Management and prevention of mastitis: A multifactorial approach with a focus on milking, bedding and data-management
Sarne De Vliegher , Ian Ohnstad, Sofie Piepers
1 M-team and Mastitis and Milk Quality Research Unit, Department of Reproduction, Obstetrics, and Herd Health, Faculty of Veterinary Medicine, Ghent University, Merelbeke 9820, Belgium
2 MEXCELLENCE BVBA, Gontrode Heirweg 168 bus 1.2., Merelbeke 9820, Belgium
3 The Dairy Group, Taunton TA12PX, United Kingdom
1. Introduction
Mastitis is the result of micro-organisms, typically bacteria,entering the bovine mammary glandviathe teat canal,establishing an intramammary infection (IMI) and resulting in an inflammatory reaction. The disease can present in a clinical and subclinical form. Clinical mastitis is characterized by abnormal milk and swelling or pain in the udder and may be accompanied by systemic signs such as elevated rectal temperature, lethargy and anorexia (Harmon 1994). Subclinical mastitis is the form in which there is no detectable change in the udder and there are no observable abnormalities in the milk. Still, milk production decreases,bacteria are present in the secretion and composition is altered (Harmon 1994). In this case, tests have to be used to detect the presence of IMI either directly (culturing of the causative bacterium) or indirectly (by showing inflammatory responses including an elevated somatic cell count). In either form, mastitis in dairy cows is a costly disease due to depression of milk yield, milk withdrawal, extra treatment and labour costs, and early culling. It should be prevented rather than cured (Halasaet al.2007; van Soestet al.2016).
Mastitis is an extremely important disease not only for the dairy farmer but also for the dairy industry, as a number of issues threaten the image of milk as a healthy product from healthy animals. Antibiotic usage on dairy farms is most often related to udder health as the majority of medicines are used in prevention and control of mastitis (Lamet al.2011;Stevenset al.2016). Although blanket dry cow treatment remains a backbone of any successful mastitis prevention and control plan (NMC 2017), it is already the subject of discussion in many countries including the Netherlands(Scherpenzeelet al.2014) and will become a subject of discussion in the future in many other countries and regions(van den Borneet al.2017). Antimicrobials remain vital for treatment of bacterial infections in dairy cattle but in the light of the upcoming debate instigated by the perceived link between the use of antimicrobial products and the development of antimicrobial resistance in both animal and human pathogens,the industry will have to act in a responsible and proactive way. It is important to note that cows suffering from severe cases of clinical mastitis are in pain (de Boyer des Rocheset al.2017). Improving prevention as well as taking better account of pain alleviation through the use of appropriate treatment, are key to addressing the issue of animal welfare related to clinical mastitis.
Milking cows on a farm struggling with udder health problems (an elevated bulk milk somatic cell count, reflecting problems with subclinical mastitis, or a high incidence of clinical mastitis) is without doubt very frustrating and stressful for the farmer. Treating infected cows also increases labor and causes stress of which the consequences should not be underestimated. They are both perceived as the two most annoying aspects of mastitis by farmers (Jansenet al.2009).Any udder health advisor should realise these aspects are probably the most important for farmers to start tackling a mastitis problem at his farm.
2. Multifactorial disease
2.1. Multiple players
Multiple players have a role in the development and outcome of mastitis. Bacteria, farmer (management) and host are all involved. A certain cow (of a certain age,breed, at a certain lactation stage, with a certain immune competence), managed by a particular farmer (deciding on a specific nutrition, implementing certain milking procedures) within a specified environment (characterised by a certain type of housing, hygiene, etc.) is exposed to a diversity of mastitis pathogens (contagious or opportunistic in nature and with different virulence features) able to cause disease. When the balance tilts in favour of the pathogen,mastitis occurs.
2.2. Multiple factors
Mastitis is a so-called multifactorial disease. Not only cow characteristics explain variability between cows in their susceptibility to (intramammary) infection. Factors at the herd-level (management, environment) explain some of the variation as well; e.g., if a farm does not practise post-milking teat disinfection, the cows will be more likely to contract an IMI (typically caused by a contagious pathogen) compared to cows milked on another farm where post-milking teat-dipping is part of the milking routine (Lamet al.1996; Dufouret al.2011). As a cow rarely has mastitis in all four quarters at a time, it is likely there is some variability between quarters within a cow in their susceptibility to IMI. Identification of quarter-level factors related to IMI will explain some of this variation. Some studies have found pathogen-specific risk factors (RF) at the quarter-level (e.g., Zadokset al.2001). Obviously, anything that increases the risk of IMI withStaphylococcus aureusis not necessarily a RF for IMI withStreptococcus uberisas the epidemiology can be very different.
It is useful to try to determine at what level of the hierarchy(herd, cow, or quarter) most of the variability in the outcome(e.g., somatic cell count, presence of IMI, presence of clinical mastitis) resides as interventions targeted at that level will have the greatest chance of success (Dohooet al.2001b).When designing studies to describe variation (by identifying factors associated with the outcome), the focus should be on the level where most of that variation resides. A large unexplained variation in the early lactation somatic cell count between heifers indicates substantial room for improvement at the heifer level if it is understood why some heifers do better than others (De Vliegheret al.2004b).
Much previous work has concentrated on identifying RF at the herd-level for clinical mastitis (typically using clinical mastitis incidence data as the outcome variable) (e.g.,Schukkenet al.1990; Barkemaet al.1999b; Peeleret al.2000; O’Reillyet al. 2006). A number of the significant herd-level variables were average cow-level features such as “percentage of cows leaking milk increasing the incidence of clinical mastitis” (Schukkenet al.1990). Interpreting this finding as “cows leaking milk are more susceptible to clinical mastitis” could be erroneous (so called “ecologic fallacy”; Dohooet al.2001a). Only well-designed cow- and quarter-level studies substantiating this finding could allow for such conclusion. Only limited work has been published on herd RF for subclinical mastitis (Barkemaet al.1999a;Sampimonet al.2009).
Bovine defence mechanisms against intramammary infection(1) Teat characteristics. The first line of defence against invading bacteria is the teat (canal). Changes in teat end condition may favour penetration of bacteria into the healthy gland. Glands with severe teat end hyperkeratosis or where the teat had been traumatised or leaked milk had higher rates of infection (Sieber and Farnsworth 1981).Based on more recent studies, the relevance of the teat end condition for the cows’ udder health is however less straightforward. Neijenhuiset al. (2001) demonstrated that quarters and cows with clinical mastitis had significantly higher teat end callosity scores than cows and quarters that did not develop clinical mastitis. In a UK study, quarters with moderate and very severe hyperkeratosis of the teat end were at significantly increased risk of developing clinical mastitis caused byEscherichia coli(Breenet al.2009a). Also, quarters with very severe hyperkeratosis of the teat end were significantly more likely to develop clinical mastitis due toS.uberis(Breenet al.2009a). Smaller changes in teat end condition were of less importance forS.uberismastitis which might hypothetically explain why no association was found between teat end hyperkeratosis andS.uberisIMI by Zadokset al.(2001). In the latter study, teat ends were classified as smooth or rough and no distinction was made between slightly, moderately and severely rough teat ends. Interestingly but not yet explainable is the finding that the risk of new IMI withS.aureuswas only significantly higher in udder quarters with rough teat ends if corynebacteria were present simultaneously and in teats with extreme thick callosity rings around the orifice. In a German study, a positive association was found between the teat end hyperkeratosis score and the microbial load of the teat canal byE.coliandS.uberis(Paduchet al.2012).Generally, a teat with a highly calloused teat end had an increased teat canal load by environmental pathogens compared with a contralateral low calloused teat end within the same cow. No such association could be found for teat end hyperkeratosis and theS.aureusteat canal load(Paduchet al.2012). Finally, in a longitudinal study in which the impact of teat condition on the risk of new IMI in dairy cows was investigated, no effect could be observed of any variable describing the teat end condition, including teat end hyperkeratosis, on the risk of new IMI, high somatic cell count or clinical mastitis (Zoche-Golobet al.2015).
Quarters that had a cracked teat end at some time between 2 weeks prior to drying-off and 6 weeks of the dry period had higher odds of developing new IMI during the dry period than those without cracks (Dingwellet al.2004).Quarters that closed within 6 weeks of the dry period (a process that was less likely to happen when the cow was high-producing at dry-off) were less likely to develop a new infection (Dingwellet al.2004). Also, the probability of an IMI withStreptococcispp. increased significantly with an increase in quarter peak flow rate (Grindalet al.1991). Milk yield and peak flow rate are higher in rear than those in front quarters (Weisset al.2004) which could be an explanation for the finding that IMI and high somatic cell count are found more often in rear than those in front quarters (Barkemaet al.1997). However, quarter position was not a RF in a study looking for predictors of pathogen specific IMI (Zadokset al.2001).
(2) Cellular immunity. Cellular immunity, the second line of defence, is a major component explaining variability in susceptibility to IMI between cows. Phagocytic neutrophils in milk are the key cells in the battle between host and mastitis-causing bacteria with blood vessel endothelial cells,mammary epithelial cells, and milk macrophages playing important roles as well in the local inflammatory response(Burton and Erskine 2003; Schukkenet al.2011). The role of the T- and B-lymphocytes in the response of the udder to a mastitis pathogen is yet less well defined (Schukkenet al.2011). Blood and milk from cows with confirmed staphylococcal and streptococcal mastitis show dramatic changes in the numbers and distribution of T-lymphocytes.Based on the current knowledge, it is suggested that distinct T-cell subsets are involved in the host defence of the udder against IMI and that selective recruitment of these T-cell subsets depends on the infectious agent involved and most probably also on the stage of lactation (Schukkenet al.2011). Immune suppression makes cows more vulnerable to infectious disease and can occur as a consequence of several factors (Kehrliet al.2009). Natural physiological conditions such as pregnancy, parturition and peak lactation and primary infectious disease predispose cattle to mastitis and other infections. Various types of stress (natural or induced) and environmental factors such as nutritional deficiencies, shipping, and commingling also have influence.Immune competence is, therefore, potentially related to and influenced by many different variables and has a genetic component as well making selection for resistance to mastitis possible (Pighetti 2009).
(3) Breed. Jersey cows are less likely to be culled for mastitis than Holstein cows with prevalence studies supporting the hypothesis that breed differences exist in susceptibility to IMI (Bannermanet al.2008a, b). The fact that Jersey cows have higher milk somatic cell count than Holstein-Friesian cows could explain this finding as milk somatic cells within the healthy gland (mainly lymphocytes and macrophages) confer protection against IMI by initiating the inflammatory response after detecting invading pathogens. Possibly these differences in somatic cell count reflect the ability of these breeds to respond to an IMI although a differential prevalence of underlying IMI between the breeds could be true as well. Recent work demonstrated that innate immunity afterE.coliandS.aureuschallenge is very similar between Jersey and Holstein cows (Bannermanet al.2008a, b). Thus the innate immune response of Holstein and Jersey cows to IMI remains highly conserved despite previously reported differences in mastitis prevalence, as well as genotypic and phenotypic traits that exist between the two breeds. By contrast, introduction of crossbreeding in a Holstein herd substantially reduces the incidence of clinical mastitis and increases the cows’ longevity, suggesting that crossbreeds are less vulnerable for diseases such as mastitis than pure Holstein cows (Dezetteret al.2017).
