Effect of alcohol infusion of Cissus populnea root on testicular function and serum hormone of male Wistar rats
Tomilola D. Olaolu, Damilare E. Rotimi, Ayotunde P. Olaolu
1Biochemistry Unit, Department of Biological Sciences, College of Science and Engineering, Landmark University, PMB 1001, Omu-Aran, Kwara State, Nigeria
2Obstetrics and Gynaecology Unit, Federal Teaching Hospital, PMB 201, Ido-Ekiti, Ekiti State, Nigeria
1. Introduction
The testis is a pair of oval-shaped organs which are found in the scrotal sac and situated directly behind the penis, in front of the anus[1]. It is a part of the male reproductive organ that is responsible for the production of male gametes through spermatogenesis and the secretion of male sex hormones via steroidogenesis. The testis is made up of the tubular compartment (made up of seminiferous tubules, peritubular cells and sertoli cells) and the interstitial compartment (made up of the leydig cells, immune cells, nerves,fibroblasts, loose connective tissue, blood and lymph vessels).The seminiferous tubules are the site for spermatogenesis while the interstitial compartment is the site for steroidogenesis[2]. The hypothalamo-pituitary-testicular axis regulates the testicular functions, and this comprises pituitary gonadotropins which are the luteinizing hormone, follicle stimulating hormone[3].
Several common plants were reported for their ability to modulate male reproductive functions either through their fertility-enhancing properties or antispermatogenic and antisteroidogenic nature; some plants that have been used include Rauvolfia vomitoria[4], Lophira lanceolata[5], Xylopia aethiopica[6], and Bulbine natalensis[7].
Cissus populnea of Vitaceae/Amplidacea family is native to West Africa. It is locally known in Nigeria as ‘Okoho’ by the Idoma, Igala and Igbo tribes of Nigeria, ‘Ogbolo or Ajara’ by the Yorubas and‘Dafaaraa or Latutuwa’ by the Hausas[8]. Cissus populnea plant is a savannah shrub with a height of about 10 cm long and with 7.5 cm in diameter. Abundant watery sap emanates from its stem when cut,and it has creamy flowers with blackish-purple fruits when ripe. The roots are succulent in nature and could be used for building when dried[9]. The stem bark contains tannins, cyanogenic glycosides,anthraquinones, saponins, carbohydrates, cardiac glycosides and flavonoids[10]. Methanolic extract of Cissus populnea was reported to have increased proliferation of sertoli cells TM4 in in vitro studies[11].Cissus populnea is one of several herbs sold in public areas by herb sellers in Nigeria with claims that it enhances fertility and reproductive activities. This study aimed at examining the ethanol infusion effect of Cissus populnea root on some reproductive indices of male Wistar rats.
2. Materials and methods
2.1. Plant material
Cissus populnea root was purchased from herb seller at Olunlade market, Ilorin, Nigeria and authenticated at the Plant Biology Department, Faculty of Life Sciences, University of Ilorin, Ilorin,Nigeria. A voucher specimen with voucher number UILH/001/1019 was deposited at the herbarium. The herb prepared in the form of infusion by soaking the plant material in dry gin containing 43%ethanol was allowed to stand for 72 h. This procedure used was the same as that of the local herb sellers on the preparation of the sample.
2.2. Experimental setup
Twenty albino male Wistar rats of Rattus norvegicus strain with weights [(100.0 ±25.5) g] from the Animal Holding Unit, Biological Sciences Department, Landmark University, Omu-Aran, Nigeria.The rats were kept in clean cages with access to tap water and rat pellets after their daily doses throughout the experimental period.The animals were allowed to acclimatize for 14 days before the start of the experiment.
The experimental rats were selected into 4 different groups containing 5 animals in each group. These animals were administered 0.20 mL of distilled water, 0.05 mL, 0.10 mL, and 0.20 mL/kg body weight of ethanol infusion in each group, respectively. Administration was done by using oropharyngeal cannula orally once daily for 14 days between 09:00-09:45 am. During the experimental setup, the animals’ weights were taken twice a week and recorded. The animals were then sacrificed on day 15, their blood samples collected, testis excised and homogenates obtained.
