancreatic cancer has turned the spotlights to immunotherapy.Despite of many disappointments in several clinical trials, immunotherapy has become an established modality for treatment of other cancer types such as melanoma, breast and lung cancer. Clinical trials testing anticancer vaccines showed promising results to treat pancreatic cancer, however most of them have failed to demonstrate a significant efficacy in improving patient's overall survival and quality of life.
As already discussed, a more comprehensive understanding of cancer microenvironment and the chemical communication between cancer cells and immune cells can result in new molecules targets and pathways,which could be used to increase the immune responses against tumoral cells. These hypothetical targets may ultimately lead, alone or combined with a proper chemotherapy scheme, to a massive cancer cells elimination, improving quality of life and significantly extending overall survive of patients.
DECLARATIONS
Authors’ contributions
Searched bibliographic references data: Pereira NP, Corrêa JR Edited partial scientific text: Pereira NP, Corrêa JR
Reviewed scientific text: Corrêa JR
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
Both authors declare no conflicts of interest in association with this study.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2018.
 Journal of Cancer Metastasis and Treatment2018年6期
Journal of Cancer Metastasis and Treatment2018年6期
- Journal of Cancer Metastasis and Treatment的其它文章
- Pancreaticoduodenectomy for gastric cancer
- Cancer, circulating tumor cells, and metastasis:could protein-derived peptide fragments impede brain metastases?
- Immunotherapy in the treatment of colorectal cancer: a new kid on the block
- Bioconjugate structures vs. composite nanoparticulate carriers: the battle for the future of smart, effective and safe cancer management
- Update on targeted therapy and immune therapy for gastric cancer, 2018
