Pancreaticoduodenectomy for gastric cancer
Rie Makuuchi, Tomoyuki Irino, Yutaka Tanizawa, Etsuro Bando, Taiichi Kawamura, Masanori Terashima
Division of Gastric Surgery, Shizuoka Cancer Center, Shizuoka 411-8777, Japan.
Abstract Pancreaticoduodenectomy (PD) is performed to achieve an R0 resection for gastric cancer with pancreatic and/or duodenal invasion. Several retrospective case series have been published, but the sample cohorts in each study were heterogeneous and small. Moreover, the absence of prospective studies results in a lack of solid evidence that will help determine who can benefit from this procedure. Although the morbidity and mortality of PD have been reported by most studies to be acceptable and that the procedure is feasible, these remained to be much higher than those of standard gastrectomy. Therefore, careful selection of patients should be considered. Based on a review of previous case series and our own experience, PD appears to be beneficial to patients with gastric cancer with pancreatic invasion when R0 resection is possible. In addition, multidisciplinary treatment such as neoadjuvant chemotherapy, is anticipated to improve survival. Nevertheless, considering that prospective randomized studies are difficult to perform, a large-scale multicenter retrospective cohort study is required to evaluate this highly invasive procedure.
Keywords: Gastric cancer, pancreaticoduodenectomy, multivisceral resection
INTRODUCTION
Gastric cancer is the fifth most common cancer and is the third leading cause of cancer deaths worldwide[1].Its incidence is higher in Eastern Asia, including Japan, Korea, and China, than in Western countries.Although approximately 50% of the patients in Japan are diagnosed during the early stages of gastric cancer,several patients are diagnosed in the advanced stages[2]. For gastric cancer treatment, radical surgical resection with lymph node dissection is the established standard and complete surgical resection without residual disease (R0 resection) is the cornerstone. For tumors that invade adjacent organs, combined resection is necessary for achieving complete tumor clearance.
The pancreas is the organ most frequently invaded by gastric cancer[3-6]. When a tumor and/or lymphadenopathy invades the pancreatic head or infiltrates the duodenum, pancreaticoduodenectomy(PD) is the only possible treatment for achieving R0 resection. However, PD is a highly invasive procedure that cannot be performed on all patients. Since the first reported case of a patient who underwent PD for gastric cancer in 1978[7], all case series published[8-17]were retrospective and single-center studies and no prospective study has been done. Because of the limited number of patients and heterogeneous data of the studies, definite indications for PD have not been established. Here we reviewed the literature on PD for gastric cancer and our own experience to clarify short- and long-term outcomes and the role of PD in gastric cancer.
METHODS OF LITERATURE SEARCH
We conducted a literature search on PubMed using keywords “gastric cancer”, “pancreaticoduodenectomy”,and “multivisceral resection” considering articles published until November 2017. We excluded inaccessible abstracts or articles not written in English. In addition, we reviewed patients who underwent distal or total gastrectomy with PD at Shizuoka Cancer Center (Shizuoka, Japan) between September 2002 and December 2015. We collected patients’ characteristics and pathological and surgical findings from our database and individual patients’ electronic medical records. In addition, we statistically analyzed our data using R Statistics version 3.4.0 (R Foundation for Statistical Computing, Vienna, Austria). Furthermore, we calculated 5-year survival rates using the Kaplan-Meier method and compared them between the groups using the log-rank test. The statistical significance of data was defined as P < 0.05.
SHORT-TERM SURGICAL OUTCOMES
PD is a highly invasive procedure that requires high surgical skills. When Buchholtz et al.[7]first reported PD for gastric cancer in 1978, they concluded that this treatment should not be performed because of the unacceptable risk without an additional and greater degree of palliation or likelihood of cure; however, they did not discuss their reasons in detail. Several studies have demonstrated short-term surgical outcomes of PD, including intraoperative blood loss, operation time, morbidity, and mortality [Table 1][8-17]. The median amount of blood loss was reported to be > 1000 mL and the median operation time was as long as 7 h.
Although several studies have concluded that PD for gastric cancer is feasible in terms of safety, the incidence of postoperative complications ranged widely from 22% to 74%, probably because of discrepancies in the definitions of complication. No study defined the exact criteria for postoperative complications because many of these reports were published before the definitive criteria for postoperative complications,the Clavien–Dindo classification[18], were established. The mortality rate of PD was reported to be from 0%to 13%; however, the definition of the period of operative death differed among the studies; some defined mortality as death from any cause within 30 days after surgery, whereas the others did not mention the period. The study by Nunobe et al.[14], who defined mortality as death from any cause before discharge,reported the highest mortality of 13%.