(4) Genotype. The importance of genotype as a factor explaining variability in susceptibility to IMI has been often demonstrated, e.g., Schukkenet al. (1999), with new studies concentrating on in this very exciting and promising research area. A significant association was detected between the CXCR1 single nucleotide polymorphism (SNP) +777 genotype and prevalence of subclinical mastitis cases in Holsteins. Holsteins expressing genotype GG had less subclinical mastitis with genotype CC cows having more subclinical mastitis (Youngermanet al.2004). Significant differences in clinical mastitis incidence were not detected between the genotypes. Cows expressing the CXCR1+777 CC genotype had impaired neutrophil migration and adhesion molecule up-regulation compared to cows of the GG genotype (Rambeaud and Pighetti 2005).More recently, Verbekeet al. (2014) demonstrated that CXCR1 polymorphism can influence somatic cell count and neutrophil viability following experimental IMI withStaphylococcus chromogenesin dairy heifers. Quarters from heifers with genotypes c.980AG and c.980GG both developed subclinical mastitis but showed differences in the early response at 6–18 h post challenge. Bacterial count at 18 h post challenge tended to be higher in quarters from c.980AG heifers compared to c.980GG heifers (Verbekeet al.2014). Somatic cell count was higher at 6 h post challenge and tended to be higher at 9 h post challenge in c.980AG heifers compared to c.980GG heifers. Additionally,milk neutrophils of c.980AG heifers showed more apoptosis at 9 h post challenge and tended to show more necrosis at 6, 9 and 12 h post challenge than those of c.980GG heifers(Verbekeet al.2014). Knowledge of the role of specific genes in the aetiology of IMI is still limited. The technology has advanced rapidly in recent years and because less costly methods to study large numbers of genes are available, significant progress can be expected. Most likely,these studies will find a difference in aetiology and also a different role of specific genes in response to different mastitis causing pathogens. The difference in susceptibility to IMI between breeds could be the result of the difference in the prevalence of specific genes.
(5) Age. Older cows (and quarters belonging to older cows) are at an increased risk of clinical mastitis (Barkemaet al.1998; Pantojaet al.2009) and IMI (Zadokset al.2001). Potential explanations are that the older cows have more concurrent problems (e.g., lameness) compared to the younger herd mates making them more susceptible to (environmental) IMI as they spend more time laid down(Breenet al.2009a). It may be that there are anatomical changes in the teat over time that cause disruption of the natural defence mechanisms or there may be a systematic reduction in immune capability associated with ageing that increases susceptibility to infection (Greenet al.2007).There may also be a risk that chronic infection survives through lactations as well as dry periods and results in an accumulated risk of recrudescence of clinical disease with increasing age (Greenet al.2007). By contrast and not yet explainable, quarters of fresh heifers are more likely to be infected with the more relevant non-aureusstaphylococci includingS.chromogenes,S.simulansandS.xylosusthan cows in higher parity (De Vissheret al.2016). Even more strikingly is that the incidence of clinical mastitis in the first week after calving is even higher in heifers compared with that in cows (Barkemaet al.1998; Verbekeet al.2014).
(6) Stage of lactation. It is clear from the results of Bradleyet al. (2015) that the most susceptible times for dairy cows to acquire new IMI are the early dry period and around parturition. In both, the mammary gland is undergoing vast remodelling, first during the gradual involution following the rapid cessation of milking at dry-off and then during the onset of colostrogenesis (Burton and Erskine 2003).The aetiology of susceptibility in the two high-risk periods,however, appears to be very different. The dry period has been identified as being the time of greatest risk for the acquisition of both new Gram-negative and Gram-positive IMI (Smithet al.1985; Bradleyet al.2015). Some particular factors at the cow- and quarter-level play a role. The early lactation period is a time of increased risk for clinical mastitis(Barkemaet al.1998; Verbekeet al.2014) and the rate of new IMI may reflect periparturient immune suppression.But infections acquired during dry cow period, rather than immune suppression facilitating new IMI, can also explain a proportion of the cases of clinical mastitis encountered in early lactation (Bradley 2002). The degree and duration of the periparturient immune depression differs between cows and is influenced by factors such as genetics, nutrition and management (Kehrliet al.2009).
(7) Somatic cell count. An inflammatory response is initiated in the mammary gland when bacteria enter through the teat canal and multiply in the milk. One of the initial components of this response is the influx of polymorphonuclear neutrophils into the mammary tissue and the associated increase in somatic cell count (Harmon 1994). Somatic cell count is considered to be one of the most important RF for clinical mastitis (Steeneveldet al.2008). Actually, both elevated somatic cell count (Breenet al.2009a) and very low somatic cell count (Greenet al.2004; Suriyasathapornet al.2000b) have been found to increase risk of subsequent clinical mastitis. In experimental mastitis challenge studies, the severity of mastitis is increased in cows with low pre-infection somatic cell count(Suriyasathapornet al.2000a). The link between a low somatic cell count and an increased risk of clinical mastitis could be that the outcome of pathogen invasion of the mammary gland depends on the leukocyte/bacterium ratio in the early phase of an infection (van Werven 1999). Normal counts of immune cells in healthy mammary quarters range between 20 000 and 100 000 cells mL–1(Greenet al.2006).Low numbers of leucocytes might increase the probability that bacterial invasion results in a true IMI and clinical signs(Peeleret al.2000). Mammary quarters with lower cells counts tended to response less efficient to an intramammary challenge (Wellnitzet al.2010). Still, low quarter somatic cell count was not associated with an increased rate of IMI forS.uberisorS.aureus(Zadokset al.2001). Relatively few quarters in that study were in the lowest somatic cell count categories which could have prevented detection of the effect found by Greenet al. (2004). The increased risk for clinical mastitis in quarters with an elevated somatic cell count most probably reflects subclinical infections becoming clinical at a certain point in time when the equilibrium between host immunity and pathogens is disturbed. An elevated somatic cell count in the last months before drying off, increased the risk of clinical mastitis after calving (Greenet al.2007). This could indicate a failure to cure an existing IMI during the dry cow period eventually becoming clinical in early lactation. Increased somatic cell count was also consistently associated with elevated risk of new major pathogen infections by Reyheret al. (2012a), but this was assumed to be the result of low sensitivity of bacteriology to diagnose new IMI with major pathogens expediently and accurately. A higher pre-infection quarter somatic cell count and an existing IMI withCorynebacterium bovisprotected against experimentalS.aureusinfection (Schukkenet al.1999). Still, the increased somatic cell count associated withC.bovisinfections only partially explained the protective effect against experimentalS.aureusIMI, indicating that other mechanisms play a role. Intramammary infections with non-aureusstaphylococci have also been associated with a protective effect against IMI with major pathogens(Matthewset al.1991). Non-aureusstaphylococci are a heterogenous group of different staphylococcal species.They are commonly considered to be minor mastitis pathogens because of their limited potential to cause mastitis. Interestingly, some isolates inhibit the growth of major pathogensin vitro(De Vliegheret al.2004c; Braemet al.2013). A surprising finding in literature is that heifers(first lactating cows) infected with non-aureusstaphylococci in early lactation had a lower incidence of clinical mastitis and higher milk production in their first lactation compared to non-infected heifers (Pieperset al.2010, 2013). Similar as for IMI withC.bovis, a moderate but constant increase in somatic cell count and thus a continuous influx of immune cells in quarters infected with some specific species or strains has been suggested as one of the potential mechanisms behind the protective effect (Greenet al.2004), besides competitive exclusion and the production of bacteriocins.All of these findings suggest a potential positive role of specific commensal non-aureusstaphylococci strains in safeguarding mammary glands from becoming infected.Recent work demonstrated the ability of specific non-aureusstaphylococcal strains to inhibit biofilm formation of mastitisrelated pathogens, through the production of bioactive compound with a protein nature (Isaacet al.2017). As well,IMI with minor pathogens have not always been associated with protection against clinical mastitis (Greenet al.2004)or IMI with major pathogens (Reyheret al.2012a). Overall,the protective effects of IMI with minor pathogens against IMI with major pathogens seem to be more pronounced in challenge studies, specifically when major pathogens were introduced into the mammary glandviamethods bypassing the teat end, than in observational studies (Reyheret al.2012b).
(8) Milk yield. High milk yield is a risk factor for clinical mastitis (Houbenet al.1993), although, within-breed differences in milk production do not affect the severity ofE.colimastitis (Kornalijnslijperet al.2003). Milk secretion in the dairy cow has a high metabolic priority and is clearly maintained at the cost of other reproductive and metabolic processes (Fleischeret al.2001). High milk yield at dryoff was significantly associated with environmental IMI at calving (Rajala-Schultzet al.2005). In line with the latter finding, a higher milk yield at dry off was recently found to be associated with higher somatic cell scores in the following lactation (Gottet al.2017). An explanation could be that high milk yield at drying-off may mean leakage of milk and slower formation of the protective keratin plug, thus allowing an open entry for bacteria to the udder (Dingwellet al.2004).However, contrary to those findings, slower teat closure or failure of teat closure was not associated with an increase in the risk of IMI in a more recent study of Bradleyet al. (2015).
(9) Energy balance. Due to the rapid increase of milk production after calving, cows require more energy for maintenance, milk production and growth than they are able to obtain through feed. This leads to a temporary state of negative energy balance (NEB). The NEB is more pronounced in high producing cows (Kornalijnslijperet al.2003). The severity of NEB during the transition period,which is characterised by an increased concentration of circulating non-esterified fatty acids (NEFA) and β-OH-butyrate, and a decrease in glucose, may contribute to suppression of immune system function (Moyeset al.2009).Several factors, including body condition score (BCS),NEFA, the fat/protein ratio in milk, and ketone bodies, are signs of NEB (Suriyasathapornet al.2000a). No association was seen between BCS and clinical mastitis risk (Breenet al.2009a) or rate of IMI withS.aureusandS.uberis(Zadokset al.2001). In another study, cows with a BCS<1.5 or BCS>3.5 (using a 5-point scale) were at higher risk of having an elevated somatic cell count (Breenet al.2009b).Also, dairy heifers losing 0.25 points or more of their body condition in periparturient period had higher proportions of apoptotic (and thus less viable) blood PMN in early lactation compared with heifers losing less than 0.25 points (Pieperset al.2009). Ionophore use pre-calving in heifers resulted in higher BCS at calving, lower β-OH-butyrate and NEFA concentrations, but did not alter the prevalence of subclinical mastitis at calving or reduce incidence of clinical mastitis(McDougallet al.2004). Intriguingly, mid-lactating cows subjected to dietary-induced NEB had minimal alterations in immune function following mastitis challenge (Moyeset al.2009) and no effect on clinical symptoms was observed following acute endotoxin-induced mastitis (reviewed by Sordillo 2013).
(10) Nutrition. Inadequate dietary vitamin E or Se decreases neutrophil function during the periparturient period which could be related to a higher risk for mastitis(Spears and Weiss 2008). Cows that received a dietary supplement with about 1 000 IU d–1of vitamin E had 30%less clinical mastitis than did cows receiving a supplement of 100 IU d–1of vitamin E. The reduction was 88% when cows were fed 4 000 IU d–1of vitamin E during the last 14 days of the dry period. All cows were supplemented with 0.1 mg Se kg–1diet (Weisset al.1997). Experimental mastitis withE.coliwas more severe and of longer duration in cows receiving 0.04 mg Se kg–1diet compared with those receiving 0.14 mg Se kg–1diet (Erskineet al.1989). Seleniumdeficient cows had greater peak bacteria concentrations in milk than Se-supplemented cows after challenge withS.aureus(Erskineet al.1990). Supplementation of a commercial mineral/vitamin mix to pregnant dairy heifers before calving was associated with a better blood and milk neutrophil viability near calving, presumably related to the higher blood selenium concentrations that were observed(Pieperset al.2009).
