Effect of different concentration of fish oil in skim milk-egg yolk extenders on postthawed semen qualities of Kalang swamp buffalo bull
Abdul Malik, Jaelani A, Neni Widaningsih, Gt Khairun Ni’mah, Raviani, Sakiman, Sasongko N
1Department of Animal Science, Faculty of Agriculture, Islamic University of Kalimantan, Banjarmasin-South Kalimantan, Indonesia
2Office of Animal Husbandry District of Banjar, South Kalimantan Province, Indonesia
3Centre of Artifical Insemination, Banjarbaru, South Kalimantan Province, Indonesia
1. Introduction
Kalang swamp buffalo is a species of swamp buffalo, a native from Amuntai district, South Kalimantan province, Indonesia. Its pattern of maintenance differs from other types of swamp buffalo in the world. Everyday early in the morning, the buffalo go out of the cage(Kalang) to soak in the swamp for a day and then afternoon climb into the cage for rest. The population of Kalang swamp buffalo during five years has decreased, it is due to the number of cuts that is unbalanced with the development of the population and the low fertility[1]. One of the important factors to increase kalang swamp buffalo population is a estrus synchronization program trailed by artificial insemination (AI), because Kalang buffalo has unclear symptoms of estrus. While, an significant factor that determines the success of AI is the qualities of post-thawing semen that will affect conception and percentage of pregnancy[2].
Application of AI program in swamp buffaloes has been reported,but the results are not as good as in the application of AI in cows.The main cause of low AI success in swamp buffaloes is due to low quality of post-thawed sperm and low fertility level of female buffalo[3-7]. Furthermore, Andrabi[8] reported that there were many aspects that could affect the sperm motility, integrity of membrane and sperm viability through storage including cryopreservation and thawing process.
The freezing process of semen needs a step-by-step process that begins with microscopic and macroscopic semen evaluation,dilution, cooling, equilibration and freezing weakens the naturally existing seminal antioxidant capacity. Consequently, many strategies have been employed to progress the efficiency of cryopreservation process. However, the overall success to increase the effectiveness of the cryopreservation of buffalo semen remains relative low. In this condition, the exogenous antioxidants addition in extender has provided a great opportunity to increase sperm quality to struggle the oxidative stress during cryopreservation[9].
One of the ingredients that can be used to help increase the sperm quality of buffalo post-thawing is fish oil. Fish oil contains several active ingredients such as omega 3 which is the main source of eicosapentaenoic acid, docosahexaenoic acid, saturated fatty acid and polyunsaturated fatty acids (PUFA)[10,11]. Several researches have been done on the effects of using fish oil to increase the semen quality either through feed or mixed with diluents in domestic animals. Meanwhile, the use of fish oil containing saturated fatty acids has been stated to be used to increase spermatozoa survival in general, membrane integrity and motility in boar[12] and cattle[13,14].However, the influence of fish oil on Kalang buffalo spermatozoa has never been conducted. Therefore, the objective of the present study was carried out to determine the influence of fish oil supplementation in the extender on the sperm motility, viability,abnormality and integrity of membrane of cryopreserved buffalo bull sperm.
2. Materials and methods
The study was conducted at the center of artificial insemination,province of South Kalimantan at Banjarbaru, Indonesia. The approval of the animal ethics committee on the conduct of this study was not necessary because there no invasive techniques on animals.Semen was used in the process of collection, evaluation and freezing by using standard method under the central artificial insemination program.
A total of 4 Kalang swamp buffalo bulls aged 3-5 years and weighed about 340-360 kg were used in the study. All bulls were kept in individual cage with the same feed. Forage was given to livestock about 10% of body weight of buffalo bull. Concentrate containing approximately 16% crude protein and 2.6% crude fat was given 3 kg/buffalo/day to each buffalo bull and water ad libitum. Semen was regularly collected from all bulls once a week by an artificial vagina.A total of 24 samples were poised and then transferred at 37 ℃ in water bath to the center of artificial insemination of Banjarbaru for evaluation.
