Analgesic effect of AG490, a Janus kinase inhibitor, on oxaliplatin-induced acute neuropathic pain
Shuang-Feng Li , Bi-Shan Ouyang, Xin Zhao, Ya-Ping Wang
Abstract Neuropathic pain often occurs during chemotherapy with oxaliplatin. AG490 has been shown to exert an antagonistic effect on in flammatory pain, but its effect on oxaliplatin‐induced neuropathic pain remains poorly understood. This study sought to observe the analgesic effect of AG490 on acute neuropathic pain induced by a single oxaliplatin treatment and to address the possible mechanism. In this study, we estab‐lished a model of oxaliplatin‐induced acute neuropathic pain by intraperitoneal injection of 6 mg/kg oxaliplatin. On day 2 after injection,models were intraperitoneally injected with 1, 5, or 10 mg/kg AG490. Paw withdrawal threshold to mechanical stimuli and tail withdrawal latency to cold stimuli were determined. Western blot assay was performed to detect the expression of spinal phosphorylated signal transducer and activator of transcription 3 (p‐STAT3). Immunohistochemistry was used to determine the immunoreactivity of p‐STAT3 and inter‐leukin‐6. Results demonstrated that paw withdrawal threshold and tail withdrawal latency were signi ficantly increased by the treatment of AG490 in rats. There was no signi ficant difference in the effect among the different doses of AG490. AG490 10 mg/kg decreased the expression of p‐STAT3, the immunoreactivity of p‐STAT3 and interleukin‐6 in spinal cord of acute neuropathic pain rats. These findings con firm that AG490 can attenuate oxaliplatin‐induced acute neuropathic pain and is associated with the inhibition in the JAK/STAT3 signaling pathway.
Key Words: nerve regeneration; AG490; analgesia; oxaliplatin; cancer chemotherapy; neuropathic pain; phosphorylated STAT3; JAK/STAT signaling pathway; interleukin-6; neural regeneration
Introduction
Oxaliplatin, a chemotherapeutic agent, often causes adverse side effects, such as diarrhea, emesis, muscle pain, joint pain,and neuropathic pain (Argyriou et al., 2013). Neuropathic pain is one of the most severe adverse side effects during can‐cer chemotherapy, and controlling the symptoms of neuro‐pathic pain is a major clinical problem (Argyriou et al., 2013).There are no clear alternative interventions for the effective treatment of neuropathic pain in patients that are refractory to available opioid and non‐opioid analgesics (Inoue et al.,2011; Nagashima et al., 2014), or who develop severe side effects that result in withdrawal from chemotherapy. These concerns have prompted the identification of novel targets and experimental strategies to test potent interventions in well‐validated animal models of neuropathic pain.
The signal transducer and activator of transcription 3(STAT3) protein is a prominent cascade for cellular signal transduction. Several studies have shown that STAT3 is ac‐tivated after nerve injury in animal models of neuropathic pain, and that it is present in multiple cell types (Yamauchi et al., 2006; Dominguez et al., 2008, 2010; Wang et al., 2014;Popiolek‐Barczyk et al., 2015). Inhibition of microglial JAK/STAT3 signaling attenuated mechanical allodynia in neu‐ropathy induced by ligation of spinal nerves in rats (Domin‐guez et al., 2008). AG490 is a synthetic derivative of ben‐zylidenemalononitrile and is a speci fic and potent inhibitor of JAK/STAT signaling (Levitzki, 1990). The oxaliplatin‐in‐duced acute neuropathic pain model in rats is widely used to study acute neuropathic pain or the effect of analgesic drugs(Ling et al., 2007; Lim et al., 2013). A study has shown that AG490 possesses an antiallodynic effect on inflammatory pain (Cheppudira et al., 2015). Therefore, we studied the an‐algesic effect of AG490 on acute neuropathic pain induced by a single oxaliplatin treatment in rats and addressed the possible mechanism.