Although Min et al.[16]reported the lowest complication rate of 22% among the reported rates of the previous studies, they also demonstrated one of the highest mortality rates, which was 11%. These results meant that half of the patients who suffered from postoperative complications died; this 50% mortality rate among patients who suffered postoperative morbidity seemed to be a bit high, which was possibly due to the variable definitions of all the complications. At the same time, Yonemura et al.[8]reported a 23% incidence of pancreatic fistula, but did not report the incidence of all complications.
Saka et al.[11]reported the highest complication rate of 74%, with pancreatic fistula being the most frequent in 44% of patients; all patients recovered with conservative management and none reported operation-related
death. Nunobe et al.[14]featured the largest number of patients, including 31 patients with gastric cancer who underwent PD. Although their center is one of the largest high-volume centers in Japan, with > 300 cases of gastrectomy performed during one year, the mortality rate of PD was as high as 13%. The most frequently observed complication was pancreatic leakage (13%), followed by intraabdominal abscess (6%) and colitis (6%);however, they did not report the rates of the other postoperative complications.
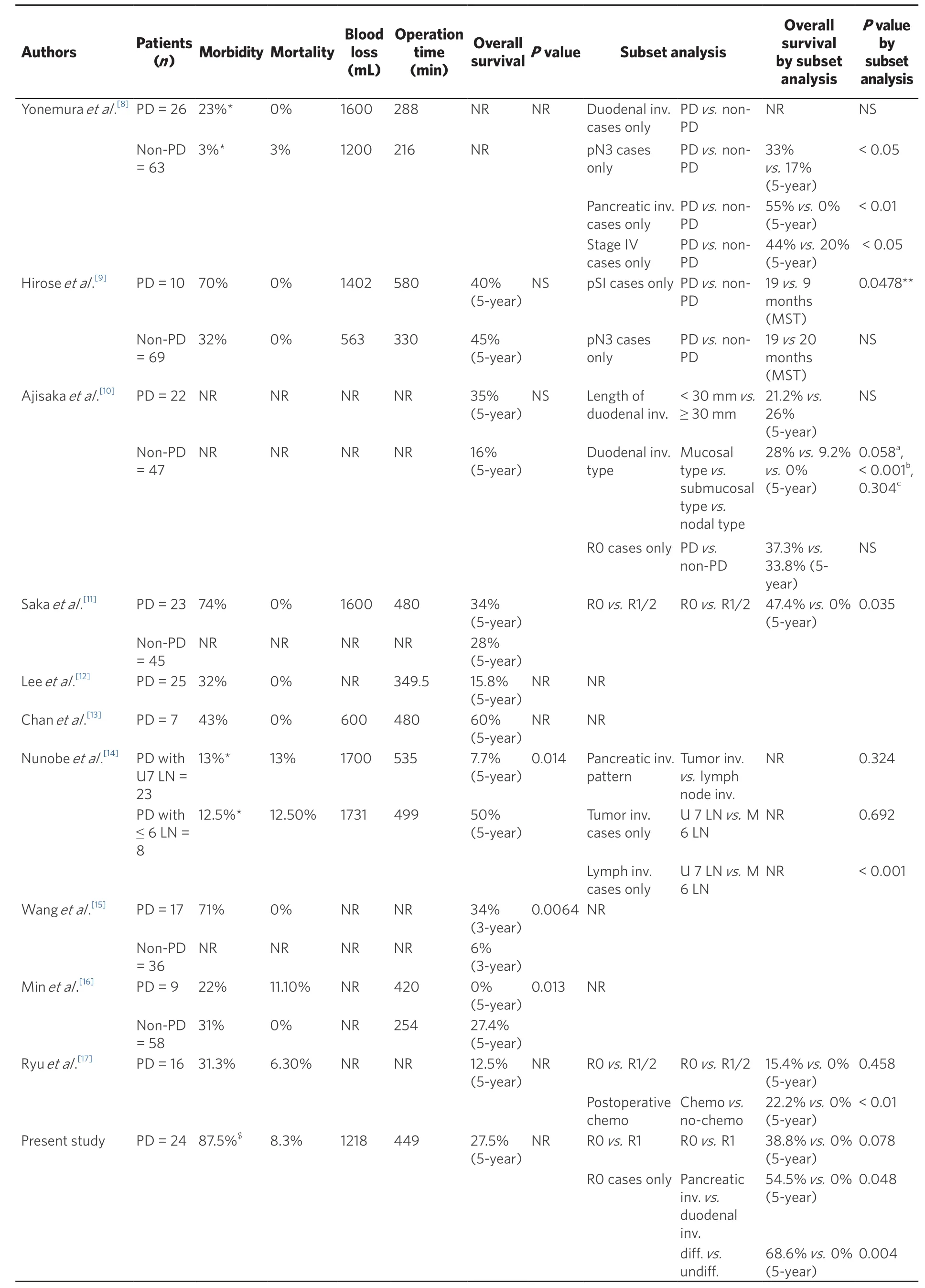
Table 1. Summary of studies on pancreaticoduodenectomy for gastric cancer
In our center, 24 gastric cancer patients underwent PD from 2002 to 2016; 19 patients underwent distal gastrectomy and 5 patients underwent total gastrectomy. Differentiated adenocarcinoma was noted in 15 patients and undifferentiated adenocarcinoma was noted in nine. The median blood loss was 1218 mL and the median operative time was 449 min. R0 resection was performed on 17 patients (70.8%) and R1 was performed on 7 patients (29.2%) owing to positive lavage cytology (CY1). There were no patients with tumorpositive resection margins. Four patients had a small number of peritoneal deposits adjacent to the stomach,which were completely resected during operation.
SURVIVAL BENEFITS OF PD FOR PATIENTS WITH GASTRIC CANCER
Several studies have evaluated the survival outcomes of patients undergoing PD for gastric cancer [Table 1].However, conflicting results were reported, mainly because of heterogeneous data and small sample size in each study.