2.1. Semen handling
The extender base used comprises of skim milk and egg yolk,containing 10% skim mlik, 5% (v/v) egg yolk, 1% (w/v) fructose(Scharlau, Barcelona, Spain), 8% (v/v) glycerol (Merck, Darmstadt,Germany), penicillin (1 000 IU/mL) and streptomycin (1 000 mg/mL).The samples were designed to compare the effect of different concentrations of fish oil (liquid) (Manufacture Pty, Ltd. Minna Close Belrose, Australia) with the dose of 0 mg (control), 50 mg,100 mg, 150 mg or 200 mg in the 100 mL skim milk-egg yolk extender. Semen was diluted with final sperm concentration 25×106/mL. Diluted semen was gradually cooled to 4 ℃ for 2 h.After equilibration, cooled semen was wrapped in 0.5 mL French straws, then placed on top of liquid nitrogen vapor (about 5 cm above liquid nitrogen) for 10 min. The straws were then dipped continuously and stored into liquid nitrogen. Later one week of kept in liquid nitrogen, five semen straws from each treatment group in each replicate were melted at 37 ℃ for 30 s and analyzed for the following parameters.
2.2. Evaluation of sperm qualities
Motility, viability, and abnormality parameters was measured on fresh semen and post-thawing semen. Motility of sperm was evaluated when a small droplet (10 µL) of fresh, pre-freezing sperm and post-thawing sperm was placed in the center of a pre-warmed slide and then covered with cover slip, and observed under phase contrast microscope at 400××magnification. Morphology of sperm and viability were observed by using eosin-nigrosin stain as adopted by Khumran et al[15]. At least of 200 sperms were counted in four microscopic diverse fields adopted by Memon et al[16]. Morpholog of sperm was observed using the same slide used for sperm viability.The normal of sperm cells was counted from a total sperm cells examined. The sperm integrity of membrane was assessed in fresh,pre-freezing semen, and post-thawed semen. Integrity of membrane was evaluated using hypo osmotic swelling test as defined by Kaka at al[17].
2.3. Statistical analysis
The effects of difference concentration of fish oil in the extender on motility, viability, integrity of membrane, and abnormality were analyzed by one-way analysis of variance (ANOVA). The collected data were presented as mean ±SD, and were significant at P<0.05.
3. Results
Observation of fresh semen by macroscopic and microscopic evaluation showed the following results: the average volume was(2.90±0.09) mL, pH was 6.80±0.15 and semen concentration was 1.5×106/mL. The average percentages of fresh semen were(71.00±1.04)%, (78.00±0.81)%, (15.00±1.02)% and (73.00±0.93)%for sperm motility, viability, abnormality and integrity of membrane,respectively. The sperm motility of Kalang swamp buffalo bull semen stored for 4 h before freezing at dose of 150 mg fish oil was higher (P<0.05) than those at dose of 50 mg and 100 mg fish oil;however, the treatment with 200 mg fish oil caused a significant(P<0.05) reduction in motility (Table 1).
Significant (P<0.05) increases were detected in semen motility and viability of post frozen-thawed sperm at dose of 150 mg fish oil added, while other doses caused a decrease in semen motility and viability (Table 2).
Another parameter of the study was to determine of the integrity of membrane frozen-thawed. Based on the data on Table 2 in this study,fish oil had no significant effect on membrane integrity.
4. Discussion
AI programs in large livestock including Kalang swamp buffaloes generally use frozen semen. There are various benefits of using frozen semen such as easy transportation, kept in long term, and efficient use of bulls to increase the potential of gene. Success of the semen freezing program can faciliate the improvement of swamp buffaloes population, for sperm can be kept for a long time and can be used as superior genetic stock. Sperm motility is a significant parameter in assessing the quality of spermatozoa, as it will affect the success of fertilization. Therefore, it is required to increase the high motility in post-thawing semen.