(11) Viral infections. Certain selected pathogens may induce immune suppression (Kehrliet al.2009). The suppressive effects can lead directly to secondary disease or can add to the degree and duration of an already existing immune suppression in, e.g., early lactation. Bovine herpes virus 4 (BVH4)-positive animals had a higher rate of IMI withS.aureusthan BHV4-negative animals (Zadokset al.2001). Perhaps the reduction in phagocytic capacity of udder monocytes and macrophages explains the increased susceptibility. Acute infections with non-cythopatic Bovine viral diarrhoea virus (BVDV) suppress both innate and acquired immune responses. In this regard, Laureynset al.(2013) found a positive association between herd exposure to BVDV-infection and bulk milk somatic cell count of Flemish Dairy Farms, Belgium. Essentially BVDV-negative farms had a significantly lower bulk milk somatic cell count than BVDV-positive farms (i.e., positive antibody titre in bulk milk). Bovine leukaemia virus is able to deregulate the host immune system at humoral and cellular levels (Kehrliet al.2009).
Exposure to mastitis pathogensExposure to mastitis pathogens can originate from several sources, including the environment of the cow, existing or previous IMI, and teat skin flora (Pankeyet al.1989).
(1) Hygiene. Cows with a very dirty udder, reflecting poor cow hygiene and housing, are at an increased risk of developing clinical mastitis especially infections caused by environmental pathogens (Breenet al.2009a). Also,cows with a dirty udder were more likely to have subclinical mastitis caused by major pathogens compared with cows with a clean udder (Schreiner and Ruegg 2003). Herds where at least 50% of the cows had an udder hygiene score of 3 or 4 had 1.49 more risk of clinical mastitis caused by any pathogen and 2.57 more risk of clinical mastitis caused byE.colithan herds where less than 50% of the cows had an udder hygiene score of 3 or 4 (Verbekeet al.2014).
(2) Existing/Previous intramammary infections. Cows that have had clinical mastitis once have a greater risk for clinical mastitis later during lactation (Houbenet al.1993;Steeneveldet al.2008). Quarters that had at least one case of clinical mastitis during the previous lactation were 4.2 times more likely to have a first case of clinical mastitis in the current lactation than quarters that did not have clinical mastitis in the previous lactation (Pantojaet al.2009). In a more recent study, multiparous cows were at greater risk of a second clinical mastitis case if they had suffered from a first clinical mastitis case that was caused by the same pathogen as the second case (Chaet al.2016). In contrast,a second clinical mastitis case generally put the cows at greater risk of a third case, irrespective of whether the third case was caused by the same or a different pathogen. It was concluded that a previous case of pathogen specific clinical mastitis did not protect against a recurrent case. Quarters that had recovered fromS.uberisorS.aureusmastitis had a higher rate of infection with both pathogens than quarters that had not experienced infection before (Zadokset al.2001) and quarters belonging to a cow of which one of the other quarters was infected withS.uberisorS.aureushad a higher rate of IMI withS.uberisorS.aureus, respectively(Zadokset al.2001). The authors concluded that some quarters are more susceptible to infection than others,irrespective of pathogen. Indeed, one should be cautious in the interpretation of the abovementioned positive associations between exposure to previous IMI and the risk of recurrent IMI as the observations might also be explained by differences in local quarter immunological events. This was substantiated by the finding that some variability in milk neutrophil-viability exists in non-infected quarters from heifers in early lactation (Pieperset al.2009).
(3) Teat skin flora. The probability of a new IMI is highly correlated with the number of mastitis pathogens on the teat end at milking (Pankey 1989). However, the presence of prepartum teat apex colonisation in heifers withS.chromogeneswas not associated with IMI early postpartum with the same bacterium. Contrarily, prepartum teat apex colonization withS.chromogenes, a very common species in milk samples from cows, was associated with improved udder health in early lactating dairy heifers (De Vliegheret al.2003). A finding that was substantiated later showing that teat apex colonization with non-aureusstaphylococci as a group in prepartum dairy heifers was associated with a lower likelihood of intramammary infection with major pathogens in the first days after calving in the corresponding udder quarters (Pieperset al.2011). This intriguing finding might be explained by the fact that the milk neutrophil apoptosis is less pronounced in early lactation in quarters having teat orifices colonised with non-aureusstaphylococci before calving whereas milk neutrophil concentration was increased (Pieperset al.2009).
3. Multifactorial approach
3.1. Mastitis prevention and control programs
Improving udder health at the farm level is based on the application of two basic principles: (1) Reduction in duration of existing IMI (E), and (2) lowering the incidence of new IMI (N).
As mastitis is a complex, multifactorial disease,motivating the farmer to implement these basic principles,means the successful udder health advisor should have certain characteristics. He or she must fully understand the complexity of the disease, should know the principles of prevention and control, is motivated and determined,motivates his/her client (the dairy farmer), and should be able to translate (complex) knowledge into practice.
Improvement of udder health will be obtained through working with a mastitis prevention and control program,such as the one promoted by the National Mastitis Council(NMC 2017).
3.2. Milking machine and teat condition
Direct and indirect milking machine effects may account for up to 20% of new IMI in some herds, and probably not much more than about 10% in an average herd these days –provided that the machine settings are correct (NMC 2017).One of the main ways that a milking machine can influence new infection rates is by changing the resistance of the teat canal to bacterial invasion. The risk of new infections by contagious pathogens as well as environmental pathogens such asS.uberisis increased by machine-induced changes in teat condition. Teat condition is affected by many factors associated with the milking machine, including the working vacuum level at which the system operates, the degree of over-milking, the fit of the liner to the teats, the type of liner used (shape and material), and the adjustment of the pulsation (Ohnstad 2012).
Liner design and teat end hyperkeratosisAs the milking liner is intimate contact with the teat, the choice of the liner and the vacuum level at which it is used is highly relevant when examining machine induced effects on teat condition.A critical point when choosing a liner for a specific herd is how well the liner fits the teats. Still, one should realize that while only one liner will be selected to milk all cows on a herd, the teat size and teat shape within a herd vary largely. Liner compression is the primary milking machine influence on teat end hyperkeratosis. For any individual liner, liner compression increases with the milking vacuum level. The latter can be explained by the fact that the pressure difference across the liner is increased during the liner closed (d-phase) of a pulsation cycle. Both liner compression and overpressure are highly correlated with teat end hyperkeratosis as was demonstrated by Zucaliet al. (2008). In the latter study, a quarter-udder experiment was performed with four liners each applied one quarter of 75 Holstein cows for a period of 3 weeks. Teat end hyperkeratosis was assessed weekly. Interestingly, the risk of developing hyperkeratosis was higher with liners that applied greater pressure to the teat end when closed. The risk of developing teat end hyperkeratosis was also highly affected by the duration of milking and the initial teat end hyperkeratosis score (Zucaliet al.2008).The latter finding was confirmed by the results of a survey conducted on commercial dairy farms in Wisconsin in which was observed that liners with the highest overpressure measurements were responsible for more than 80% of teats having rough or very rough hyperkeratosis scores. In contrast, liners with the lowest overpressure measurements produced less than 20% of teats that were rough or very rough. Using teat liners that apply a lower compressive load, applying enough stimulation during udder preparation (Weiss and Bruckmaier 2005), ensuring sufficient prep-lag time in the milking routine (Watterset al.2012), and adjusting the threshold settings in order to shorten the average unit on time per cow (Rasmussen 1993; Edwardset al.2013) are some of the milking management factors that will reduce the risk of teat end hyperkeratosis.
Teat conditionOne of the main determinants of teat congestion during milking is the level of vacuum applied to the teat tissue during milking (Ohnstad 2012). Congestion can be defined as an accumulation of circulatory fluids within the teat. In case that congestion is severe and persistent,edema will occur. Congestion might occur either at the teat end or at the teat barrel. The level of both teat end and barrel congestion is strongly affected by the teat size and teat shape, independently of the type of liner. Short teats receive less liner compression around the teat apex, as they do not penetrate into the liner as deeply as long teats(Meinet al.2001). Changes in the teat barrel diameter during milking have been associated with the quarter milk somatic cell count (Zwertvaegheret al.2013). Negative changes in the diameter of the teat barrel during milking(i.e., thinner teats postmilking compared with premilking)were associated with lower quarter milk somatic cell count,whereas positive changes (i.e., thicker teats postmilking compared with premilking) were associated with higher quarter milk somatic cell count (Zwertvaegheret al.2013).Teat barrel congestion might be at least partly solved by reducing the vacuum level in the liner mouthpiece. In a recent study, quarters were subsequently exposed to low-risk conditions for teat-barrel congestion and to highrisk conditions for teat-barrel congestion. The low-risk condition for teat-barrel congestion was created by venting the liner mouthpiece chamber to atmosphere. In the highrisk condition for teat-barrel congestion, the mouthpiece chamber was connected to the short milk tube vacuum.The latter conditions were designed to impair circulation in the teat barrel. The calculated teat canal cross-sectional area was used to assess congestion of teat tissue. The main effect of the teat-barrel treatment was a reduction in teat canal cross-sectional area of 9.7% between the lowrisk conditions for teat-barrel congestion and the high-risk conditions for teat-barrel congestion (Penryet al.2017).The degree of teat end congestion can be affected by the pulsation settings. Uptonet al.(2016) recently quantified the effect of d-phase duration of pulsation on the teat canal cross-section area during the period of peak milk flow from bovine teats. As in case of excessively long d-phases(>250 ms), a greater percentage of the pulsation cycle will be in a massaging rather than milking phase, they can reduce milking speed. An increase in the length of the liner open phase (b-phase) increases the degree of teat end congestion. The latter finding was confirmed in an experimental study conducted by Penryet al. (2017).
最后,加大对大数据搜集、利用、整理和分析的技能培养。大数据时代要求财务人员掌握如上技能和模式,在接受教育的过程中,增加实践训练和练习机会,使得财务人员的实践性能力和技能有所提高。
Selection of linersMost variation in teat dimensions occurs at the cow or within-cow level, and not at the herd level,indicating that choosing a teat cup liner that is identical for all cows in a herd is far from optimal (Zwertvaegheret al.2012). Quarter position, parity and stage of lactation are some factors that have been identified to be associated with teat length and teat diameters. Generally, front teats were longer and broader than hind teats. Teat length and diameters increased with parity. After the first 30 days in milk, teat length substantially and significantly increased,whereas teat diameters decreased (Zwertvaegheret al.2012). There is a general trend towards breeding for short teats. Heifers might even have teats less than 30 mm long in their resting state (Zwertvaegheret al.2012) which often results in discomfort in heifers at the end of the milking, and high levels of edema and discoloration on heifers’ teats after milking.
3.3. Bedding and environment
Stall bedding is very closely related to the bacterial exposure of the cows taking into account that teats of dairy cattle may be in direct contact with bedding materials for 40 to 60%of the day (Hogan and Smith 2012). Bedding materials are primary sources of mastitis causing environmental pathogens (Hogan and Smith 2012). Populations of these bacteria in bedding are related tot the number of bacteria on teat ends (Hogan and Smith 1997; Zdanowiczet al.2004)as well as to the incidence rate of clinical mastitis (Hoganet al.1989). Therefore, reducing the number of bacteria in bedding generally results in a decrease in environmental mastitis (Hoganet al.1989). The criteria for the selection of bedding for dairy cows have changed drastically over the last 30 years (Hogan and Smith 2012). Bedding costs are one of the greatest variable expenses on the farm. Bedding materials historically were by-products of the dairy or other local industries that provided inexpensive and readily available product. Deficiencies associated with some of these products were tolerated as a balance to their low cost and local accessibility. Most bedding materials that are currently available and commonly used are organic in nature. A major drawback of organic by-products such as sawdust, wood shavings, and straw is their ability to harbour and cultivate mastitis pathogens (Hogan and Smith 2012).