According to studies that evaluated multivisceral resection for gastric cancer clinically invading the adjacent organs (T4b) or for pathologic T4b gastric cancer, R0 resection and lymph node status were the independent prognostic factors[3,4,6,19]; however, few studies have shown poor survival outcomes for patients who underwent combined resection of the pancreas or a tumor invading the pancreas[16,20]. It is important to note that, in these studies, the number of patients who underwent PD was few or unknown. Among these, the retrospective study on the prognostic factors in patients with T4b gastric cancer by Min et al.[16]reported the highest number of patients who underwent PD; there were a total of 243 T4b gastric cancer patients, including 67 patients that had tumor invasion to the pancreas. In that study, pancreatic invasion was identified as an independent unfavorable prognostic factor by multivariate analysis. Moreover, among the operation methods used for pancreatectomy in the pancreatic invasion group, the PD group (n = 9) had a significantly lower 5-year survival rate, compared with that of the other pancreatectomies group (n = 58) (0%vs. 27.4%, P = 0.013). Therefore, they did not recommend PD for T4b gastric cancer invading the pancreatic head.
In contrast, studies that compared PD and gastrectomy alone for T4b gastric cancer have found a therapeutic benefit of PD. Wang et al.[15]evaluated 53 patients with gastric cancer and pancreaticoduodenal region involvement and found that PD improved the 3-year survival rate, compared with that of palliative gastrectomy (34% vs. 5.6%, P = 0.0064). Hirose et al.[9]showed that among patients with gastric cancer invading the pancreatic head, the median survival time (MST) was better in the PD group than in the palliative gastrectomy group (19 months vs. 9 months, P = 0.0478). Yonemura et al.[8]also demonstrated that, compared with gastrectomy alone, PD with right hemicolectomy improved the 5-year survival rate of patients with pancreatic invasion (55% vs. 0%, P <0.01). Saka et al.[11]investigated 23 patients who underwent R0 resection with PD for gastric cancer macroscopically infiltrating the pancreatic head and showed that the 5-year survival rate was significantly better in patients without incurable factors, such as para-aortic lymph node metastasis, positive lavage cytology (CY1), and peritoneal dissemination, than in those with incurable factors (47.4% vs. 0%, P = 0.035). It should be noted that in that study, CY1 cases were treated as R0 resection,which is considered an R1 resection according to the 7th edition UICC TNM classification.
In patients undergoing PD, there are two patterns of invasion to the pancreatic head, including direct invasion of the primary tumor and invasion via metastatic lymph nodes. Although most studies have not investigated survival according to the pattern of pancreatic invasion, the study by Nunobe et al.[14]showed no difference in survival between these two patterns of invasion (P = 0.324). According to these studies,if R0 resection is considered possible, PD should be performed for patients with either primary tumor or metastatic lymph node invasion to the pancreatic head.