Materials and Methods
Animals
A total of 56 12‐week‐old specific‐pathogen‐free male Sprague‐Dawley rats were purchased from Tianqin Biotech‐nology Company in China (license No. SCXK (Xiang) 2014‐0010). Experimental animals were raised in clean facilities with free access to food and water. Rats were housed in a room maintained at 22 ± 1°C with a 12‐hour light—dark cycle (lights on 8:00 a.m. to 8:00 p.m.). The animal experi‐ment was conducted in accordance with animal protocols approved by the Institutional Animal Care and Use Com‐mittee of Xiangya Research Laboratory, China (approval No. IACUC‐2016‐0157). Every effort was made to minimize the numbers and any suffering of animals used in the study.Rats were habituated to handling by investigators and to all testing procedures for a week before starting the exper‐iments. The researchers performing the behavioral studies were blinded to the treatment administered.
Model establishment
An oxaliplatin‐induced acute neuropathic pain model was used in this study. Sixteen rats randomly received either one intraperitoneal injection of oxaliplatin (Hengrui Medicine,Lianyugang, Jiangsu Province, China; 6 mg/kg,n= 8) or 5% glucose solution (6 mg/kg,n= 8) (Ling et al., 2007; Lim et al., 2013). All behavioral tests were performed 0, 1, 2, 3,4, and 5 days after the administration of oxaliplatin or 5%glucose. The rats exhibited the most signi ficant increase in mechanical and cold allodynia on day 2. Thus, we selected this time point for subsequent experiments.
Drug administration
An additional 40 rats were randomly assigned to five groups.The control group received the 5% glucose solution injection.The other four groups received an injection of 6 mg/kg oxal‐iplatin. AG490 (Sigma, St. Louis, MO, USA) was dissolved in 3.5% dimethyl sulfoxide. Two days after the rats had re‐ceived the oxaliplatin they were randomly and equally divid‐ed into four groups, and received a single injection of either AG490 (1, 5 or 10 mg/kg, intraperitoneally) or vehicle (3.5%dimethyl sulfoxide, intraperitoneally). All rats were eutha‐nized by an overdose of pentobarbital sodium at the end of the experiment.
Behavioral tests
Behavioral tests representing different sensory components of neuropathic pain were performed 0, 1, 2, 3, 4, and 5 days after oxaliplatin administration, and 4 hours after AG490 injection.Thermal allodynia was assessed using the tail‐immersion test in water maintained at low (10°C) or high (42°C) tem‐perature (Ling et al., 2007). The tails of the rats were immersed in water maintained at the speci fied temperatures until the tail was withdrawn. The duration of tail immersion was recorded and a cut‐off time of 20 seconds was used. Mechanical allody‐nia was measured by using the electronic von Frey pain mea‐suring instrument (IITC Life Science, Woodland Hills, CA,USA). A punctate stimulus was delivered to the mid‐plantar area of each posterior paw below the mesh floor with a rigid von Frey fiber, and the withdrawal threshold was automatical‐ly displayed on the screen when the paw was withdrawn. Stim‐uli were applied to each posterior paw at 5‐second intervals.The cut‐off threshold was recorded as 80 g. All measurements were repeated five times and the mean value was obtained.
Western blot assay
Immediately following the behavioral tests on the indicat‐ed days, rats were deeply anesthetized with pentobarbital(100 mg/kg, intraperitoneally). The lumbar enlargements of the spinal cord were removed immediately and placed into liquid nitrogen for rapid freezing. The tissue was put into radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitor cocktails (Sigma‐Aldrich) to be homogenized on ice. Following centrifugation at 4°C, the su‐pernatants were collected. Proteins in the samples were sep‐arated by sodium dodecyl sulfate‐polyacrylamide gel electro‐phoresis and electrotransferred onto polyvinylidene fluoride membrane (Merck Millipore, Darmstadt, Hesse‐Darmstadt,Germany). The membrane was blocked by 5% non‐fat milk for 1 hour at room temperature, then incubated overnight at 4°C with the primary antibodies for phosphorylated STAT3(Tyr705: rabbit polyclonal antibody IgG; 1:500; Cell Signal‐ing Technology, Boston, MA, USA) and α‐tubulin (rabbit polyclonal antibody IgG; 1:500; Cell Signaling Technology,Boston, MA, USA). The membrane was then incubated with the secondary antibodies (horseradish‐peroxidase goat an‐ti‐rabbit IgG; 1:3000; Bioworld Technology, St. Louis Park,MN, USA) for 1.5 hours at room temperature, developed in enhanced chemiluminescence solution and exposed onto X‐ray film. The density values were computed using Gel‐Pro analyzer 4 software (Media Cybernetics, Bethesda, MD,USA) and were standardized with α‐tubulin expression.
