A novel chronic nerve compression model in the rat
Zhen-Yu Liu, Zhen-Bing Chen, Jiang-Hai Chen
Department of Hand Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei Province, China
Abstract Current animal models of chronic peripheral nerve compression are mainly silicone tube models. However, the cross section of the rat sciatic nerve is not a perfect circle, and there are differences in the diameter of the sciatic nerve due to individual differences. The use of a silicone tube with a uniform internal diameter may not provide a reliable and consistent model. We have established a chronic sciatic nerve compression model that can induce demyelination of the sciatic nerve and lead to atrophy of skeletal muscle. In 3‐week‐old pups and adult rats, the sciatic nerve of the right hind limb was exposed, and a piece of surgical latex glove was gently placed under the nerve.N‐butyl‐cyanoacrylate was then placed over the nerve, and after it had set, another piece of glove latex was placed on top of the target area and allowed to adhere to the first piece to form a sandwich‐like complex. Thus, a chronic sciatic nerve compression model was produced.Control pups with latex or N‐butyl‐cyanoacrylate were also prepared. Functional changes to nerves were assessed using the hot plate test and electromyography. Immuno fluorescence and electron microscopy analyses of the nerves were performed to quantify the degree of neuropathological change. Masson staining was conducted to assess the degree of fibrosis in the gastrocnemius and intrinsic paw muscles.The pup group rats subjected to nerve compression displayed thermal hypoesthesia and a gradual decrease in nerve conduction velocity at 2 weeks after surgery. Neuropathological studies demonstrated that the model caused nerve demyelination and axonal irregularities and triggered collagen deposition in the epineurium and perineurium of the affected nerve at 8 weeks after surgery. The degree of fibrosis in the gastrocnemius and intrinsic paw muscles was signi ficantly increased at 20 weeks after surgery. In conclusion, our novel model can reproduce the functional and histological changes of chronic nerve compression injury that occurs in humans and it will be a useful new tool for investigating the mechanisms underlying chronic nerve compression.
Key Words: nerve regeneration; chronic nerve compression; carpal tunnel syndrome; nerve conduction velocity; N-butyl-cyanoacrylate;hypoesthesia; demyelination; remyelination; intrinsic muscles; collagen deposition; axonal irregularity; neural regeneration
Introduction
Chronic nerve compression (CNC) injuries, such as carpal tunnel syndrome, cubital tunnel syndrome, and tarsal tun‐nel syndrome, are very common (Levine et al., 1993; Papa‐nicolaou et al., 2001; Flanigan and DiGiovanni, 2011). Many complications, such as neuropathic pain, numbness, skel‐etal muscle atrophy, and motor disability, can result from CNC injury. Much of the current understanding of CNC is derived from studies involving animal models, especially animal models in which the sciatic nerve is banded with Silastic tubing. Mackinnon et al. (1984) described a rodent CNC model in which Silastic tubes with internal diameters ranging from 0.6 mm to 1.5 mm were used to band the sci‐atic nerve of adult Sprague‐Dawley rats. This induced con‐sistent histological changes in the affected nerve over several months. However, tubes with an internal diameter ranging from 0.9 mm to 1.1 mm have a smaller diameter than the nerve (whose mean diameter is 1.3 mm) and, thus, can in‐duce actual physical compression and nerve injury. Further‐more, tubes with an internal diameter of 1.5 mm are likely to induce mechanical stimulation or foreign body reactions in the nerve. O’Brien et al. (1987) banded the sciatic nerve with a tube whose internal diameter was similar to that of the nerve (1.3 mm) and reconstituted the tube with 3 6‐0 Pro‐lene sutures. They noted apparent histological changes and slowing of nerve conduction velocity (NCV) at 5 months after surgery. They also noted that long periods of compres‐sion caused connective tissue fibrosis and thickening, as well as fibroblast proliferation‐induced epineural scarring, result‐ing in nerve fiber pathology. Decreases in axonal numbers and function were also noted in the late post‐injury period.Thus, the histopathological characteristics of chronic com‐pression injury differed from those characterizing Wallerian degeneration, which is precipitated by axonal degeneration.The placement of the sutures as well as the knotting of the sutures may have changed the shape of the tube. Gupta and Steward (2003) recently introduced a new CNC model, a modified version of O’Brien’s model, in which a Silastic tube with an internal diameter of 1.3 mm was atraumatical‐ly placed on the sciatic nerve (approximately 1−1.1 mm in diameter) without sutures. This model eventually induced chronic nerve injury characterized by local demyelination and remyelination, and phenotypic switching of the neu‐rons within the dorsal root ganglia (Gupta et al., 2004, 2012;Chao et al., 2008). The model mimics the constrained spaces through which normal human peripheral nerves must pass but does not duplicate the increased pressure found at sites of anatomical narrowing.