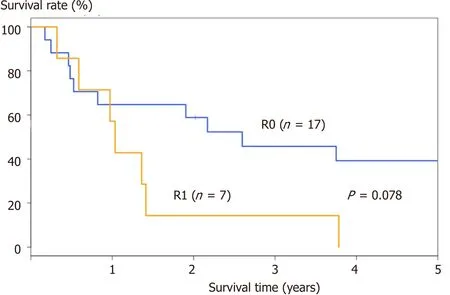
Figure 1. OS curve of 24 patients. There were 17 patients who underwent R0 resection and 7 patients who underwent R1 resection. The 5-year OS was better in patients who underwent R0 resection (38.8%) than in those who underwent R1 resection (0%), although the difference was not statistically significant (P = 0.078). OS: overall survival
Regarding the therapeutic benefit of PD for patients with tumors infiltrating the duodenum, no unified view has been obtained so far. Yonemura et al.[8]reported a survival benefit of PD over gastrectomy for T4b tumors, but not for tumors with duodenal invasion. Ajisaka et al.[10]evaluated 69 gastric cancer patients with duodenal invasion; among them, 22 patients underwent PD and 47 patients underwent gastrectomy alone.When a negative resection margin was achieved (i.e., R0 resection), the 5-year survival rates were almost the same (37.3% for PD vs. 33.8% for gastrectomy alone), although patients who underwent PD had more frequent adjacent tissue infiltration and significantly longer extent of duodenal invasion. They also found that survival was worse when duodenal invasion was from lymph node metastasis than from the primary tumor. Therefore, they concluded that curative PD for gastric cancer improved the survival of patients with duodenal invasion, except when duodenal invasion was of the nodal type.
Two studies have investigated the survival benefit of PD for patients with extensive lymph node metastases.Yonemura et al.[8]reported that PD improved the 5-year survival rate of patients with N3 lymph node metastasis (33% vs. 17%, P < 0.05). They used the first English edition of the Japanese Classification of Gastric Carcinoma[21], in which there were five N stages, with N3 referring to metastases in the hepatoduodenal, preand retropancreatic, and superior mesenteric nodes. In contrast, Hirose et al.[9]demonstrated that compared with palliative gastrectomy, PD had a tendency to not improve MST for patients with N3 lymph node metastases (19 months vs. 20 months, the differences were not significant). Therefore, it is difficult to reach a conclusion from these opposing results.
The other reported factors associated with better survival in patients who underwent PD included welldifferentiated histologic type[15], adjuvant intravenous chemotherapy[17], and metastatic lymph nodes less than seven[14]. Based on our experience of patients who underwent PD for gastric cancer, the 5-year overall survival (OS) rate was 27.5% and the MST was 17.2 months. The 5-year OS rate was 38.8% in patients who underwent R0 resection (n = 17) and 0% in those who underwent R1 resection (n = 7), although this difference was not statistically significant (P = 0.078), possibly due to the small sample size [Figure 1].The OS curves of patients who underwent R0 resection are shown in Figure 2. The 5-year survival rate was significantly higher in patients with predominantly pancreatic invasion than in those with duodenal invasion (n = 11, 54.5% vs. n = 6, 0%; P = 0.048) [Figure 2A]. Likewise, the 5-year OS rate was significantly higher in patients with differentiated tumors than in those with undifferentiated tumors (n = 10, 68.6% vs. n= 7, 0%; P = 0.004) [Figure 2B]. The univariate analysis of patients who underwent R0 resection is shown in Table 2.

Figure 2. OS curves of 17 patients who underwent R0 resection. The 5-year OS rate was significantly better (A) in patients with pancreatic invasion than in those with duodenal invasion (54.5% vs. 0%; P = 0.048) and (B) in patients with differentiated tumors than in those with undifferentiated tumors (68.6% vs. 0%; P = 0.004). OS: overall survival
Although conclusive results are difficult to obtain from previous studies, which had limited number of patients and heterogeneous data, it appeared that R0 resection is the minimum requirement for cure and that PD should not be performed in cases of CY1. In addition, tumors with duodenal invasion have little chance for cure; therefore, in cases with a positive resection margin, palliative surgery followed by chemotherapy or radiotherapy may be an alternative to PD. However, evidence proving this hypothesis is lacking.