Sand beddingThe use of washed sand as bedding for dairy cows dramatically reduces the exposure of teat ends to coliform mastitis pathogens compared with common organic bedding materials (Hoganet al.1989; Zdanowiczet al.2004; Rowbotham and Ruegg 2016b), and resulted in a reduction in clinical mastitis in lactating cows on nine commercial dairy herds in the US (Hoganet al.1989).Also, quarters of primiparous cows bedded with new sand tended to have a lower risk of clinical mastitis than quarters of primiparous cows bedded with deep-bedded manure solids or recycled sand (Rowbotham and Ruegg 2016a).The effectiveness of washed sand in reducing exposure of mastitis pathogens to mammary glands is due to its inorganic properties. The most common component of washed sand from inland sources is silica. Mastitis pathogens derive energy from carbon based materials and cannot oxidize silica. Hence, the ability of environmental mastitis pathogens to multiply in sand bedding is directly associated with the carbon-rich organic material contamination, although a pathogen-specific effect might exist. In a recent study performed on 161 large Chinese dairy farms, it was found thatStreptococcus dysgalactiaewas more often isolated from clinical mastitis cases using sand bedding, whereasKlebsiellaspp. and other streptococci were more common in herds using organic bedding (Gaoet al.2017). Also,exposure to large numbers ofStreptococciandStreptococcilike organisms was consistent across four different bedding types including deep-bedded new sand, deep-bedded recycled sand, deep-bedded manure solids and shallowbedded manure solids over foam core mattresses. The latter suggested that the exposure to streptococci spp. is less affected by the bedding type than the exposure to coliforms(Rowbotham and Ruegg 2016a). Interestingly, recycled sand bedding had intermediate counts of Gram-negative bacteria compared to new sand and deep-bedded manure solids. The findings are in line with the observations of anin vitrostudy in which, after 72 h of incubation, numbers ofKlebsiella pneumoniawere 10 and 20 times greater in recycled sand and digested manure, respectively, than in new sand. Also, digested manure solids and recycled sand were able to maintain populations ofEnterococcus faeciumfor 72 h while new sand was not with bacteria entering a death phase shortly after inoculation (Goddenet al.2008).On the contrary, no significant differences were found in the numbers of Gram-negative bacteria, coliforms,Klebsiellaspp., andStreptococcusspp. between new and recycled sand when compared with each other at any time up to 7 days after bedding (Kristulaet al.2005). Bacterial counts also differ among the depth strata of sand in a stall. Bacterial populations were lower on the surface 25 mm compared with sand at a depth of 50 to 75 mm (Hoganet al.2012). The increase in bacteria counts in the deeper layers of a sand pack is most probably related to the increase in organic matter and moisture in these environments.
Organic beddingLittle advantage exists in using one organic material over the use of another. Chopped straw bedding tended to have the highest counts ofStreptococciwhile sawdust had the highest counts in comparison to the other organic bedding materials on nine commercial dairy herds in the US (Hoganet al.1989). Two management strategies are commonly applied to use organic beddings in free stalls: deep packs and daily replacement. Sorteret al.(2014) have shown that daily replacement of both sawdust and recycled manure solids in the rear of stalls decreased exposure of cows’ teats to coliform bacteria. Samples taken from daily replacement stalls in a trial investigating recycled manure solids had lower coliform counts compared with deep pack stalls. This reduction was particularly seen forKlebsiellaspp. which was reduced approximately 10-fold each day in daily replacement stalls compared with deep packed recycled manure under comparable, controlled housing conditions. Still, daily replacement of recycled manure bedding appeared not to be an effective approach to reducing exposure to streptococci. The reason for the discrepancy between effects of daily bedding replacement on coliform counts compared with streptococcal counts is unknown. Composting has been proposed as beneficial method of decreasing initial bacterial load in organic materials such as recycled manure solids. Composting is the process of breaking down organic material by bacteria, which helps decrease the populations of potential pathogens in materials coming in contact with plants and animals. Effective composing heats recycled manure solids to approximately 60°C to kill coliforms and other bacteria commonly associated with bovine mastitis. Still,in an experiment conducted to compare bacterial counts of environmental mastitis pathogens in composted recycled manure solids bedding with those in fresh recycled manure solids, only Gram-negative bacterial counts on day 1 were reduced in composted recycled manure solids compared with fresh recycled manure solids. Despite the increase in ash after composting, bacterial counts of mastitis in composted recycled manure solids were comparable with those in fresh recycled manure at day 2 and 6 (Cole and Hogan 2016). Interestingly, the manure from the alley taken into the stalls on cow legs, and hooves was from a common source of contamination to both composted and fresh recycled manure solids. It was hypothesized that the similar bacterial counts between both bedding treatments was due to contamination of the beddings by the faecal bacteria derived from manure in the common use alley for both treatments.
Bedding conditionersA common practice on herds using organic bedding materials is to add hydrated lime to the stalls to control bacterial population. Treatment of sawdust bedding with a commercial alkaline conditioner reduced the teat skin bacterial counts ofS.uberis,E.coliand other coliform bacteria but not ofS.aureus(Paduchet al.2013). An investigation on the effect of free-stall mattress bedding treatments on the mastitis bacterial growth found the lowest counts ofKlebsiellaspp.,E.coli,andStreptococcusspp. on mattresses bedded with lime.Mattresses bedded with a commercial acid conditioner had the next lowest counts for coliforms (Kristulaet al.2008). Strikingly, hydrated lime was the only treatment that significantly reduced bacterial counts on both mattresses and teat ends. Still, other controlled trials have shown the addition of hydrated lime to all organic bedding had minimal effect on controlling bacterial populations. Lime had a bactericidal effect in organic materials prior to placement in stalls, but the pathogen load in bedding treated with lime rapidly increased to that comparable in untreated bedding.Alkaline conditions were most effective in recycled manure solids which as near neutral pH. As the pH of the recycled manure solids neutralized during use, the antibacterial effects of the alkaline conditioners diminished (Hoganet al.2007). In contrast, acidic conditions were more effective in sawdust, with pH 4, compared with recycled manure solids. A commercial acid-bedding conditioner reduced the pH in sawdust compared with the untreated sawdust for 2 days corresponding with the bacteriostatic effect of the treatment (Hoganet al.2007). Sawdust bedding treated with a clay-based acid bedding conditioner, compared with the untreated sawdust, had lower counts of total Gramnegative bacteria and streptococci, but not coliform counts.Teat end bacterial counts were lower for cows bedded on treated sawdust for streptococci, coliforms, andKlebsiellaspp. compared with cows bedded on the untreated sawdust(Proiettoet al.2013).
3.4. Data-driven management changes
The solutions for achieving and maintaining good udder health at a dairy farm are well-known and are included in the standard 10-point mastitis prevention and monitoring program (NMC 2017). Nonetheless, in practice it often still proves difficult to structurally improve and regularly monitor udder health at a dairy farm (Barkemaet al.2013).One of the reasons is that we often focus too closely on“solutions” rather than the “problem”. We want anything that is not done by the book to be changed, so the dairy farmer becomes overwhelmed and demotivated. Moreover,such an approach often leads to frustration. After all, the focus is often on the most obvious causes, such as the milking technique, the milking machine or the hygiene of the lactating animals’ accommodation, and all sorts of things get changed, whereas the actual problem may lie with the dry cows or the young (pregnant) heifers. The range of solutions for improving udder health is the same for all dairy farms, but the actual problem and causes of the problem often differ from farm to farm. If you really want to succeed in improving the udder health at a dairy farm, it is important to first analyse the problem and find and offer the most effective, evidence-based solution for each farm-specific problem, based on facts and information (Barkemaet al.2013). Individual cow somatic cell count measurements at a regular basis (i.e., every 4 to 6 weeks) as well as a good clinical mastitis recording are therefore indispensable to further improve and monitor the udder health on a dairy farm.This chapter gives some examples of how farm-specific data and parameters derived from those data can be helpful in unravelling the farm-specific cause of udder health issues and in finding the most appropriate solution.
Infection dynamicsThe bulk milk somatic cell count is determined by the percentage of cows with an elevated somatic cell count (typically ≥200 000 cells mL–1). Cows with a high somatic cell count either contracted a new IMI or did not cure from an existing IMI since the previous milk recording. The (spontaneous) cure rate is calculated as the number of cows that experienced a decrease in somatic cell count typically from ≥200 000 cells mL–1at the previous milk test to <200 000 cells mL–1at the current milk test,multiplied by 100 and divided by the number of cows with high somatic cell count (typically ≥200 000 cells mL–1) at the previous milk test (cows ‘at risk’ to cure). One should strive for a (spontaneous) cure rate >40% per month which can be translated to an average infection duration of 2.5 months.Cows that did not (spontaneously) cure are considered as chronically infected cows. The percent chronic infection is calculated as the number of cows with a somatic cell count≥200 000 cells mL–1both at the previous and current milk recording, multiplied by 100 and divided by all lactating cows on the herd. One should strive for a percent chronic IMI≤10%. The percent of new high somatic cell count cows is calculated as the number of cows that experienced an increase in somatic cell count typically from <200 000 cells mL–1at the previous milk test to ≥200 000 cells mL–1at the current milk test, multiplied by 100 and divided by all lactating cows on the herd. On average, herds with a monthly milk test and a bulk milk somatic cell count around 200 000 cells mL–1have a percent of new high somatic cell count cows of approximately 8%. It is the balance between the percentage of new high somatic cell count cows and (spontaneously)cured high somatic cell count cows, the so-called infection dynamics, that determines the bulk milk somatic cell count on a dairy farm. Fig. 1 shows a farm with a high bulk milk somatic cell count due to a high percent of new high somatic cell count cows in combination with a low (spontaneous)cure rate. This pattern is indicative for farms withS.aureusmastitis problems. On the contrary, Fig. 2 shows a farm with a moderate bulk milk somatic cell count although a high percentage of new high somatic cell counts. The high percent of new IMI on this farm is outweighed by the high(spontaneous) cure rate and the short infection duration of less than 2 months. This pattern is indicative for farms on which the cows are highly exposed to environmental pathogens but easily cure thanks to an optimal immunity.