Immunohistochemistry
Rats were deeply anaesthetized by intraperitoneal injection of pentobarbital (100 mg/kg). Cold saline followed by 4% para‐formaldehyde in 0.1 M PBS (pH 7.2—7.4, 4°C) were perfused through the ascending aorta. Following perfusion, the spinal cord lumbar enlargement was quickly removed, post‐ fixed in the same fixative for 2 hours, and cytoprotected in 30% su‐crose for two nights. Transverse spinal sections (5 μm) were cut in a cryostat, mounted on poly‐lysine‐coated slides and processed for immunohistochemistry/immunofluorescence.All the sections were blocked with 2% goat or donkey serum in 0.1 M Tris‐buffered saline/0.3% Triton X‐100 for 1 hour at room temperature. First, the tissue sections were mounted in 4′,6‐diamidino‐2‐phenylindole solution (1.0 mg/mL; So‐larbio, Beijing, China) for dyeing. Second, the tissue sections were incubated over two nights at 4°C with either the primary antibody for p‐STAT3 (Tyr705; rabbit polyclonal antibody IgG; 1:500; Cell Signaling Technology, Boston, MA, USA) or interleukin‐6 (IL‐6) (mouse monoclonal IgG; 1:2000; Abcam Plc, Cambridge, MA, USA). Following incubation, the tissue sections were washed and incubated for 3 hours at room tem‐perature in a secondary antibody solution (Alexa Fluor 594,goat anti‐mouse IgG; 1:1000; Molecular Probes, Eugene, OR,USA). The stained sections were observed using a fluores‐cence microscope (Olympus, Tokyo, Japan), and the images were captured by a CCD spot Camera.
Statistical analysis
Data are presented as the mean ± standard error of mean.Statistical evaluation was performed with SPSS 22.0 software(IBM SPSS, Chicago, IL, USA). Data from the experiments were validated using one‐way analysis of variance, followed by Tukey’s multiple comparison. A value ofP< 0.05 was considered statistically signi ficant.
Results
In fluence of oxaliplatin on cold, heat and mechanical allodynia and expression of spinal p-STAT3 in rats
The effects of a single administration of oxaliplatin (6 mg/kg, in‐traperitoneally) was investigated on behavioral tests and on the JAK/STAT3 signaling in rats. Before oxaliplatin injec‐tion, there were no signi ficant differences in mean thresholds between the oxaliplatin‐treated group (6 mg/kg, intraperitone‐ally) and the control group (5% glucose, intraperitoneally) in any of the tests. The oxaliplatin administration signi ficantly reduced paw withdrawal threshold to mechanical stimuli and tail withdrawal latency to cold stimuli compared with the control group. A signi ficant cold and mechanical allody‐nia was observed on day 1, which was more severe on day 2 and lasted for the 5 days of the experiment after oxaliplatin injection, compared with day 0 or the control group (P<0.05; Figure 1A, B). Exposure to a heat stimulus (42°C) did not display a signi ficant difference for the oxaliplatin‐treated group in heat allodynia compared with the control group (P> 0.05; Figure 1C).
The effect of oxaliplatin treatment on the JAK/STAT3 signaling was measured by western blot assay. This showed that p‐STAT3 was markedly increased on day 1, peaked on day 2 and lasted for 5 days after the injection of oxaliplatin.Compared with p‐STAT3 protein expression on day 0, there were signi ficant differences in the p‐STAT3 expression from day 1 to day 5 (P< 0.05; Figure 2). In addition, p‐STAT3 immunoreactivity increased topographically in the spinal cord of rats induced by oxaliplatin treatment compared with the control group (Figure 3). Therefore, 2 days after a single injection of oxaliplatin (6 mg/kg, intraperitoneally) was cho‐sen as the rat model of oxaliplatin‐induced acute neuropath‐ic pain for the following tests. Heat allodynia was not tested in the following experiments.
AG490 administration alleviated cold and mechanical allodynia through inhibiting the activation of JAK/STAT3 signaling and depressing neuroin flammation in oxaliplatin-induced acute neuropathic pain of rats
In comparison with the oxaliplatin group, an intraperitoneal injection of AG490 clearly increased the cold and mechanical stimulus threshold in all three AG490 groups, but there was no signi ficant difference among three AG490 groups (Figure 4).