DIAGNOSIS OF PANCREATIC INVASION BEFORE OR DURING OPERATION
Intraoperative diagnosis of tumor invasion to the pancreas has been reported to be difficult, with an accuracy rate ranging from 39% to 56.7%[5,6,22]. Adhesions secondary to desmoplastic reaction or tumor inflammation can be mistaken for local invasion[23], which could lead to patients being subjected to unnecessary multivisceral resection and result in increased morbidity and mortality without oncologicalbenefit. In our experience, pancreatic invasion from a tumor was suspected intraoperatively in 11 patients,but it was confirmed pathologically in only 8 patients (72.7%). In patients who were suspected to have pancreatic invasion of the tumor, the 5-year survival rate tended to be poor in patients with pathologically positive invasion than in those with pathologically negative invasion (66.7% vs. 12.5%, P = 0.150).

Table 2. Univariate analysis of the factors affecting the survival of patients who underwent R0 resection
Preoperative imaging, including multidetector computed tomography (MDCT)[24]and endoscopic ultrasound (EUS)[25], may facilitate identification of pathological invasion. However, the accuracy of MDCT and EUS for the assessment of pathological tumor depth was low and varied between 77.1%–88.9% and 65%–92.1%, respectively[26].
PREOPERATIVE CHEMOTHERAPY
Neoadjuvant chemotherapy had been described by only one study; Chan et al.[13]reviewed nine patients with locally advanced gastric cancer involving the duodenum and/or pancreatic head. All patients underwent diagnostic laparoscopy or exploratory laparotomy prior to the surgery to exclude peritoneal metastases. Two patients did not undergo PD because of disease progression with liver metastasis and patient refusal. Of the seven remaining patients who underwent PD, three did not receive neoadjuvant chemotherapy due to patient refusal and bleeding from the tumor. Although the study involved quite a small number of patients and its follow-up was short, it showed a significantly better survival in patients who received neoadjuvant chemotherapy than in those who did not receive neoadjuvant chemotherapy (log-rank test; P = 0.039).
In our experience, the benefit of neoadjuvant chemotherapy was difficult to assess because only 2 of the 24 patients received the treatment. Nevertheless, one of those patients survived longer than 5 years after surgery without recurrence and the other one remained alive at the end of this study period. Therefore, neoadjuvant chemotherapy seems to be a promising treatment to improve the survival of patients with gastric cancer who undergo PD.
Another therapeutic option for patients with initially incurable or unresectable gastric cancer is conversion therapy, which is defined as surgical resection intending to achieve radical cure following chemotherapy and/or radiotherapy[27]. Several studies have reported positive outcomes from this treatment[28-32], although none of them evaluated conversion therapy for patients who underwent PD. As we previously demonstrated,PD has a high morbidity and mortality, and its survival benefit appears to be limited. Therefore, neoadjuvant chemotherapy and conversion therapy should be considered as an alternative treatment strategy for patients requiring PD for curative resection.
CONCLUSIONS
Although there is currently no solid evidence that PD may be recommended for advanced gastric cancer with pancreatic invasion when R0 resection is possible, but the high morbidity and mortality should be considered. In addition, multidisciplinary treatment, such as neoadjuvant chemotherapy, is anticipated to improve survival. Nevertheless, a large-scale multicenter cohort study is required to evaluate this highly invasive procedure.
DECLARATIONS
Authors’ contributions
Designed the study, reviewed the literature, and wrote the manuscript: Makuuchi R
Contributed to writing the manuscript, drafting, critical revision, editing, and final approval of the final version: Terashima M
Contributed to critical reversion of the manuscript and final approval of the final version: Irino T, Tanizawa Y,Bando E, Kawamura T
Availability of data and materials
Not applicable.
Financial support and sponsorship
This study was supported in part by a scientific research grant for multi-institutional trials to establish a new standard treatment for solid tumors in adults from the National Cancer Center Research and Development Fund (29-A-3).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2018.
 Journal of Cancer Metastasis and Treatment2018年6期
Journal of Cancer Metastasis and Treatment2018年6期
- Journal of Cancer Metastasis and Treatment的其它文章
- Cancer, circulating tumor cells, and metastasis:could protein-derived peptide fragments impede brain metastases?
- Immunotherapy in the treatment of colorectal cancer: a new kid on the block
- Bioconjugate structures vs. composite nanoparticulate carriers: the battle for the future of smart, effective and safe cancer management
- Pancreatic cancer: treatment approaches and trends
- Update on targeted therapy and immune therapy for gastric cancer, 2018