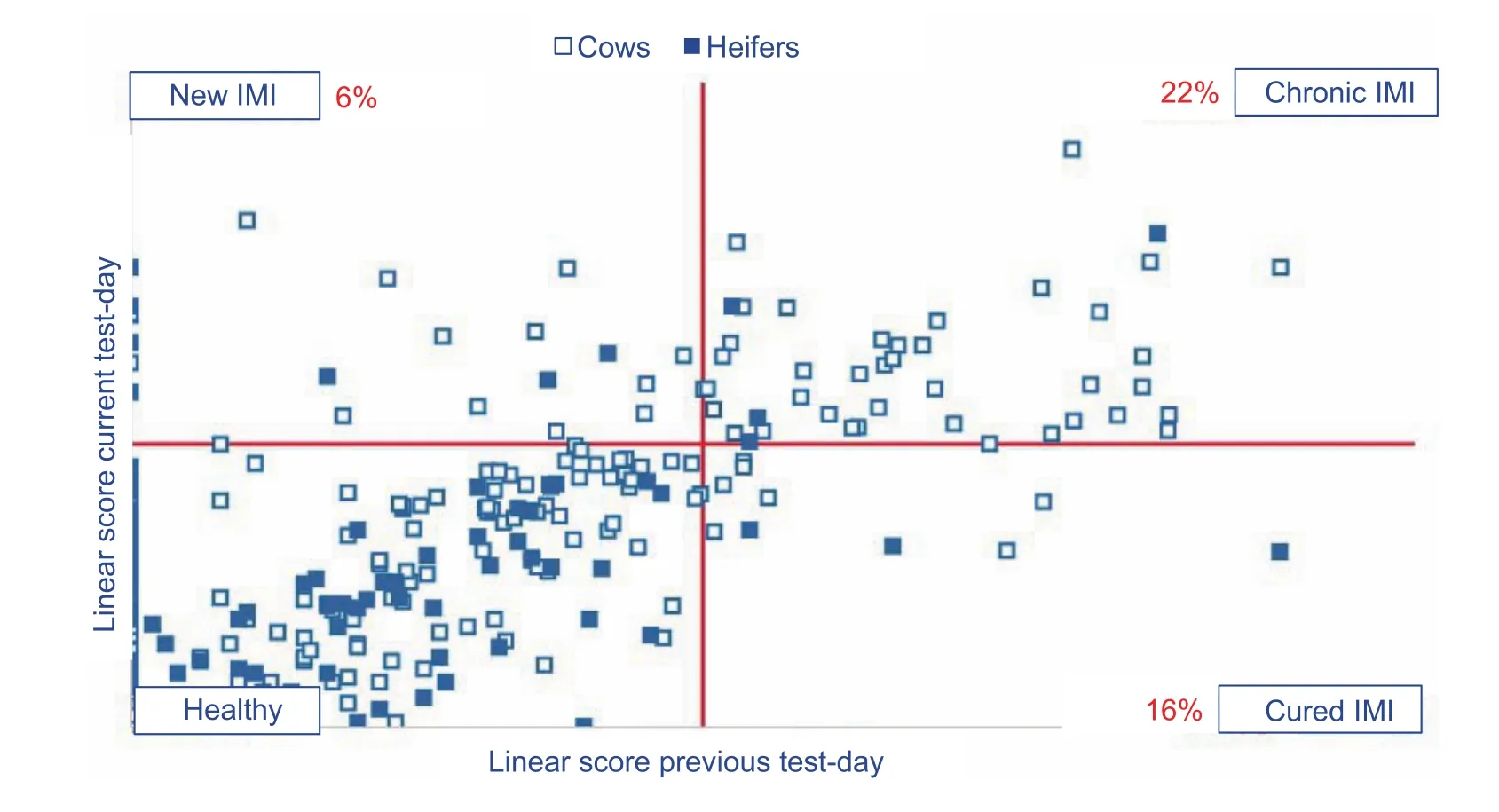
Fig. 1 Graph representing a dairy farm with a high bulk milk somatic cell count (SCC) due to a high percent of new high somatic cell count cows in combination with a low (spontaneous) cure rate (based on Keno™-M, Ghent University, Ghent, Belgium). The somatic cell count is expressed as a linear score [(ln(SCC/100)/ln2)+3].

Fig. 2 Graph representing a dairy farm with a moderate bulk milk somatic cell count although the high percent of new high somatic cell count (SCC) (based on Keno™-M, Ghent University, Ghent, Belgium). The somatic cell count is expressed as a linear score[(ln(SCC/100)/ln2)+3].
Contagious vs. environmentalIntramammary infections are caused by either contagious or environmental mastitis pathogens. Contagious mastitis causing bacteria includingS.aureusandStreptococcus agalactiaeneed the cow/udder to survive and multiply. The mammary gland and/or teat skin are the predominant reservoirs of infection. Contagious mastitis causing bacteria are easily transmitted from the carrier cow or quarter to the teats of non-infected cows/quarters during the milking processviahands, cloths or the teat liners. As the contagious mastitis pathogens are welladapted to the cow and mammary gland environment, they often cause chronic IMI. Those chronically infected cows are in turn a source of IMI for their herdmates. On the contrary,environmental or opportunistic mastitis causing pathogens includingS.uberisandE.colido not need the cow/udder to survive or multiply. The environment is the reservoir of infection. New IMI occur in between milkings by transfer of bacteria from the environment to the teats when the cow lays down and the teats are in close contact with the environment.Penetration of the teat canal can also occur by propulsion on a reverse flow of milk (i.e., the bacteria on the teat skin come loose during milking). Environmental mastitis causing pathogens are generally less well adapted to the cow and mammary gland environment and cause therefore less often persistent or chronic IMI compared to the contagious mastitis pathogens. A high rate of new IMI caused by environmental pathogens suggests poor hygiene before, during or after milking. Bacteriological culturing of milk samples collected from cows with clinical or subclinical mastitis is still the best way to identify the bacterial cause of the udder health issues on a dairy farm. Often, recent milk culture results are not available. As a start, a bulk tank sample can be submitted for bacteriological culture to determine whether one of the contagious pathogensS.agalactiaeorMycoplasmaspp.play a role in the problem. Occasionally, mastitis due toS.uberisorE.colican also be detected through use of bulk tank milk samples (Zadokset al.2004, 2005). Given the abovementioned difference in epidemiology between contagious and environmental mastitis pathogens, the probable cause of the udder health issues on a dairy farm can also be derived from the correlation between the percent new high somatic cell count cows and the percent chronic high somatic cell count cows. A high correlation between the percentage of new high somatic cell count cows and chronic high somatic cell count cows strongly indicates the presence of contagious mastitis pathogens since those bacteria easily spread from one cow to the other (i.e., high new infection rate) and are difficult to (spontaneously) cure.A poor correlation between the percentage of new high somatic cell count cows and chronic high somatic cell count cows is typical for farms with environmental mastitis issues.
Heifer mastitisMany heifers freshen with an IMI. In several heifer mastitis surveys conducted throughout the world, up to 60% of the quarters harbored an IMI at the time of calving (De Vliegheret al.2012). Most of these IMI reveal themselves as subclinical mastitis characterized by an elevated somatic cell count without any visible symptoms of inflammation. In a Belgian study, 30% of heifers had a somatic cell count ≥150 000 cells mL–1in the first 14 days after calving (De Vliegheret al.2004a). The majority of those IMI are presumably caused by the minor pathogenic group of non-aureusstaphylococci (Pieperset al.2010). The proportion of heifers calving with a high somatic cell count varies considerably among herds. A herd is considered to have a heifer mastitis problem if >15% of the heifers have a somatic cell count ≥150 000 cells mL–1at the first milk recording from 10 days in milk on. An average somatic cell count ≥150 000 cells mL–1of the heifers in the first 100 days in milk is strongly indicative for persistent infections caused by major pathogens such asS.aureus(Pieperset al.2010).
Clinical mastitisA high incidence of clinical mastitis (≥2%per month) might be the result of a high rate of first clinical mastitis cases (≥10%), a high rate of recurrent mastitis cases (>30% of all cases) or a combination. The incidence of clinical mastitis is the number of cases of clinical mastitis per 100 cows per year or per month where one case is one quarter. It is a very useful indicator of mastitis incidence as it allows comparison between herds, irrespective of size.It is the balance between the exposure to bacteria and the immunity of the host that will determine the severity of the inflammatory reaction against IMI. A high rate of first cases can be due to a too high infection pressure (i.e., high number of bacteria can penetrate the udder), an impaired immunity of the cows or a combination of both. A recurrent or repeat case of mastitis refers to one or more cases of mastitis occurring in the same cow. A high recurrence rate may be due to:
(1) Problems withS.aureusinfections which can be difficult to eliminate.
(2) Poor immunity hampering the elimination of IMI.
(3) Incorrect choice of treatment, e.g., too short duration or incorrect antibiotic.
(4) Poor mastitis detection where IMI are not picked up early enough.
Based on the individual cell count before and after the clinical mastitis case, cows can be divided in four groups:
(1) New IMI cured: low somatic cell count (<200 000 cells mL–1) before clinical mastitis case and low somatic cell count(<200 000 cells mL–1) after clinical mastitis case;
(2) New IMI not cured: low somatic cell count (<200 000 cells mL–1) before clinical mastitis case and high somatic cell count (≥200 000 cells mL–1) after clinical mastitis case;
(3) Existing IMI cured: high somatic cell count before clinical mastitis case (≥200 000 cells mL–1) and low somatic cell count (<200 000 cells mL–1) after clinical mastitis case;
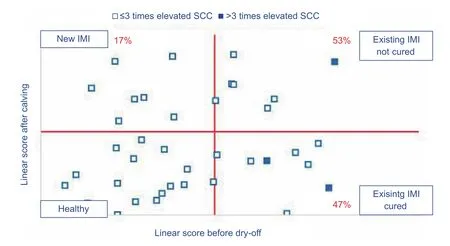
Fig. 3 Graph representing the infection dynamics across dry period of a herd where most cows that did not cure over dry period were already chronically infected (>3 times elevated somatic cell count (SCC) at the last milk recordings before dry-off) at the end of previous lactation (based on Keno™-M, Ghent University, Ghent, Belgium). The somatic cell count is expressed as a linear score [(ln(SCC/100)/ln2)+3].

Fig. 4 Graph representing the infection dynamics across the dry period of a herd where too many cows contracted a new intramammary infection (>10%) over dry period and where cows did not cure well although they were yet not chronically infected at dry-off (Keno™-M, Ghent University, Ghent, Belgium). The somatic cell count (SCC) is expressed as a linear score [(ln(SCC/100)/ln2)+3].
(4) Existing IMI not cured: high somatic cell count before clinical mastitis case (≥200 000 cells mL–1) and high somatic cell count after clinical mastitis case (≥200 000 cells mL–1).
Cows with a high somatic cell count before the clinical mastitis case can be further divided in those that had a chronically elevated cell count (>3 times elevated somatic cell count) and those that had yet not a chronically infected cell count (≤3 times elevated somatic cell count). Fig. 5 shows the distribution of the first clinical mastitis cases on a farm with a high incidence of clinical mastitis (>3% per month), and a low percentage of repeated cases (10% of all clinical mastitis cases). Most cows had a low somatic cell count before the clinical mastitis case and cured well(low somatic cell count after the clinical mastitis case). This pattern is typical for herds with a high infection pressure and a good immunity of the cows. The cows that did not cure well were those with a chronically elevated cell count(>3 times elevated somatic cell count). Fig. 6 shows the distribution of the first clinical mastitis cases on a farm with a high incidence of clinical mastitis (>3% per month), and a high percentage of repeated cases (40% of all clinical mastitis cases). Both the cows with a new IMI and those with an existing IMI did not cure well (i.e., still high somatic cell count after clinical mastitis case) although the latter group were yet not chronically infected before the clinical mastitis case and thus expected to cure. This pattern is indicative for herds where the cows suffer from an impaired immunity or cows that are housed on herds with a high infection pressure and an inappropriate treatment strategy.