In this study, the results revealed that addition of concentration 150 mg fish oil supplementation in extender remarkably enhanced sperm motility before freezing. This significant increment in postthawed motility at dose 150 mg fish oil supplementation may be related with the collective effect of chemical structure of fish oil.Thus, the presence of concentration in the extender before freezing has provided better protection to the sperm motility or viability against cryopreservation-induced damages.
Sperm qualities including motility can be protected by fish oil during freezing process, because it is related to the content of ingredients from fish oil such as eicosapentaenoic acid, docosahexaenoic acid, saturated fatty acid, and PUFA. Guthrie and Welch[18] explained that the freezing process of semen can cause physical damage and disfunction due to excessive generation of reactive oxygen species. Furthermore,Nair et al[19] and Andrabi[8] revealed that pre-dominance of PUFA in sperm plasma membrane instigates the lipid peroxidation process which in turn reduces the semen qualities including sperm viability and motility. While, the part content fish oil of PUFA,eicosapentaneoic acid and docosahexaneoic acid allegedly play a role in sperm resistance to cooling and freezing procedures and may be correlated with the type of long chain PUFA content[20].
While, the previous study[14] conducted on bovine semen revealed a significant progress in post-thawing viability and sperm motility with the addition of 100 mg fish oil in the 100 mL skim milk-eggyolk extender. That is because one of the constituents of fish oil is PUFA with long chains and has been found in the semen of different species including man, bull and ram. These fatty acids could increase the fluidity of the membrane of plasma which is then accountable for improved struggle of the sperm to conditions of cold[21]. The membrane of plasma is the central location of injury prompted by cryopreservation that issues phospholipids into the nearby medium through of cold shock and becomes transiently leaky because of lipid phase transitions[22].
In the study, as for the integrity of membrane of frozen-thawed semen, membrane integrity has not significant effect between control and all treatments after addition concentration of fish oilin the extender. These results are confirmed by previous reports by Abavisani et al[23] who revealed that the addition of PUFA straightly to extenders was not effective in protection of the sperm membrane.In conclusion, addition of 150 mg in 100 mL skim milk-egg yolk extender can give a good result to the sperm motility of post-thawed Kalang swamp buffalo semen.
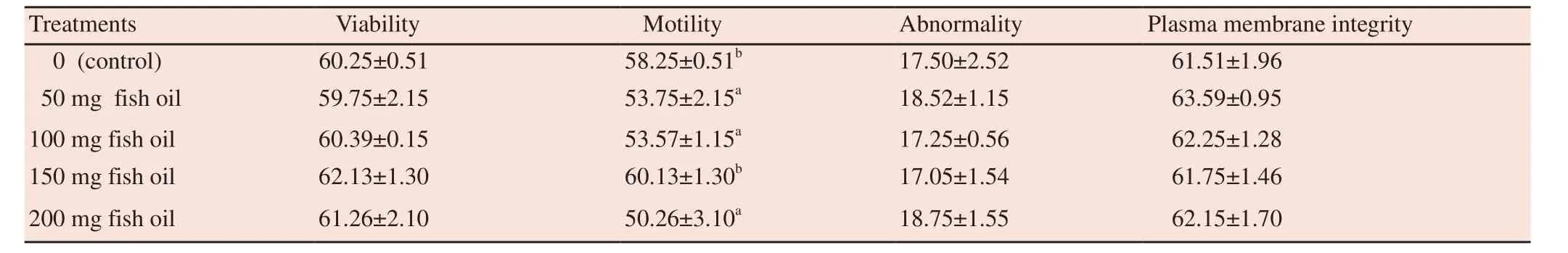
Table 1Effect of fish oil on viability, motility, abnormality and plasma membrane integrity before freezing in Kalang swamp buffalo bull semen.