The tubes used for existing models of compression neu‐ropathy are round and uniform in size. However, nerves are oblatein vivoand may have different thicknesses in different individuals. Thus, the above models do not seem to resemble human nerve compression. Moreover, in the clinic, entrapment neuropathy causes sensory dysfunction,muscle weakness and wasting in severe CNC patients. The above animal models have been used to investigate various post‐CNC injury‐related changes, such as hypoalgesia, local nerve demyelination or cutaneous neurovascular changes(Gupta et al., 2006; Lin et al., 2012; Pelletier et al., 2012).However, none of these studies reported muscle histopatho‐logical changes over observational periods of 8 or even 12 months (Mackinnon et al., 1985; O’Brien et al., 1987; Gupta and Steward, 2003). Both the carpal tunnel and the cubital tunnel are well known to be fibro‐osseous structures formed by ligaments and bones. These conditions, in which nerves become entrapped by bony and ligamentous structures,cannot be reproduced by the current models. Most CNC injuries encountered in the clinic are caused by gradually worsening compression. Herein, we propose a new technique for inducing CNC. We used pieces of latex glove and N‐bu‐tyl‐cyanoacrylate (NBCA), a tissue adhesive used for nerve repair, to enclose the sciatic nerves of rats (Choi et al., 2004;Elgazzar et al., 2007). We assessed the histological and func‐tional changes induced by the new CNC injury model and veri fied its validity.
Materials and Methods
Animals
Male Sprague‐Dawley rat pups aged 3 weeks and weighing 70—100 g (n= 70) and adult male Sprague‐Dawley rats aged 8 weeks and weighing 250—300 g (n= 20) were used. These speci fic‐pathogen‐free rats were obtained from the Labora‐tory Animal Center, Huazhong University of Science and Technology in Wuhan, China (animal license No. SYXK (E)2016‐0057). All surgical and animal care procedures were approved by the Institutional Animal Care and Use Com‐mittee at Tongji Medical College, Huazhong University of Science and Technology, China (approval No. 410).
Surgical procedures
All surgical procedures were performed under aseptic con‐ditions. A surgical latex glove (Ansell, Shanghai, China) was cut into small 5 mm × 5 mm pieces. These were immersed overnight in 75% ethanol in a sterilized Petri dish and then washed for 5 minutes three times in sterile water to mini‐mize in flammation. Rats were anesthetized by an intraperi‐toneal injection of 1% sodium pentobarbital (40 mg/kg). The NBCA compression model was established in 40 pup and 20 adult rats. The sciatic nerve of the right hind limb was exposed through a gluteal‐splitting approach, and a piece of latex glove was gently placed under the nerve to ensure that the nerve was not stretched or twisted. Before applying NBCA, the nerve and latex were soaked with fetal bovine serum (Gibco, New York, NY, USA). Using a dissecting mi‐croscope (Zeiss, Brunswick, Germany), NBCA (Histoacryl,B.Braun Surgical, Melsungen, Germany) was applied to the nerve in a dropwise manner. All the NBCA was contained by the latex glove material. Approximately 2.5 μL of NBCA was used for each pup, and approximately 5 μL of NBCA was used for each adult. After the NBCA had coagulated,another piece of surgical glove latex was placed on top of the target area and allowed to adhere to the first piece to form a sandwich‐like complex (Figure 1A, B). To exclude a direct in fluence of the latex and NBCA on the nerve, two control groups were prepared. In 15 pups, a piece of latex glove was coiled into a tube with an internal diameter of approximate‐ly 2 mm so that the nerve would not be compressed during the experiment. The right sciatic nerve was banded with the tube, and these rats served as the latex control group (CON1)(Figure 1C). In another 15 pups, the right sciatic nerve was banded with a latex glove tube (2 mm internal diameter)whose inner wall was coated with NBCA. These rats served as the NBCA control group (CON2) (Figure 1D). The con‐tralateral sciatic nerve of each rat was exposed but was not treated and was replaced within the host muscle bed. This nerve served as a sham‐operated control. Each wound was closed with 3‐0 polyester sutures upon completion of the procedure. Animals were killed for histological studies by anesthetic overdose (100 mg/kg) of sodium pentobarbital.Sciatic nerves and muscles were harvested from the surgical and nonsurgical sides at different time points after surgery.

Figure 1 Establishment of the rat chronic nerve compression model.