Decision making at cow levelObtaining and maintaining good udder health depends on two basic principles:shortening the duration of existing infections and limiting the number of new infections. Early detection of cows with IMI along with bacteriological culturing and implementation of specific measures based on the outcome is still a cornerstone in the control of mastitis at the herd level(Hillertonet al.1995). Making the optimal decision (wait or test) for cows with an existing infection is however not easy,in particular not for cows with a recently elevated somatic cell count. On the one hand, the spontaneous cure rate of a recently acquired subclinical IMI was estimated at 41%(van den Borneet al.2010). On the other hand, cows with a high composite somatic cell count have a 2- up to 4-fold higher hazard than cows with a low composite somatic cell count (van den Borneet al.2011). With the latter in mind,the first decision in a mastitis monitoring program should be whether or not a particular cow still has a chance to spontaneously cure (i.e., without antimicrobial treatment).Animals that still have a high chance to spontaneously cure should not immediately be tested. Cases that are unlikely to spontaneously cure but that still can benefit from antimicrobial treatment should be tested. The chance to(spontaneously) cure depends on several factors such as the parity, the somatic cell count, the history of elevated somatic cell counts, the days in milk, and the pathogen that is involved (van den Borneet al.2010). Overall, IMI caused by major pathogens such asS.aureusare obviously more difficult to (spontaneously) cure than IMI caused by minor pathogens such as non-aureusstaphylococci. Much research on cow-level factors and the probability of cure for subclinical mastitis cases has been focused onS.aureus.Cow-level parameters that need to be taken into account when estimating the probability of (spontaneous) cure of cows with subclinical mastitis are:

Fig. 5 Graph representing the distribution of the first clinical mastitis cases on a farm with a high incidence of clinical mastitis(>3% per month), and a low percentage of repeated cases (10% of all clinical mastitis cases). Most cows had a low somatic cell count before the clinical mastitis case and cured well (low somatic cell count after the clinical mastitis case) (Keno™-M, Ghent University, Ghent, Belgium). The cows that did not cure well were those with a chronically elevated cell count (>3 times elevated somatic cell count). The somatic cell count (SCC) is expressed as a linear score [(ln(SCC/100)/ln2)+3].
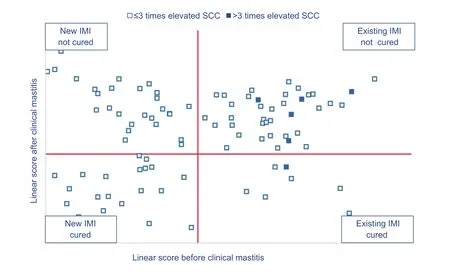
Fig. 6 Graph representing the distribution of the first clinical mastitis cases on a farm with a high incidence of clinical mastitis (>3%per month), and a high percentage of repeated cases (40% of all clinical mastitis cases). Both the cows with a new IMI and those with an existing IMI did not cure well (i.e., still high somatic cell count after clinical mastitis case) although the latter group were yet not chronically infected before the clinical mastitis case and thus expected to cure. The somatic cell count (SCC) is expressed as a linear score [(ln(SCC/100)/ln2)+3].
(1) Lactation number: Older cows are more difficult to cure than younger ones (Solet al. 1997; Deluykeret al.2005);
(2) Chronicity: Cows with a chronic infection (≥3 times high somatic cell count at test-day) have a lower probability of cure than cows with a recent infection (Solet al.1997);
(3) Somatic cell count: The chance of (spontaneous)cure decreases with increasing somatic cell count (Solet al.1997);
(4) Number of infected quarters: Animals of which two or more quarters are infected are more difficult to cure than animals of which only one quarter is infected (Solet al.1997);
(5) Quarter position: A hind quarter is more difficult to cure than a front quarter (Solet al.1997);
(6) Number of colonies: An increasing number of bacteria in the mammary gland results in a lower chance of cure(Dingwellet al.2003; Deluykeret al.2005);
(7) Stage of lactation: In some studies, an increasing cure rate was observed after antimicrobial treatment with increasing days in milk (Solet al.1997; Deluykeret al.2005) while in other studies no differences in cure rate were observed between animals in early, mid and late lactation(van den Borneet al.2010).
Recently, a preliminary study commenced to evaluate the prediction of the likelihood of spontaneous cure of a first elevated somatic cell count by an in-house developed software application as part of a novel mastitis management concept (Keno™-M, UGent, Belgium) taking into account some of the abovementioned parameters. A total of 362 primiparous and 439 multiparous dairy cows with a first elevated somatic cell count from 24 randomly selected Flemish dairy farms were included. Animals were considered to have an elevated somatic cell count if the somatic cell count exceeded 150 000 and 250 000 cells mL–1for primiparous and multiparous cows, respectively. For each animal, an advice was generated based on the herd milk somatic cell count, the individual composite somatic cell count, days in milk and parity. The association between the advice (waitvs. culture) and the time to spontaneous cure(i.e., again low somatic cell count) was determined fitting a Cox frailty model. Overall, 53% of the animals with a first elevated somatic cell count were spontaneously cured at the first next somatic cell count test-day (28–65 days). The chance of spontaneous cure of a first elevated somatic cell count within 120 days was 1.24 (95% confidence interval 1.02–1.51) higher in animals for which the advice ‘wait’was generated than those for which the advice “culture”was given. Primiparous cows had a numerically higher chance to spontaneously cure than multiparous cows (1.10;95% confidence interval 0.93–1.30) and their likelihood of spontaneous cure was up to 2.1 times (95% confidence interval 1.32–3.27) higher if the software application recommended to wait until next test-dayvs. culture.
4. Conclusion
Taking into account several herd- and cow-level parameters in selecting cows with subclinical mastitis for further testing might therefore be helpful in assisting vets to take objective and more precise decisions for high somatic cell count cows.
Bannerman D D, Kauf A C, Paape M J, Springer H R, Goff J P.2008a. Comparison of Holstein and Jersey innate immune responses toEscherichia coliintramammary infection.Journal of Dairy Science,91, 2225–2235.
Bannerman D D, Springer H R, Paape M J, Kauf A C, Goff J P. 2008b. Evaluation of breed-dependent differences in the innate immune responses of Holstein and Jersey cows toStaphylococcus aureusintramammary infection.Journal of Dairy Research,75, 291–301.
Barkema H W, Van der Ploeg J D, Schukken Y H, Beiboer M L, Benedictus G, Brand A. 1999a. Management style and its association with bulk milk somatic cell count and incidence rate of clinical mastitis.Journal of Dairy Science,82, 1655–1663.
Barkema H W, Schukken Y H, Lam T J G M, Beiboer M L,Benedictus G, Brand A. 1999b. Management practices associated with the incidence rate of clinical mastitis.Journal of Dairy Science,82,1643–1654.
Barkema H W, Schukken Y H, Lam T J G M, Beiboer M L,Wilmink H, Benedictus G, Brand A. 1998. Incidence of clinical mastitis in dairy herds grouped in three categories by bulk milk somatic cell counts.Journal of Dairy Science,81, 411–419.
Barkema H W, Schukken Y H, Lam T J G M, Galligan D T,Beiboer M L, Brand A. 1997. Estimation of interdependence among quarters of the bovine udder with subclinical mastitis and implications for analysis.Journal of Dairy Science,80,1592–1599.
Barkema H W, De Vliegher S, Piepers S, Zadoks R N. 2013.Herd level approach to high bulk milk somatic cell count problems in dairy cattle.Veterinary Quarterly,33, 82–93.
van den Borne B H P, van Schaik G, Lam T J G M, Nielen M.2010. Therapeutic effects of antimicrobial treatment during lactation of recently acquired bovine subclinical mastitis:Two linked randomized field trials.Journal of Dairy Science,93, 218–233.
van den Borne B H P, van Soest F J S, Reist M, Hogeveen H.2017. Quantifying preferences of farmers and veterinarians for National Animal Health Programs: The example of bovine mastitis and antimicrobial usage in Switzerland.Frontiers in Veterinary Science,4, 82–95.
van den Borne B H P, Vernooij J C M, Lupindu A M, van Schaik G, Frankena K, Lam T J G M, Nielen M. 2011. Relationship between somatic cell count status and subsequent clinical mastitis in Dutch dairy cows.Journal of Dairy Science,102, 265–273.
de Boyer des Roches A, Faure M, Lussert A, Herry V, Rainard P, Durand D, Foucras G. 2017. Behavioral and pathophysiological response as possible signs of pain in dairy cows duringEscherichia colimastitis: A pilot study.Journal of Dairy Science,100, 8385–8397.
Bradley A J. 2002. Bovine mastitis: An evolving disease.The Veterinary Journal,164, 116–128.
Bradley A J, De Vliegher S, Green M J, Larrosa P, Payne B, van de Leemput E S, Samson O, Valckenier D, Van Werven T,Waldeck H W F, White V, Goby L. 2015. An investigation of the dynamics of intramammary infections acquired during the dry period on European dairy farms.Journal of Dairy Science,98, 6029–6047.
Braem G, De Vliegher S, Verbist B, Piessens V, Van Coillie E,De Vuyst L, Leroy F. 2013. Unraveling the microbiota of teat apices of clinically healthy lactating dairy cows, with special emphasis on coagulase-negative staphylococci.Journal of Dairy Science,96, 1499–1510.
Breen J E, Green M J, Bradley A J. 2009a. Quarter and cow risk factors associated with the occurrence of clinical mastitis in dairy cows in the United Kingdom.Journal of Dairy Science,92, 2551–2561.
Breen J E, Green M J, Bradley A J. 2009b. Quarter and cow risk factors associated with a somatic cell count greater than 199,000 cells per milliliter in United Kingdom dairy cows.Journal of Dairy Science,92, 3106–3115.
Burton J L, Erskine R J. 2003. Immunity and mastitis - Some new ideas for an old disease.Veterinary Clinics of North America:Food Animal Practice,19,1–45.
Cha E, Hertl J, Schukken Y, Tauer L, Welcome F, Gröhn Y.2016. Evidence of no protection for a recurrent case of pathogen specific clinical mastitis from a previous case.Journal of Dairy Research,83, 72–80.
Cole K J, Hogan J S. 2016. Short communication: Environmental mastitis pathogen counts in freestalls bedded with composted and fresh recycled manure solids.Journal of Dairy Science,99, 1501–1505.
Deluyker H A, Van Oye S N, Boucher J F. 2005. Factors affecting cure and somatic cell count after pirlimycin treatment of subclinical mastitis in lactating cows.Journalof Dairy Science,88,604–614.
Dezetter C, Bareille N, Billon D, Côrtes C, Lechartier C, Seegers H. 2017. Changes in animal performance and profitability of Holstein dairy operations after introduction of crossbreeding with Monbéliarde, Normande and Scandinavian Red.Journal of Dairy Science,100, 8239–8264.
Dingwell R T, Leslie K E, Duffield T F, Schukken Y H, DesCoteaux L, Keefe G P, Kelton D F, Lissemore K D, Shewfelt W, Dick P, Bagg R. 2003. Efficacy of intramammary tilmicosin and risk factors for cure ofStaphylococcus aureusinfection in dry period.Journal of Dairy Science,86,159–168.
Dingwell R T, Leslie K E, Schukken Y H, Sargeant J M, Timms L L, Duffield T F, Keefe G P, Kelton D F, Lissemore K D,Conklin J. 2004. Association of cow- and quarter-level factors at drying-off with new intramammary infections during the dry period.Preventive Veterinary Medicine,63,75–89.
Dohoo I R, Martin W, Stryhn H. 2001a. Ecologic and group-level studies. In:Veterinary Epidemiological Research. AVC,Charlottetown, Prince Edward Island, Canada. pp. 561–580.
Dohoo I R, Martin W, Stryhn H. 2001b. Introduction to clustered data. In:Veterinary Epidemiological Research. AVC,Charlottetown, Prince Edward Island, Canada. pp. 459–472.
Dufour S, Fréchette A, Barkema H W, Mussell A, Scholl D T.2011. Invited review: Effect of udder health management practices on herd somatic cell count.Journal of Dairy Science,94, 563–579.
Edwards J P, O’Brien B, Lopez-Villalobos N, Jago J G. 2013.Overmilking causes deterioration in teat end condition of dairy cows in late lactation.Journal of Dairy Research,80,344–348.
Erskine R J, Eberhart R J, Grasso P J, Scholz R W. 1989.Induction ofEscherichia colimastitis in cows fed seleniumdeficiency or selenium supplemented diets.American Journal of Veterinary Research,40, 2093–2100.