Our results showed that p‐STAT3 played an important role in oxaliplatin‐induced acute neuropathic pain, so we observed the effect of AG490 on the expression of p‐STAT3 of spinal cord in oxaliplatin‐treated rats. Western blot as‐say showed that AG490 treatment significantly attenuated the increased level of p‐STAT3 protein, compared with the oxaliplatin group. There were no significant differences in p‐STAT3 protein expression among the three AG490 groups(Figure 5). Moreover, the level of p‐STAT3 immunoreactiv‐ity decreased topographically in the oxaliplatin + AG490 10 mg/kg group compared with the oxaliplatin group (Figure 3).
We also examined the effect of AG490 on the attenuation of neuroinflammation of oxaliplatin‐induced acute neuro‐pathic pain. The expression of IL‐6 of spinal cord in rats was determined by immunohistochemical staining in the con‐trol, oxaliplatin and oxaliplatin + AG490 10 mg/kg groups.The expression of IL‐6 in the oxaliplatin + AG490 10 mg/kg group was signi ficantly reduced as compared with the oxal‐iplatin group (Figure 6).
Discussion
This study used a neuropathic pain model induced by a single intraperitoneal injection of 6 mg/kg oxaliplatin. The intraperitoneal injection route was selected based on the re‐sults of previous studies (Holmes et al., 1998; Jamieson et al.,2005; Ling et al., 2007; Li et al., 2015). In anticancer clinical trials, the oxaliplatin dosages used were 85 mg/m2every 2 weeks or 130 mg/m2every 3 weeks, and the maximum toler‐ated dose was 200 mg/m2, which corresponds approximately to 6 mg/kg (Raymond et al., 1998; Carrato et al., 2002).Hence, we considered this dose (6 mg/kg, intraperitoneally)was appropriate for use in our pain studies. Oxaliplatin‐in‐duced neuropathic pain represents a major obstacle to suc‐cessful cancer treatment because of its prevalence in cases of individual as well as cumulative dosages. In this study, a single dose of oxaliplatin (6 mg/kg, intraperitoneally) significantly increased the cold and mechanical allodynia in rats. Signifi‐cant cold and mechanical allodynia was observed on day 1,peaked on day 2, and lasted for 5 days after a single oxaliplatin injection. Simultaneously, p‐STAT3 levels markedly increased on day 1, peaked on day 2, and lasted for 5 days. Therefore, we chose the 2 days post a single injection of oxaliplatin (6 mg/kg,intraperitoneally) to generate our rat model of oxaliplatin‐in‐duced neuropathic pain for subsequent experiments. There was no significant difference in heat allodynia between the oxaliplatin and control groups, so we excluded testing of heat allodynia in subsequent experiments.
Tyrosine kinase inhibitors, including AG490, are effective for the treatment of various malignancies. Previous studies have demonstrated that administration of AG490 attenuates mechanical allodynia and/or thermal hyperalgesia in painful diabetic neuropathy, peripheral nerve injury, cyclophospha‐mide‐induced bladder pain model and in λ‐carrageenan‐in‐duced in flammatory pain (Dominguez et al., 2008; Cheppu‐dira et al., 2009; Kou et al., 2013; Cheppudira et al., 2015).Our findings are in agreement with the findings of these studies. In our cold and mechanical allodynia tests, AG490 administration significantly increased the paw withdrawal threshold and tail withdrawal latency at all doses tested, but there was no dose‐dependency among the three AG490 dose groups. Future studies might investigate lower dosages to check for dose‐dependency.