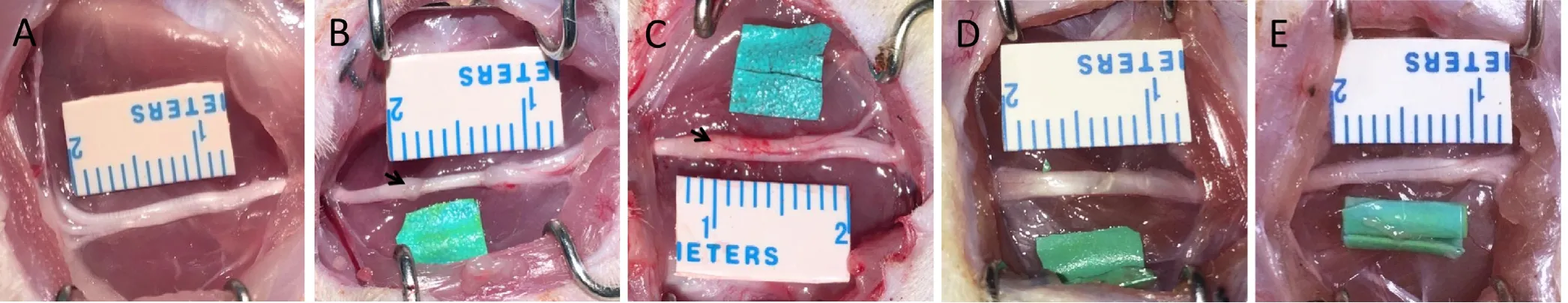
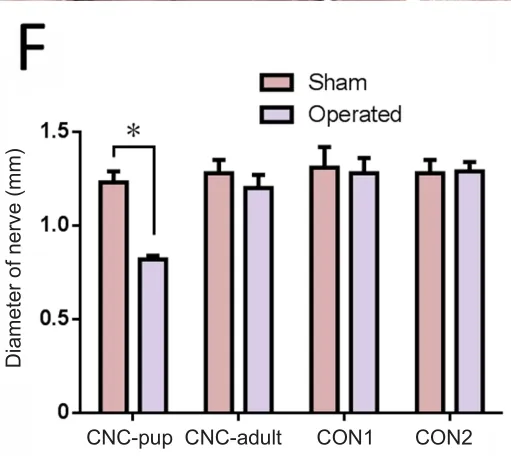
Figure 2 Sciatic nerve after removal of the bundles at 8 weeks post-operation in the novel chronic nerve compression model.
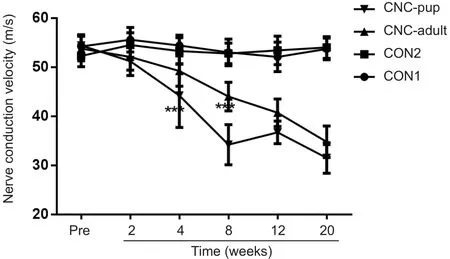
Figure 3 Change of nerve conduction velocity in the novel chronic nerve compression (CNC) model.
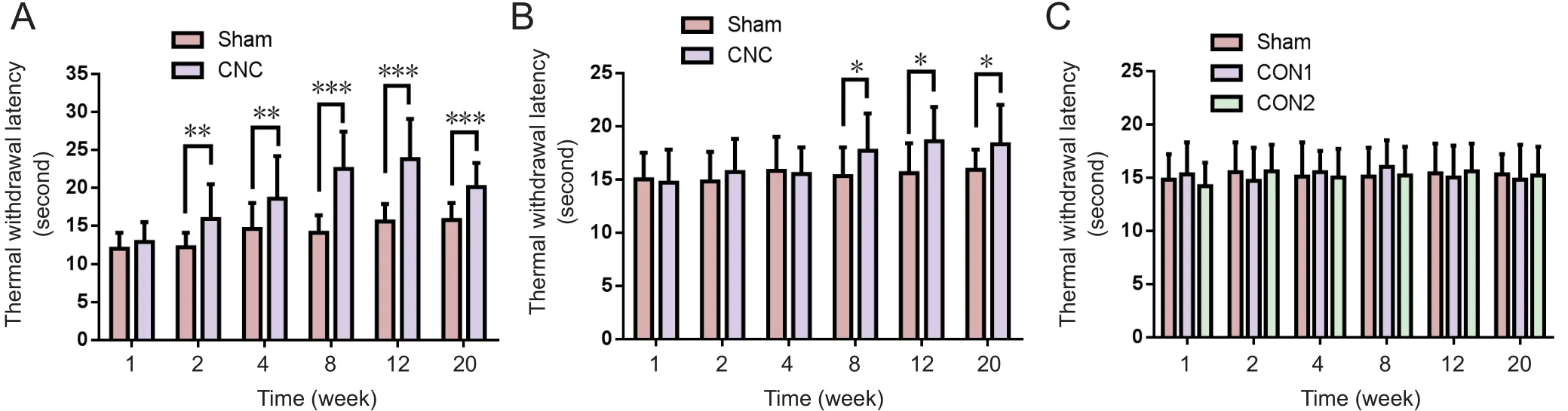
Figure 4 Change in hindpaw withdrawal latency in the novel CNC model in the hotplate test.