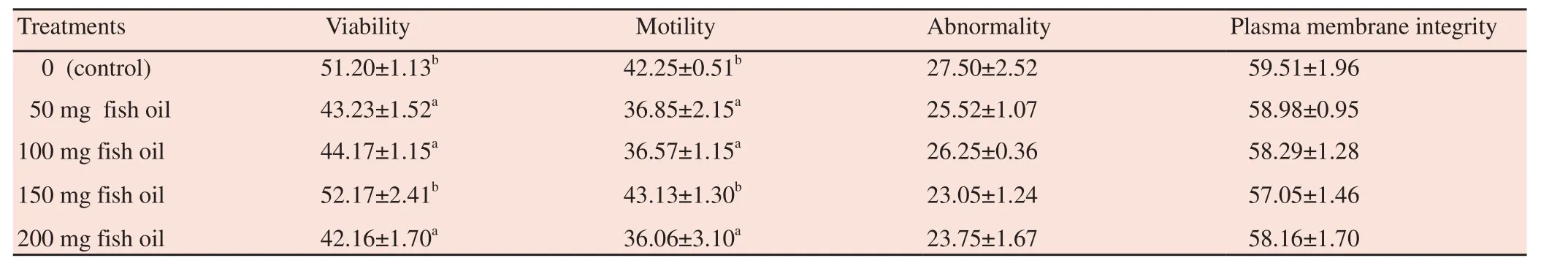
Table 2Effect of fish oil on viability, motility, abnorality and plasma membrane integrity of post-thawed Kalang swamp buffalo bull semen.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
We would like to thank the staff from the Central of Artificial Insemination of Banjarbaru, South Kalimantan Province, Indonesia.
[1] Triwidanningsih E, Praharani L. Buffaloes in Indonesia. Bogor, Indonesia:Research Institute for Animal Production; 2005, p.114-120.
[2] Malik A, Laily M, Muhammad IZ. Effects of long term storage of seemen in liquid nitrogen on the viability, motility and abnormality of frozen thawed Frisian Holstein bull spermatozoa. Asian Pac J Reprod 2015; 4(1):22-25.
[3] Andrabi MH, Ansari MS, Ullah N, Anwar M, Mehmood A, Akhter S. Duck egg yolk in extender improves the freezability of buffalobull spermatozoa. Anim Reprod Sci 2008; 104: 427-433.
[4] Qaisar S, Muhammad UM, Hamayun K, Asmaul H, Saima Q, Asima A,et al. Royal jelly supplementation in semen extender enhances post-thaw quality and fertility of Nili-Ravi buffalo bull sperm. Anim Reprod Sci 2016;167: 83-88.
[5] Pradeep K, Dharmendra K, Sikka P, Singh P. Sericin supplementation improves semen freezability of buffalo bulls by minimizing oxidative stress duringcryopreservation. Anim Reprod Sci 2015; 152: 26-31.
[6] Mohamed AE, Abd Elmonem M, Mamdouh H, Ashraf El, Magdy B,Abd Elraouf H, Ahmed B, Samy MZ. The effects of cholesterol loaded cyclodextrins on post-thawed quality of buffalo semen in relation to sperm DNA damage and ultrastructure. Reprod Biol 2017; 17: 42-50.
[7] Kumaresan A, Ansari MR, Abhishek G. Modulation of post-thawed sperm functions with oviductal proteins in buffaloes. Anim Reprod Sci 2005; 90:73-84.
[8] Andrabi SMH. Factors affecting the quality of cryopreserved buffalo(Bubalus bubalis) bull spermatozoa. Reprod Dom Anim 2009; 44: 552-569.
[9] Bansal AK, Bilaspuri GS. Impacts of oxidative stress andantioxidants on semen functions. Vet Med Int 2011; 6: 861-867.
[10] Stoeckel K, Nielsen LH, Fuhrmann H, Bachmann L. Fatty acid patterns of dog erythrocyte membranes after feeding of a fish-oil based DHA-rich supplement with a base diet low in n-3 fatty acids versus adiet containing added n-3 fatty acids. Acta Vet Scand 2011; 6: 53-57.