Some studies have observed that AG490 treatment re‐duces the levels of cytokine production, inflammatory cell infiltration and nitric acid production (Levitzki, 1990;Dimitrova and Ivanovska, 2008). Research on the possible mechanism underlying the protective effect of AG490 in ox‐aliplatin‐induced acute neuropathic pain model of rats has mainly focused on the correlation between AG490 and the JAK/STAT3 signaling. Peripheral nerve injury evokes the activation of JAK/STAT3 with increased phosphorylation of STAT3, contributing to neuropathic pain (Yamauchi et al.,2006; Dominguez et al., 2008, 2010; Wang et al., 2014; Popi‐olek‐Barczyk et al., 2015). Overexpression of p‐STAT3 could be the mechanism that generates more severe pain behaviors in our oxaliplatin model rats. Spinal IL‐33/ST2 signaling also aggravates neuropathic painviaactivating the JAK/STAT3 signaling (Liu et al., 2015). Both AG490 and JAK/STAT3 in‐hibitor‐1 induce less dorsal horn astrocyte proliferation and promote tactile allodynia recovery, in a study of rats with spinal nerve injury (Tsuda et al., 2011). Another promising agent for treating neuropathic pain, WP1066, is an inhibitor of STAT3 signaling and relieves the pain behavior of rats with chronic constriction injury (Xue et al., 2014). All these references suggest that JAK/STAT3 signaling is activated in both peripheral and spinal nerve injury, while its inhibition alleviates neuropathic pain. Similarly, in our study, p‐STAT3 protein was overexpressed in rats with oxaliplatin‐induced acute neuropathic pain. Our experimental findings indicat‐ed that STAT3 was signi ficantly phosphorylated from day 1 to day 5 after a single oxaliplatin treatment, peaking on day 2. These data suggested that JAK/STAT3 signaling was ac‐tivated in the spinal cord after a single oxaliplatin injection.AG490 treatment inhibited STAT3 phosphorylation in the spinal cord of rats with oxaliplatin‐induced acute neuro‐pathic pain.

Figure 2 Expression of p-STAT3 protein in spinal cord of oxaliplatintreated (6 mg/kg, intraperitoneally) rats detected by western blot assay.

Figure 1 In fluence of oxaliplatin on mechanical (A), cold (B), and heat (C) allodynia in rats.
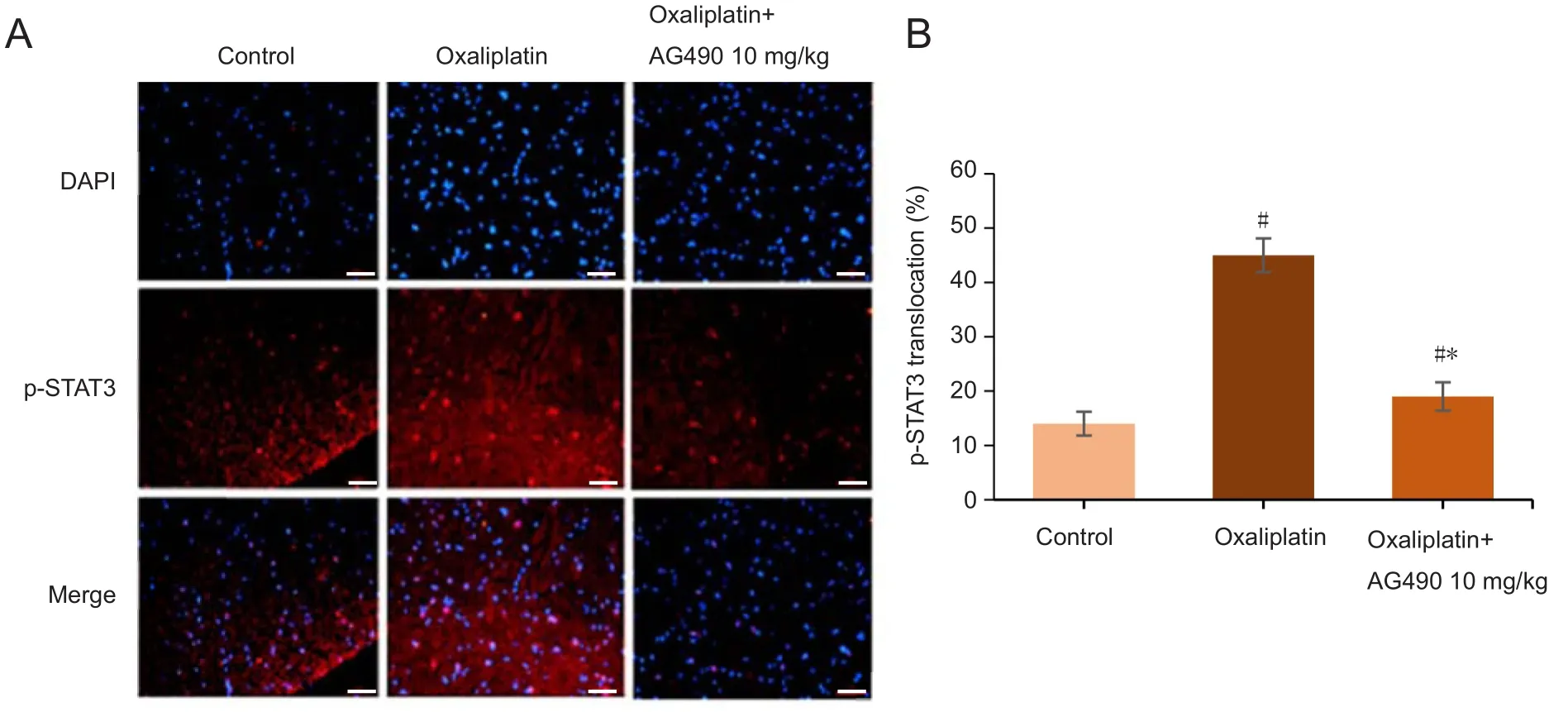
Figure 3 Effect of Janus kinase inhibitor AG490 on the expression of p-STAT3 in the spinal cord tissue following oxaliplatin-induced acute neuropathic pain.