Electrophysiological studies
The NCV of the sciatic nerve was measured using an NID‐092 electronic medical instrument (Haishen, Shanghai,China) at pre‐operation, and at 2, 4, 8, 12 and 20 weeks post‐operation. The stimulating electrode was placed on the proximal and distal sides of the compression area of the sciatic nerve (stimulation duration: 0.1 ms; frequency: 1 Hz;stimulus intensity: 5 mA), and the recording electrode was placed on the dorsum of the foot. Latency was evaluated us‐ing data obtained directly from the sciatic nerves.
Hotplate test
Sensory studies were conducted at 1, 2, 4, 8, 12 and 20 weeks post‐operation. The surface of the hotplate was heated to a maximum temperature of 52.5−53.5°C, and then the rat was placed on the testing apparatus (Yuyan Instruments,Shanghai, China) and a timer was started. The latency to respond to the thermal stimulus applied to the hindpaw was determined by recording the time that elapsed between the point at which the stimulus was applied and that at which the rat licked its hindpaw. The rat was immediately removed from the apparatus once the licking response was observed.If the rat did not respond to the stimulus within 30 seconds,the test was terminated to prevent burns, and the rat was re‐moved from the hotplate (Woolfe, 1944).
Immuno fluorescence
At different time points post‐surgery (4, 8, 12, and 20 weeks), NBCA‐treated nerves from each group were har‐vested for immunofluorescence. Frozen 10 μm transverse and longitudinal sections of the sciatic nerve were mounted on slides, dried and then immersed in phosphate‐buffered saline (PBS) containing 0.3% Triton X‐100 for 5 minutes.Sections were then blocked in PBS containing 10% donkey serum for 1 hour at 37°C. Transverse sections were subse‐quently incubated with mouse monoclonal anti‐neurofila‐ment (NF) 200 antibody (1:200 dilution; Abcam) overnight at 4°C. Longitudinal sections were incubated with rabbit anti‐collagen I monoclonal antibody (1:200; Abcam) over‐night at 4°C. The sections were then washed three times in PBS before being incubated with the appropriate secondary antibody (donkey anti‐mouse Alexa‐Fluor 488 or donkey anti‐rabbit Alexa‐Fluor 594) for 1 hour at 37°C. Nuclei were counterstained with 4′6‐diamidino‐2‐phenylindole (DAPI)for 5 minutes (1:500; Sigma). The slides were then washed for 5 minutes three times in PBS (pH 7.4) and coverslips mounted with Fluoromount (Abcam). The primary antibody was omitted in the negative control samples. The sections
were visualized using a confocal laser scanning microscope(TCS‐SP5; Leica, Mannheim, Germany). The percentage of the transverse nerve section that stained positive for NF and the collagen volume fraction in the sciatic nerve (excluding the epineurium) were evaluated using Image‐Pro Plus 6.0 image analysis software (Media Cybernetics, Rockville, MD,USA). To prevent observer bias, the slides were randomly numbered. Moreover, the slides were interpreted in a blind‐ed manner, and each slide was assessed by three observers.
Transmission electron microscopy
To evaluate myelin sheath changes, nerve samples were eval‐uated via standard transmission electron microscopy anal‐ysis at 8, 12, and 20 weeks post‐operation. Nerve samples were embedded for transverse section analysis. The sections were assessed qualitatively with an Eclipse E800 upright compound microscope (Nikon, Tokyo, Japan) equipped with a Spot camera using an oil immersion Plan Fluor 3100/1.3 N.A. objective lens. Ultrathin sections were imaged using a JEOL JEM 1230 transmission electron microscope.Myelin thickness was measured using ImageJ software(National Institutes of Health, USA). Only myelin regions exhibiting no signs of non‐compaction and featuring no fix‐ation artifacts were included in the analysis.