Erskine R J, Eberhart R J, Scholz R W. 1990. Experimentally inducedStaphylococcus aureusmastitis in seleniumdeficient and selenium supplemented dairy cows.American Journal of Veterinary Research,51, 1107–1111.
Fleischer P, Metzner M, Beyerbach M, Hoedemaker M, Klee W.2001. The relationship between milk yield and the incidence of some diseases in dairy cows.Journal of Dairy Science,84, 2025–2035.
Gao J, Barkema H W, Zhang L, Liu G, Deng Z, Cai L, Shan R, Zhang S, Zou J, Kastelic J P, Han B. 2017. Incidence of clinical mastitis and distribution of pathogens on large Chinese dairy farms.Journal of Dairy Science,100,4797–4806.
Godden S, Bey R, Lorch K, Farnsworth R, Rapnicki P. 2008.Ability of organic and inorganic bedding materials to promote growth of environmental bacteria.Journal of Dairy Science,91, 151–159.
Gott P N, Rajala-Schultz R J, Schuenemann G M, Proudfoot K L, Hogan J S. 2017. Effect of gradual or abrupt cessation of milking at dry off on milk yield and somatic cell score in the subsequent lactation.Journal of Dairy Science,100,2080–2089.
Green L E, Schukken Y H, Green M J. 2006. On distinguishing cause and conqequence: Do high somatic cell counts lead to lower milk yield or does high milk yield lead to lower somatic cell count?Preventive Veterinary Medicine,76, 74–89.
Green M J, Bradley A J, Medley G F, Browne W J. 2007. Cow,farm, and management factors during the dry period that determine the rate of clinical mastitis after calving.Journal of Dairy Science,90, 3764–3776.
Green M J, Burton P R, Green L E, Schukken Y H, Bradley A J, Peeler E J, Medley G F. 2004. The use of Markov chain Monte Carlo for analysis of correlated binary data: Patterns of somatic cells in milk and the risk of clinical mastitis in dairy cows.Preventive Veterinary Medicine,64, 157–174.
Grindal R J, Walton A W, Hillerton E J. 1991. Influence of milk flow rate and streak canal length on new intramammary infection in dairy cows.Journal of Dairy Research,58,383–388.
Halasa T, Huijps K, Osteras O, Hogeveen H. 2007. Economic effects of bovine mastitis and mastitis management: A review.Veterinary Quarterly,29,18–31.
Harmon R J. 1994. Physiology of mastitis and factors affecting somatic cell counts.Journal of Dairy Science,77, 2103–2112.
Hillerton J E, Bramley A J, Staker R T, McKinnon C H. 1995.Patterns of intramammary infection and clinical mastitis over a 5-year period in a closely monitored herd applying mastitis control measures.Journal of Dairy Research,62, 39–50.
Hogan J S, Smith K L. 1997. Bacteria counts in sawdust bedding.Journal of Dairy Science,80, 1600–1605.
Hogan J S, Smith K L. 2012. Managing environmental mastitis.Veterinary Clinics of North America:Food Animal Practice28, 217–222.
Hogan J S, Raubenolt L, McCormick J L, Weiss W P. 2012.Evaluation of propane flaming for reducing bacterial counts in sand bedding.Journal of Dairy Science,95, 6152–6159.
Hogan J S, Smith K L, Hoblet K H, Todhunter D A, Schoenberger P S, Heuston W D, Pritchard D E, Bowman G L, Heider L E,Brockett B L, Conrad H R. 1989. Bacterial counts in bedding materials used on nine commercial dairies.Journal of Dairy Science,72, 250–258.
Hogan J S, Wolf S L, Petersson-Wolfe C S. 2007. Bacterial counts in organic materials used as free-stall bedding following treatment with a commercial conditioner.Journal of Dairy Science,90, 1058–1062.
Houben E H P, Dijkhuizen A A, Van Arendonk J A M, Huirne R B. 1993. Short- and long-term production losses and repeatability of clinical mastitis in dairy cattle.Journal of Dairy Science,76, 2561–2578.
Isaac P, Bohl L P, Breser M L, Orellano M S, Conesa A, Ferrero M A, Porporatto C. 2017. Commensal coagulase-negative Staphylococcus from the udder of health cows inhibits biofilm formation of mastitis-related pathogens.Veterinary Microbiology,207, 259–266.
Jansen J, van den Borne B H P, Renes R J, van Schaik G, Lam T J G M, Leeuwis C. 2009. Explaining mastitis incidence in Dutch dairy farming: The influence of farmers’ attitudes and behaviour.Preventive Veterinary Medicine,92, 210–223.
Kehrli M E, Ridpath J F, Neill J D. 2009. Immune suppression in cattle contributors and consequences. In:NMC Annual Meeting Proceedings. National Mastitis Council, New Prague, Minnesota, United States. pp. 103–112.
Kornalijnslijper E, Beerda B, Daemen I, van der Werf J, van Werven T, Niewold T, Rutten V, Noordhuizen-Stassen E.2003. The effect of milk production level on host resistance of dairy cows, as assessed by the severity of experimentalEscherichia colimastitis.Veterinary Research,34,721–736.
Kristula M A, Dou Z, Toth J D, Smith B I, Harvey N, Sabo M.2008. Evaluation of free-stall mattress bedding treatments to reduce mastitis bacterial growth.Journal of Dairy Science,91, 1885–1892.
Kristula M A, Rogers W, Hogan J S, Sabo M. 2005. Comparison of bacteria populations in clean and recycled sand used for bedding in dairy facilities.Journal of Dairy Science,88,4317–4325.
Lam T J, De Jong M C, Schukken Y H, Brand A. 1996.Mathematical modelling to estimate efficacy of postmilking teat disinfection in split-udder trials of dairy cows.Journal of Dairy Science,79, 62–70.
Lam T J G M, Olde Riekerink R G M, van Engelen E, Hage J J. 2011. Towards a proactive approach of antibiotic use in cattle practice. In:Proceedings of the 6th European Congress of Bovine Health Management. Société Belge Francophone de Buiatrie & Vlaamse vereniging voor Buiatrie, Belgium. pp. 17–26.
Laureyns J, Piepers S, Ribbens S, Sarrazin S, De Vliegher S,Van Crombrugge J M, Dewulf J. 2013. Association between herd exposure to BVDV-infection and bulk milk somatic cell count of Flemish dairy farms.Preventive Veterinary Medicine,109, 148–151.
Matthews K R, Harmon R J, Langlois B E. 1991. Effect of naturally occurring coagulase-negative staphylococci infections on new infections by mastitis pathogens in the bovine.Journal of Dairy Science,74, 1855–1859.
McDougall S, Young L, Anniss F M. 2004. Production and health of pasture-fed dairy cattle following oral treatment with the ionophorel Lasalocid.Journal of Dairy Science,87,2967–2976.
Mein G A, Neijenhuis F, Morgan W F, Reinemann D J, Hillerton J E, Baines J R, Ohnstad I, Rasmussen M D, Timms L, Britt J S, Farnsworth R, Cook N, Hemling T. 2001. Evaluation of bovine teat condition on commercial dairy herds: 1. Noninfectious factors. In:Proceedings, AABP-NMC International Symposium on Mastitis and Milk Quality. Vancouver, BC,Canada.
Moyes K M, Larsen T, Friggens N C, Drackley J K, Ingvarsten K L. 2009. Identification of potential markers in blood for the development of subclinical and clinical mastitis in dairy cattle at parturition and during early lactation.Journal of Dairy Science,92, 5419–5428.
Neijenhuis F, Barkema H W, Hogeveen H, Noordhuizen J P. 2001. Relationship between teat-end callosity and occurrence of clinical mastitis.Journal of Dairy Science,84, 2664–2672.
NMC (National Mastitis Council, A global organization for mastitis control and milk quality). 2017.Current Concepts of Bovine Mastitis. 5th ed. National Mastitis Council, New Prague, Minnesota, United States.
Ohnstad I. 2012. Liner mapping and teat health. In:Proceedings of the British Mastitis Conference. Institute of Animal Health,United Kingdom, Worcester. pp. 7–14.
O’Reilly K M, Green M J, Peeler E J, Fitzpatrick J L, Green L E. 2006. Investigation of risk factors for clinical mastitis in British dairy herds with bulk milk somatic cell counts less than 150,000 cells/ml.Veterinary Record,158, 649–653.
Paduch J H, Mohr E, Kromker V. 2012. The association between teat end hyperkeratosis and teat canal microbial load in lactating dairy cattle.Veterinary Microbiology,158,353–359.
Paduch J H, Mohr E, Kromker V. 2013. The association between bedding material and the bacterial counts ofStaphylococcus aureus,Streptococcus uberisand coliform bacteria on teat skin and in teat canals in lactating dairy cattle.Journal of Dairy Research,80, 159–164.
Pankey J W. 1989. Premilking udder hygiene.Journal of Dairy Science,72, 1308–1312.
Pantoja J C F, Hulland C, Ruegg P L. 2009. Somatic cell count status across the dry period as a risk factor for the development of clinical mastitis in the subsequent lactation.Journal of Dairy Science,92, 139–148.
Peeler E J, Green M J, Fitzpatrick J L, Morgan K L, Green L E.2000. Risk factors associated with clinical mastitis in low somatic cell count British dairy herds.Journal of Dairy Science,83,2464–2472.
Penry J F, Upton J, Mein G A, Rasmussen M D, Ohnstad I,Thompson P D, Reinemann D J. 2017. Estimating teat canal cross-sectional area to determine the effects of teat-end and mouthpiece chamber vacuum on teat congestion.Journal of Dairy Science,10, 821–827.
Piepers S, Opsomer G, Barkema H W, de Kruif A, De Vliegher S. 2010. Heifers infected with coagulase-negative staphylococci in early lactation have fewer cases of clinical mastitis and higher milk production in their first lactation than non-infected heifers.Journal of Dairy Science,94,1873–1892.
Piepers S, Opsomer G, Demeyere K, Barkema H W, de Kruif A, De Vliegher S. 2009. Heifer and quarter characteristics associated with periparturient blood and milk neutrophil apoptosis in healthy heifers and in heifers with subclinical mastitis.Journal of Dairy Science,92, 4330–4339.
Piepers S, Peeters K, Opsomer G, Barkema H W, Frankena K,De Vliegher S. 2011. Pathogen-specific risk factors at the herd, heifer and quarter level for intramammary infections in early lactating dairy heifers.Preventive Veterinary Medicine,99, 91–101.
Piepers S, Schukken Y H, Passchyn P, De Vliegher S. 2013.The effect of intramammary infection with coagulasenegative staphylococci in early lactating heifers on milk yield throughout first lactation revisited.Journal of Dairy Science,96, 5095–5105.
Pighetti G. 2009. New horizons for boosting immune competence. In:Proceedings NMC Annual Meeting.National Mastitis Council, New Prague, Minnesota, United States. pp. 98–102.
Proietto R L, Hinckley L S, Fox L K, Andrew S M. 2013.Evaluation of a clay-based acidic bedding conditioner for dairy cattle bedding.Journal of Dairy Science,96,1044–1053.
Rajala-Schultz P J, Hogan J S, Smith K L. 2005. Association between milk yield at dry-off and probability of intramammary infections at calving.Journal of Dairy Science,88,577–579.
Rambeaud M, Pighetti G M. 2005. Impaired neutrophil migration associated with specific bovine CXCR2 genotypes.Infection and Immunity,73, 4955–4959.
Rasmussen M D. 1993. Influence of switch level of automatic cluster removers on milking performance and udder health.Journal of Dairy Research,60, 287–297.