Figure 4 Effects of AG490 on oxaliplatin-induced acute cold and mechanical allodynia in rats.
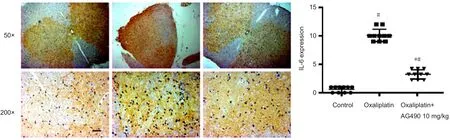
Figure 6 Expression of IL-6 in the spinal cord following oxaliplatin-induced acute neuropathic pain.

Figure 5 Western blot assay of p-STAT3 expression in the spinal cord in each group.
In models of neuropathic pain, the time course of the ex‐pression of IL‐6 has been well characterized in the peripheral and central nervous systems (Rutkowski and DeLeo, 2002; Lee et al., 2004; Marchand et al., 2005; Sacerdote et al., 2013). Up‐regulation of mRNA transcripts and immunoreactivity for the proinflammatory mediator of IL‐6 have also been observed in the spinal cord following nerve injury (Raghavendra et al.,2003; Sacerdote et al., 2013). Therefore, we determined to see whether AG490 could inhibit spinal IL‐6 expression in our rat model. We found that the spinal cord showed increased ex‐pression of IL‐6 after oxaliplatin treatment, which was down‐regulated by AG490 treatment. These results suggest that the effect of AG490 is probably anti‐neuroin flammatory.
This study has revealed a new role and indicated a potential application for AG490 in attenuating oxaliplatin‐induced acute neuropathic pain. AG490 prevents oxaliplatin‐induced acute neuropathic pain from becoming chronic neuropathic pain, allowing the continuation of oxaliplatin therapy. This study has provided signi ficant experimental and theoretical background for further clinical study. In conclusion, AG490 is an effective way in reducing oxaliplatin‐induced acute neuropathic pain. This new function of AG490 is anti‐neu‐roinflammatory and probably acts by inhibiting the JAK/STAT3 signaling; however, the detailed and precise mecha‐nism still needs further investigation.
Author contributions:SFL and YPW conceived and designed the study. XZ and BSO performed the experiments. SFL analyzed the data and wrote the paper. XZ provided reagents/materials/analysis tools. SFL wrote the paper.All authors approved the final version of the paper.
Conflicts of interest:The authors declare no con flict of interest.
Financial support:This work was supported by the National Natural Science Foundation of China, No. 81671962. The conception, design, execution, and analysis of experiments, as well as the preparation of and decision to publish this manuscript, were made independent of this funding organization.
Institutional review board statement:The study protocol was approved by the Institutional Animal Care and Use Committee of Xiangya Research Laboratory, China (approval No. IACUC-2016-0157). The experimental procedure followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1985).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- In Memoriam: Ray Grill (1966–2018)
- Reorganization of injured anterior cingulums in a hemorrhagic stroke patient
- A novel chronic nerve compression model in the rat
- Three-dimensional visualization of the functional fascicular groups of a long-segment peripheral nerve
- Novel conductive polypyrrole/silk fibroin scaffold for neural tissue repair
- Local inhibition of matrix metalloproteinases reduced M2 macrophage activity and impeded recovery in spinal cord transected rats after treatment with fibroblast growth factor-1 and nerve grafts