Masson trichrome staining
Tissue samples from both sides of the gastrocnemius muscle and the intrinsic muscles of the hindpaw were harvested at 20 weeks post‐surgery. The samples were embedded in par‐affin and 5‐μm‐thick sections prepared. Five slides of each sample were randomly selected for Masson trichrome stain‐ing, which was performed according to the manufacturer’s protocol (Masson’s Trichrome Staining Kit; Boster, Wuhan,China). Sections were evaluated at 100× and 400× magni fi‐cation to assess collagen fiber size, shape, and distribution.Five fields in each slide were randomly chosen and photo‐graphed at 400× magni fication and then analyzed to deter‐mine the average collagen volume fraction of muscle fibers using Image‐Pro Plus 6.0 image analysis software (Media Cybernetics, Rockville, MD, USA) (collagen volume fraction% = collagen area/total cross‐sectional area × 100%).
Statistical analysis
The data are presented as the mean ± SD. Statistical signi fi‐cance was analyzed by repeated measures one‐way analysis of variance. Dunnett’s post hoc test was used for multiple comparisons. Statistical analyses were performed using SPSS 16.0 software (SPSS, Chicago, IL, USA). A P‐value < 0.05 was considered statistically signi ficant.
Results
Appearance of the sciatic nerve in the novel CNC model
The sciatic nerve was not adhered to the NBCA complex at 3 days post‐operation. The nerve could be easily separated from the complex when the latex pieces were longitudinally cut along the nerve. The diameter of the compressed nerve was signi ficantly smaller than that of the contralateral nerve(P < 0.05) and swelling was present distal and proximal to the site treated with NBCA in the NBCA‐treated pup group(Figure 2B). The width of the proximal and distal nerve was measured from a photograph using ImageJ analysis software before the nerve was harvested. The average width of the nerve at the compression site was approximately 0.8 mm, while the average width of the contralateral nerve was approximately 1.2 mm. The adult NBCA rats were found to have slight swelling in the segments proximal and distal to the site of nerve compression. The width of the nerve at the compression site was similar to that of the contralateral nerve (Figure 2C). No signi ficant difference in nerve width was evident between the two control groups.
NCV decreased in the novel CNC model
Electrophysiological analysis of the NBCA‐treated pups (n= 15) showed that the NCV of the compressed nerve was relatively stable until 4 weeks post‐operation (P< 0.001)compared with pre‐operation values and those of the con‐trol group. The NCV then decreased gradually and reached 59% of the pre‐operation values at 20 weeks post‐operation.In NBCA‐treated adults (n= 5), there was a considerable decrease in the NCV until 8 weeks post‐operation. The NCV then gradually decreased for 20 weeks. No significant dif‐ference in the NCV was observed between the two control groups (n= 3; Figure 3).
Thermal withdrawal latency increased in the novel CNC model
The hotplate test was performed to evaluate the sensitivity of the CNC‐injured limbs. In pups, NBCA‐treated limbs (n= 15) displayed similar paw withdrawal latencies to those of the contralateral limbs at 1 week after CNC injury. How‐ever, the hindpaw withdrawal latencies of the compressed limbs were significantly longer than those of the contralat‐eral limbs at 2 weeks post‐surgery (P< 0.01), indicating that the NBCA‐treated pups had developed hypoesthesia of the hindpaw at this time point. Similar results were noted at 8 to 20 weeks post‐operation. We noted increases in the thermal threshold latency in the contralateral paw between 2 and 20 weeks post‐surgery, which we attributed to age‐related chang‐es (Hess et al., 1981; Ririe et al., 2003). In the NBCA‐treated adult group (n= 5), the hindpaw withdrawal latencies of the compressed limbs did not change until 8 weeks post‐surgery.The latency increased signi ficantly (P< 0.05) compared with that in the contralateral paw at 8, 12, and 20 weeks post‐sur‐gery (Figure 4B). No signi ficant changes were found in the two control groups (n= 3; Figure 4C).
Collagen deposition in sciatic nerves of the novel CNC model
At 8 weeks post‐compression, the entrapped and contralater‐al sciatic nerves of each group were analyzed to assess the de‐gree of collagen deposition. In the NBCA‐treated pup group(n= 5), immunofluorescence revealed that collagen I was present in both the margin and the center of the compressed nerve. The collagen volume fractions of the compressed sciat‐ic nerves were signi ficantly higher than those of the contralat‐eral nerves (P< 0.001). In addition, collagen I was highly ex‐pressed in areas proximal to the compressed region. Intense linear or spotted collagen staining indicated that the peri‐neurium in the corresponding region had thickened. Intense collagen staining was present mainly in the epineurium of the compressed nerve. Collagen staining in the intact nerve was relatively weak (Figure 5). We also noted collagen deposition in the epineurium and perineurium of the compressed nerve in the NBCA‐treated adults (n= 5). The collagen volume frac‐tions were higher than those of the contralateral nerves (P<0.001) but were lower than those of the pup group (P< 0.001).We noted collagen deposition in the epineurium but not in the perineurium in the two control groups (n= 3; Figure 5).The collagen volume fractions were 0.640 ± 0.028% in control group 1 and 0.553 ± 0.036% in control group 2 (P> 0.05).