Reyher K K, Dohoo I R, Scholl D T, Keefe G P. 2012a.Evaluation of minor pathogen intramammary infection,susceptibility parameters and somatic cell counts on the development of new intramammary infections with major mastitis pathogens.Journal of Dairy Science,95,3766–3780.
Reyher K K, Haine D, Dohoo I R, Revie C W. 2012b. Examining the effect of intramammary infections with minor mastitis pathogens on the acquisition of new intramammary infections with major mastitis pathogens - A systematic review and meta-analysis.Journal of Dairy Science,95,6483–6502.
Rowbotham R F, Ruegg P L. 2016a. Associations of selected bedding types with incidence rates of subclinical and clinical mastitis in primiparous Holstein dairy cows.Journal of Dairy Science,99, 4707–4717.
Rowbotham R F, Ruegg P L. 2016b. Bacterial counts on teat skin and in new sand, recycled sand, and recycled manure solids used as bedding in freestalls.Journal of Dairy Science,99, 6594–6608.
Sampimon O C, Barkema H W, Berends I M, Berend I M, Sol J, Lam T J. 2009. Prevalence and herd-level risk factors for intramammary infection with coagulase-negative staphylococci in Dutch dairy herds.Veterinary Microbiology,134, 37–44.
Scherpenzeel C G, den Uijl I E, van Schaijk G, Olde Riekerink R G, Keurentjes J M, Lam T J. 2014. Evaluation of the use of dry cow antibiotics in low somatic cell count cows.Journal of Dairy Science,97, 3606–3614.
Schreiner D A, Ruegg P L. 2003. Relationship between udder and leg hygiene scores and subclinical mastitis.Journal of Dairy Science,86, 3460–3465.
Schukken Y H, Grommers F J, Van de Geer D, Erb H N, Brand A. 1990. Risk factors for clinical mastitis in herds with a low bulk milk somatic cell count. 1. Data and risk factors for all cases.Journal of Dairy Science,73, 3463–3471.
Schukken Y H, Günther J, Fitzpatrick J, Fontaine M C, Goetze L, Holst O, Leigh J, Petzl W, Schuberth H J, Sipka A,Smith D G E, Quesnell R, Watts J, Yancey R, Zerbe H,Gurjar A, Zadoks R N, Syefert H M, MPMRC (Members of the Pfizer Mastitis Research Consortium). 2011. Hostresponse patterns of intramammary infections in dairy cows.Veterinary Immunology and Immunopathology,144,270–289.
Schukken Y H, Leslie K E, Barnum D A, Mallard B A, Lumsden J H, Dick P C, Vessie G H, Kehrli M E. 1999. ExperimentalStaphylococcus aureusintramammary challenge in late lactation dairy cows: Quarter and cow effects determining the probability of infection.Journal of Dairy Science,82,2393–2401.
Sieber R L, Farnsworth R J. 1981. Prevalence of chronic teat end lesions and their relationship to intramammary infection in 22 herds of dairy cattle.Journal of the American Veterinary Association,178, 1263–1267.
Smith K L, Todhunter D A, Schoenberger P S. 1985.Environmental pathogens and intramammary infections during the dry period.Journal of Dairy Science,68,402–417.
van Soest F J S, Santman-Berends I M G A, Lam T J G M, Hogeveen H. 2016. Failure and preventive costs of mastitis on Dutch dairy farms.Journal of Dairy Science,99, 8365–8374.
Sol J, Sampimon O C, Snoep J J, Schukken Y H. 1997. Factors associated with bacteriological cure during lactation after therapy for subclinical mastitis caused byStaphylococcus aureus.Journal of Dairy Science,80, 2803–2808.
Sordillo L. 2013. Nutritional strategies to optimize dairy cattle immunity.Journal of Dairy Science,99, 4967–4982.
Sorter D E, Koster H J, Hogan J S. 2014. Short communication:Bacterial counts in recycled manure solids bedding replaced daily or deep packed in freestalls.Journal of Dairy Science,97, 2965–2968.
Spears J W, Weiss W P. 2008. Role of antioxidants and trace elements in health and immunity of transition dairy cows.Veterinary Journal,176, 70–76.
Steeneveld W, Hogeveen H, Barkema H W, van den Broek J, Huirne R B. 2008. The influence of cow factors on the incidence of clinical mastitis in dairy cows.Journal of Dairy Science,91, 1391–1402.
Stevens M, Piepers S, Supré K, Dewulf J, De Vliegher S. 2016.Quantification of antimicrobial consumption in adult cattle on dairy herds in Flanders, Belgium, and associations with udder health, milk quality, and production performance.Journal of Dairy Science,99, 2118–2130.
Suriyasathaporn W, Heuer C, Noordhuizen-Stassen E N,Schukken Y H. 2000a. Hyperketonemia and the impairment of udder defence: A review.Veterinary Research,31,397–412.
Suriyasathaporn W, Schukken Y H, Nielen M, Brand A. 2000b.Low somatic cell count: A risk factor for subsequent clinical mastitis in a dairy herd.Journal of Dairy Science,83,1248–1255.
Upton J, Penry J F, Rasmussen M D, Thompson P D,Reinemann D J. 2016. Effect of pulsation rest phase duration on teat end congestion.Journal of Dairy Science,99, 3958–3965.
Verbeke J, Piccart K, Piepers S, Van Poucke M, Peelman L,De Visscher A, De Vliegher S. 2015. Somatic cell count and milk neutrophil viability of dairy heifers with specific CXCR1 genotypes following experimental intramammary infection withStaphylococcus chromogenesoriginating from milk.The Veterinary Journal,204, 322–326.
Verbeke J, Piepers S, Supré K, De Vliegher S. 2014. Pathogenspecific incidence rate of clinical mastitis in Flemish dairy herds, severity, and association with herd hygiene.Journal of Dairy Science,97, 6926–6934.
De Visscher A, Piepers S, Haesebrouck F, De Vliegher S.2016. Intramammary infection with coagulase-negative staphylococci at parturition: Species-specific prevalence,risk factors, and effect on udder health.Journal of Dairy Science,99, 6457–6469.
De Vliegher S, Barkema H W, Stryhn H, Opsomer G, de Kruif A. 2004a. Impact of early lactation somatic cell count in heifers on somatic cell counts over the first lactation.Journal of Dairy Science,87, 3672–3682.
De Vliegher S, Fox L K, Piepers S, McDougall S, Barkema H W. 2012. Invited review: Mastitis in dairy heifers: Nature of the disease, potential impact, prevention, and control.Journal of Dairy Science,95,1025–1040.
De Vliegher S, Laevens H, Barkema H W, Dohoo I R, Stryhn H, Opsomer G, de Kruif A. 2004b. Management practices and heifer characteristics associated with early lactation somatic cell counts of dairy heifers in Belgium.Journal of Dairy Science,87, 937–947.
De Vliegher S, Laevens H, Devriese L A, Opsomer G, Leroy J L, Barkema H W, de Kruif A. 2003. Prepartum teat apex colonization withStaphylococcus chromogenesin dairy heifers is associated with low somatic cell count in early lactation.Veterinary Microbiology,92, 245–252.
De Vliegher S, Opsomer G, Vanrolleghem A, Devriese L A,Sampimon O C, Sol J, Barkema H W, Haesebrouck F, de Kruif A. 2004c.In vitrogrowth inhibition of major mastitis pathogens byStaphylococcus chromogenesoriginating from teat apices of dairy heifers.Veterinary Microbiology,101, 215–221.
Watters R D, Schuring N, Erb H N, Schukken Y H, Galton D M.2012. The effect of premilking udder preparation on Holstein cows milked 3 times daily.Journal of Dairy Science,95,1170–1176.
Wellnitz O, Baumert A, Saudenowa M, bruckmaier R M. 2010.Immune response of bovine milk somatic cells to endotoxin in healthy quarters with normal and very low cell counts.Journal of Dairy Research,77, 452–459.
van Werven T. 1999. The role of leukocytes in bovineEscherichia coliclinical mastitis. Ph D thesis, Utrecht University, The Netherlands.
Weiss D, Bruckmaier R M. 2005. Optimization of individual prestimulation in dairy cows.Journal of Dairy Science,88,137–147.
Weiss D, Weinfurtner M, Bruckmaier R M. 2004. Teat anatomy and its relationship with quarter and udder milk flow characteristics in dairy cows.Journal of Dairy Science,87,3280–3289.
Weiss W P, Hogan J S, Todhunter D A, Smith K L. 1997.Effect of vitamin E supplementation in diets with a low concentration of selenium on mammary gland health of dairy cows.Journal of Dairy Science,80, 1728–1737.
Youngerman S M, Saxton A M, Oliver S P, Pighetti G M. 2004.Association of CXCR2 polymorphisms with subclinical and clinical mastitis in dairy cattle.Journal of Dairy Science,87, 2442–2448.
Zadoks R N, Allore H G, Barkema H W, Sampimon O C,Wellenberg G J, Gröhn Y T, Schukken Y H. 2001. Cowand quarter-level risk factors forStreptococcus uberisandStaphylococcus aureusmastitis.Journal of Dairy Science,84, 2649–2663.
Zadoks R N, Gonzalez R N, Boor K J, Schukken Y H. 2004.Mastitis-causing streptococci are important contributors to bacterial counts in raw bulk tank milk.Journal of Food Protection,67, 2644–2650.
Zadoks R N, Schulte H F, Tikofsky-Garrison L L. 2005.Molecular tools enchance the value of bulk milk monitoring.In:Proceedings 44th Annual Meeting of the NMC. Orlando,FL, USA, NMC, Verona, WI, USA. pp. 86–93.
Zdanowicz M, Shelford J A, Tucker C B, Weary D M, von Keyserlingk M A G. 2004. Bacterial populations on teat ends of dairy cows housed in free stalls and bedded with either sand or sawdust.Journal of Dairy Science,87, 1694–1701.
Zoche-Golob V, Haverkamp H, Paduch J, Klocke D, Zinke C, Hoedemaker M, Heuwieser W, Krömker V. 2015.Longitudinal study of the effects of teat condition on the risk of new intramammary infections in dairy cows.Journal of Dairy Science,98, 910–917.
Zucali M, Reinemann D, Tamburini A, Bade R. 2008. Effects of liner compression on teat end hyperkeratosis. In:ASABE Annual International Meeting. Rhode Island Convention Center, Rhode Island, USA. p. 083798.
Zwertvaegher I, De Vliegher S, Verbist B, Van Nuffel A,Baert J, Van Weyenberg S. 2013. Short communication:Associations between teat dimensions and milking-induced changes in teat dimensions and quarter milk somatic cell counts in dairy cows.Journal of Dairy Science,96,1075–1080.
Zwertvaegher I, Van Weyenberg S, Piepers S, Baert J, De Vliegher S. 2012. Variance components of teat dimensions in dairy cows and associated factors.Journal of Dairy Science,95, 4978–4988.
 Journal of Integrative Agriculture2018年6期
Journal of Integrative Agriculture2018年6期
- Journal of Integrative Agriculture的其它文章
- Improve access to the EU market by identifying French consumer preference for fresh fruit from China
- Factors influencing hybrid maize farmers’ risk attitudes and their perceptions in Punjab Province, Pakistan
- Long-term grazing exclusion influences arbuscular mycorrhizal fungi and their association with vegetation in typical steppe of lnner Mongolia, China
- Soil microbial characteristics and yield response to partial substitution of chemical fertilizer with organic amendments in greenhouse vegetable production
- Reducing nitrogen fertilization of intensive kiwifruit orchards decreases nitrate accumulation in soil without compromising crop production
- Ultrastructure of the sensilla on antennae and mouthparts of larval and adult Plutella xylostella (Lepidoptera: Plutellidae)