[11] Nichols PD, Glencross B, Petrie JR, Singh SP. Readily available sources of long-chain omega-3 oils: Is farmed Australian seafood a better source of the good oil than wild-caught seafood. Nutrients 2014; 6: 1063-1079.
[12] Hossain MS, Tareq K, Hammano KI, Tsujii H. Effect of fatty acids on boar sperm motility, viability and acrosome reaction. Reprod Med Biol 2007; 6(4): 235-239.
[13] Kiernan M, Fahey A, Fair S. The effect of the in vitro supplementation of exogenous long-chain fatty acids on bovine sperm cell function. Reprod Fertil Dev 2013; 25(6): 947-954.
[14] Malik A, Syarifdjaya M, Gunawan A, Erlina S, Jaelani A, Wibowo DB.Effect of fish oil addition to the skim milk-egg yolk extender on the quality of frozen-thawed Bali bull spermatozoa. Kafkas Univ Vet Fak Derg 2017; 23(4): 651-654.
[15] Khumran AM, Yimer N, Rosnina Y, Ariff MO, Wahid H, Kaka A, et al. Butylated hydroxytoluene can reduce oxidative stress and improve quality of frozen-thawed bull semen processed in lecithin and egg yolk based extenders. Anim Reprod Sci 2015; 163: 128-134.
[16] Memon AA, Wahid H, Rosnina Y, Goh YM, Ebrahimi M, Nadia F. Effect of antioxidants on post-thaw microscopic, oxidative stress parameter and fertility of Boer goat spermatozoa in Tris egg yolk glycerol extender.Anim Reprod Sci 2012; 136(1): 55-60.
[17] Kaka A, Wahid H, Rosnina Y, Yimer N, Khumran AM, Behan AA,Ebrahimi M. Alpha-linolenic acid supplementation in Tris extender can improve frozen-thawed bull semen quality. Reprod Domest Anim 2015;50(1): 29-33.
[18] Guthrie HD, Welch GR. Determination of intracellular reactiveoxygen species and high mitochondrial membrane potential inpercoll-treated viable boar sperm using fluorescence-activated flowcytometry. J Anim Sci 2006; 84: 2089-2100.
[19] Nair KS, RA Rizza, Brien PO. DHEA in elderly women and DHE or testosteron on elderly men. N Engl J Med 2006; 355: 1647-1659.
[20] Castellano CA, Audet I, Bailey JL, Laforest JP, Matte JJ. Dietary omega 3 fatty acids (fish oils) have limited effects on boar semen stored at 17 ℃ or cryopreserved. Theriogenology 2010; 74: 1482-1490.
[21] Wathes DC, Abayasekara DR, Aitken RJ. Polyunsaturated fatty acids in male and female reproduction. Biol Reprod 2007; 77(2): 190-201.
[22] Behera S, Harshan HM, Bhai KL, Ghosh KNA. Effect of cholesterol supplementation on cryosurvival of goat spermatozoa. Vet World 2015;8(12): 1386-1391.
[23] Abavisani A, Arshami J, Naserian AA, Sheikholeslami KMA, Azizzadeh M. Quality of bovine chilled or frozen-thawed semen after addition of omega 3 fatty acids supplementation to extender. Int J Fertil Steril 2013;7(3): 161-168.
 Asian Pacific Journal of Reproduction2018年3期
Asian Pacific Journal of Reproduction2018年3期
- Asian Pacific Journal of Reproduction的其它文章
- Missed estradiol determination resulting in oocyte retrieval and embryo development following controlled ovarian hyperstimulation at early pregnancy: Case report
- Role of preputial washing in reducing microbial load and improving bovine semen quality
- Prediction of protein structure of novel protein (116 kDa) from human sperm membrane
- Antiandrogenic activity of Calotropis procera latex in rats
- Improvement of cortical granules migration and in vitro embryo production of vitrified bovine oocyte by 9-cis retinoic acid
- Effect of alcohol infusion of Cissus populnea root on testicular function and serum hormone of male Wistar rats