To investigate axonal changes induced by CNC injury,transverse sections of the compressed sciatic nerve were im‐munostained for NF. Immuno fluorescence showed that the NF expression pattern in the compressed nerve was more ir‐regular than in the contralateral nerve of the NBCA‐treated pups at different time points (n= 20; Figure 6A), indicating that the axonal structure of the compressed nerve was dis‐rupted. Dense staining of the outermost nuclei suggested that the cells constituting the epineurium had proliferated,resulting in epineural thickening. The percentage of the total transverse sectional area that was stained positive for NF in the compressed nerve was significantly lower than that in the intact nerve beginning at 8 weeks post‐operation (P<0.05; Figures 6B–E). Immuno fluorescence showed that the axonal structure of the compressed nerve was slightly dis‐ordered in the NBCA‐treated adults (n= 20) beginning at 8 weeks post‐operation. However, the degree to which the ax‐onal structure was disrupted did not increase until 20 weeks post‐surgery (Figures 6F–I).
Myelin thickness of sciatic nerves in the novel CNC model
Electron microscopy evaluation showed compression induced changes in the myelin sheath. We noted separa‐tion of the myelin lamellae, swelling, and vacuolization in NBCA‐treated pups, and the average thickness of the sheath in the affected nerve decreased to a value that was 63% of that in the contralateral nerve (P< 0.001) at 8 weeks after surgery. This disorganization continued up to 12 weeks after compression because the sheath was thinner at 12 weeks post‐compression than at 8 weeks post‐compression. How‐ever, the thickness of the myelin sheath was increased at 20 weeks after compression compared with 12 weeks after com‐pression (P< 0.001) and was not comparable with that of the intact sheath (Figures 7A–F). The average myelin sheath thickness in the compressed nerve in the NBCA‐treated adults was 68% of that in the contralateral nerve at 8 weeks post‐compression (P< 0.001) and decreased to a value that was 59% of that in the contralateral nerve at 20 weeks post‐compression (Figures 7G–L).
Collagen fibers in the gastrocnemius muscle and theintrinsic muscles of the hindpaw in the novel CNC model
Muscle morphology in the NBCA‐treated pups (n= 5) was observed by Masson staining. The collagen volume fractions of the CNC gastrocnemius muscle were signi ficantly higher than those of the contralateral muscles (P< 0.001). Further‐more, the collagen volume fractions of the intrinsic muscles of the hindpaw were significantly higher than those of the contralateral muscles (Figure 8). No significant increases in collagen deposition in the gastrocnemius muscle and the intrinsic muscles of the hindpaw were detected at 20 weeks post‐operation in the NBCA‐treated adults (n= 5; Figure 8)or in the two control groups (data not shown).
These results demonstrated that the novel CNC model induced symptoms similar to those associated with CNC in humans.
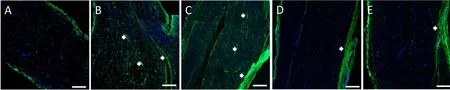
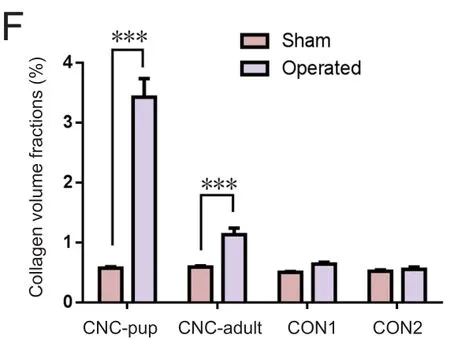
Figure 5 Collagen deposition in the sciatic nerve of the novel CNC model detected by immuno fluorescence.
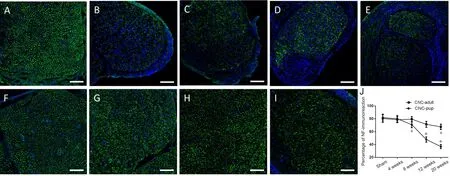
Figure 6 Immuno fluorescence staining of NF in sciatic nerves of the novel CNC model.
Discussion
CNC injury is a common clinical problem that often results in signi ficant morbidity, including loss of motor and sensory functions. CNC injury may cause amyotrophy, paresthesia,and paralysis (Thonnard et al., 1999; Tamburin et al., 2008).The pathological changes and mechanisms underlying these conditions remain to be elucidated. To date, published CNC animal models only partially reproduce the pathological characteristics of human CNC injury, which often occurs in response to soft tissue compression by semi‐solid surfaces.The present study established a new CNC rat model. We found that applying NBCA to the sciatic nerve and using a piece of latex glove to band the nerve led to sensory dys‐function and muscle changes. In this model, the NBCA and latex glove form a semi‐solid and semi‐soft structure similar to that of the human nerve canal. Previous studies have sug‐gested that NBCA is nontoxic to nerve tissues and can be used as an alternative treatment in peripheral nerve repair(Landegren et al., 2006; Elgazzar et al., 2007; Papalia et al.,2016). Choi et al. (2004) noted good axonal regeneration and a mild foreign body reaction around cyanoacrylates when a microdrop of butyl‐2‐cyanoacrylate was applied to the sur‐face of the overlapping epineurium. These results are similar to those of the present study. The results for control group 2 indicated that applying NBCA to the surface of the epineu‐rium did not lead to progressive fibrous tissue thickening or local nerve compression. The results for control group 1 indicated that latex pieces did not change nerve histology or local nerve compression. Moreover, this CNC model was only marginally effective in the NBCA‐treated adult group.Therefore, the sciatic nerve compression induced by this model is dependent on the growth forces on the nerve as the rat matures as opposed to a foreign body reaction affecting the nerve. In the 3‐week‐old pups, the sciatic nerve was lim‐ited to a fixed space, but as the nerve got thicker with age it gradually became compressed.

Figure 7 Electron microscopy analysis of sciatic nerves in the novel CNC model.

Figure 8 Light microscopy analysis of the gastrocnemius and intrinsic muscles.
Similar to other CNC models, this model showed decreas‐es in NCV and average myelin sheath thickness. Electro‐physiological changes occurred at an earlier time point (4 weeks post‐operation) in pups in this model than in other models (12 weeks post‐operation) (O’Brien et al., 1987; Gup‐ta and Steward, 2003). However, the absence of a change in NCV at 2 weeks post‐operation indicated that the compres‐sion injury induced in the new model was chronic rather than acute. The hotplate test showed that the withdrawal latency increased in CNC rats compared with control rats, indicating that neuropathic hypoesthesia developed after CNC injury.Hypoalgesia has also been observed in other CNC models(Gupta et al., 2006; Barac et al., 2013) in contrast to the hy‐persensitivity that is observed with models of neuropathic pain, such as chronic constriction injury (Bennett and Xie,1988), partial sciatic nerve ligation (Seltzer et al., 1990) and spared nerve injury (Richner et al., 2011) models. In the clin‐ic, patients with nerve compression often complain of allo‐dynia and paresthesia. The mechanism of neuropathic pain is not clear. However, according to our clinical experience, pain is not a classical symptom in CNC patients, although numb‐ness and electric shock‐like pain are so intense that they are described as painful. In addition, quantitative sensory testing has revealed thermal hypoesthesia in carpal tunnel syndrome patients (Tamburin et al., 2011; Schmid et al., 2014). In our new model, an initial increase in the ipsilateral thermal withdrawal latency appeared (2 weeks after surgery). Similar findings have been found in humans who have developed entrapment neuropathies. Paresthesia often develops earlier than electrophysiological abnormalities.
In contrast to other studies (Mackinnon and Dellon, 1986;Dellon and Mackinnon, 1991; Aanonsen et al., 1992), the entrapped nerve was thinner than the intact nerve in this study. The rats in the pup group displayed signs of muscle atrophy. Patients who suffer from compressive neuropa‐thies often present with weakness or muscle atrophy in the late stage of the disease (Nora et al., 2004; Mallette et al.,2007). Moreover, collagen deposition was detected in the gastrocnemius muscle and intrinsic muscles of the hindpaw in these rats. The collagen volume fraction of the ipsilateral muscles was higher than that of the contralateral muscles.These results indicate that the new model can induce mus‐cle atrophy. Muscle atrophy has not been noted in previous CNC models. Decreases in axon numbers and axonal de‐generation have been detected in previous studies; howev‐er, no changes in muscle fiber type or muscle morphology have been observed in existing models employing Silastic tube‐induced CNC injury (Mackinnon et al., 1984; O’Brien et al., 1987; Gupta et al., 2004, 2006). Our new model is a signi ficant improvement over previous models, as it enables the study of nerve entrapment‐induced muscle atrophy. The above phenomena observed in our model are similar to the clinical symptoms and pathological characteristics of human diseases caused by chronic peripheral nerve compression,such as carpal tunnel syndrome (Han et al., 2011; Floranda and Jacobs, 2013; Kleopa, 2015).
The adult rat group was included in the study not only to demonstrate that the NBCA and latex glove procedure would not lead to immediate compression but also to mimic popu‐lar Silastic tube CNC models (O’Brien et al., 1987; Gupta and Steward, 2003). The NBCA model produced similar results in adult rats to those of other models. Speci fically, the model induced decreases in NCV, local demyelination, and hypoes‐thesia in adult rats. The NBCA envelops the nerve very well and is not affected by differences in nerve diameter among rats. We believe that our new model is an improvement over existing models. Moreover, the Silastic tube model, including the model in which the Silastic tube is reinforced with sutures(O’Brien et al., 1987) and that in which only the tube is used(Gupta and Steward, 2003), requires an operation that may be dangerous to the nerve because the Silastic tube must be cut longitudinally and then placed around the nerve. This lon‐gitudinal slit created in the tube could cause the nerve to be crushed when the tube is placed around it.
Aguayo et al. (1971) studied chronic progressive nerve inju‐ry in young rabbits. These authors placed a large Silastic tube around the sciatic nerve of 3—5‐week‐old rabbits and placed a suture in the tube. These authors used young animals; thus, the nerve had some space in which to grow and gradually adapt to the compression, which does not occur in human diseases.Moreover, the Silastic tube is round and not a semi‐solid and semi‐soft structure and, therefore, does not accurately resem‐ble the human nerve canal. Although carpal tunnel syndrome in children is uncommon and most childhood carpal tunnel syndrome is a secondary phenomenon to conditions such as mucopolysaccharidosis, obesity and Cavanagh syndrome (Van Meir and De Smet, 2003; Potulska‐Chromik et al., 2014), these diseases increase pressure in the carpal tunnel and eventual‐ly cause median nerve compression. These patients or their parents reported improvement either in symptoms or hand function after carpal tunnel release (Yuen et al., 2007). There‐fore, the young rats used in this study provide a good nerve compression model of carpal tunnel syndrome in children. In addition, the sciatic nerve was not adhered to the NBCA com‐plex at 3 days post‐operation.
In our preliminary experiments, approximately 25% of the NBCA‐treated pups were discarded. Their gait changed with‐in 1—2 days after surgery. We found that dry latex deformed when NBCA was deposited directly onto it or onto latex wet‐ted with PBS. However, the latex remained flat when NBCA was deposited onto latex pieces previously soaked in fetal bovine serum. We suspect that the above deformation of the latex results in acute sciatic nerve compression. We, there‐fore, used the latter method to prevent acute nerve compres‐sion. In addition, the fetal bovine serum on the surface of the sciatic nerve prevents long‐term adhesion of the NBCA and contributes to the early separation of the nerve and NBCA.
The myelin thickness was recovered by 20 weeks post‐op‐eration. Demyelination and remyelination co‐exist during CNC. At early time points when the nerve is undergoing fast growth, demyelination is active. As the nerve stops growing and remyelination proceeds, the nerve may repair the inju‐ry. Although the novel CNC model might not induce con‐tinuous deterioration, it causes chronic muscle dysfunction.However, the compression force does not increase contin‐uously. When the nerves stop growing, the force no longer increases. What occurs when the nerve stops growing and whether dysfunction improves in the late stages of the novel CNC model requires further investigation.
In summary, we have established a novel CNC injury model that simulated both neuropathy and dominant mus‐cle injury. This model may be useful as a new tool for inves‐tigating the mechanisms underlying CNC. In addition, the new CNC injury model reproduces the pathological changes of CNC injury in humans.
Author contributions:ZBC and JHC conceived and designed the study and reviewed and edited the paper. ZYL performed the experiments and wrote the paper. All authors contributed to and have approved the final version of the paper.
Conflicts of interest:The authors declare that they have no con flicts of interest or financial disclosures to report.
Financial support:This study was supported by the National Natural Science Foundation of China, No. 81471270. The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement:The study was approved by the Institutional Animal Care and Use Committee at Tongji Medical College,Huazhong University of Science and Technology, China (approval No. 410).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Aldo Calliari, Universidad de la República de Uruguay, Uruguay.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- In Memoriam: Ray Grill (1966–2018)
- Reorganization of injured anterior cingulums in a hemorrhagic stroke patient
- Analgesic effect of AG490, a Janus kinase inhibitor, on oxaliplatin-induced acute neuropathic pain
- Three-dimensional visualization of the functional fascicular groups of a long-segment peripheral nerve
- Novel conductive polypyrrole/silk fibroin scaffold for neural tissue repair
- Local inhibition of matrix metalloproteinases reduced M2 macrophage activity and impeded recovery in spinal cord transected rats after treatment with fibroblast growth factor-1 and nerve grafts