Assay kits were used for determining some of the parameters;Products of Agappe Diagnostics Ltd, Switzerland were used for the determination of cholesterol while the hormonal assay kits[testosterone, follicle stimulating hormone (FSH) and luteinizing hormones (LH)] were products of Monobind Inc. Lake Forest USA.The concentrations of these parameters in the serum were evaluated using the method described by the manufacturer in the instruction manual.
Other parameters assayed for in the testicular supernatant were total protein concentration described by Gornall et al[12], total cholesterol based on the reaction outlined by Fredrickson et al[13], glycogen[14]and sialic acid[15]. Warren[16] and Wright et al[17] described the procedures for the assays to evaluate the activities of alkaline phosphatase (ALP) and acid phosphatase (ACP).
2.3. Statistical analysis
All data obtained from this study were presented as mean ±standard error of mean. They were analysed using a one-way analysis of variance (ANOVA) with multiple comparisons. This was followed by the post-hoc Tukey HSD and values at P<0.05 were considered statistically significant.
2.4. Ethics
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
3. Results
3.1. Effect of ethanol infusion of Cissus populnea root on testes-body weight
The testes-body weight were 1.38%, 1.32% and 1.31% at the various doses of 0.05, 0.10, 0.20 mL/kg body weight, respectively while the control value was 1.10%. The administration of ethanol infusion of Cissus populnea root increased significantly (P < 0.05) the testes-body weight ratio across all treated groups compared with the control.
3.2. Effect of ethanol infusion of Cissus populnea root on serum testosterone concentration
The serum testosterone levels were (2.70±0.14), (1.90±0.07) and(1.40±0.14) ng/mL at the various doses of 0.05, 0.10, 0.20 mL/kg body weight of infusion administered, respectively when compared with the control value of (1.00±0.65) ng/mL. The highest increase observed was detected in the animals that received infusion concentration of 0.05 mL/kg body weight while 0.20 mL/kg body weight of ethanol infusion had the lowest value. The serum testosterone level of the rats treated with ethanol infusion of Cissus populnea root was observed to increase significantly (P < 0.05); this increase was not observed to be significant in animals that were administered 0.20 mL/kg body weight of the infusion.
3.3. Effect of ethanol infusion of Cissus populnea root on serum LH concentration
LH levels were (3.50±0.21), (2.30±0.14) and (1.90±0.28) mlu/mL at the various doses administered when compared with the control which was (1.30±0.07) mlu/mL. The highest increase was detected in the animals that received infusion concentration of 0.05 mL/kg body weight which was 3.50 mlu/mL, 0.10 mL/kg body weight of ethanol infusion had 2.30 mlu/mL as the observed value while 0.20 mL/kg body weight of ethanol infusion had the lowest value which was observed to be 1.90 mlu/mL. When compared with the control, the level of the serum LH increase significantly (P < 0.05)but this showed a counter dose-dependent manner.
3.4. Effect of ethanol infusion of Cissus populnea root on serum FSH concentration
FSH levels were increased to (10.60±0.42), (9.20±0.28) and(4.25±0.25) mlu/mL respectively at the various doses administered when compared with the control which was (3.50±0.35) mlu/mL.The highest increase observed for the serum FSH concentration was detected in the animals that received infusion concentration of 0.05 mL/kg body weight which was observed to be 10.6 mlu/mL,0.1 mL/kg body weight of ethanol infusion had 9.2 mlu/mL while 0.2 mL/kg body weight of ethanol infusion had the lowest value observed to be 6.75 mlu/mL. A significant increase (P < 0.05) was observed in the FSH level in the animals administered with 0.05 and 0.10 mL/kg body weight of infusion when compared with the control. However, a non-significant increase was noted in animals that given 0.20 mL/kg body weight of the ethanol infusion of Cissus populnea root was not significant.
3.5. Effect of ethanol infusion of Cissus populnea root on testicular protein
Total protein levels were (78.72±2.63), (66.13±1.51) and(59.77±1.25) mg/mL respectively at the various doses administered when compared with the control which was (51.58±1.82) mg/mL.The highest increase observed was detected in the animals that received infusion concentration of 0.05 mL/kg; 0.10 mL/kg body weight of ethanol infusion had 66.13 mg/mL while 0.20 mL/kg body weight of ethanol infusion had the lowest values observed to be 59.77 mg/mL. It increased significantly (P < 0.05) the testicular protein level when compared with the control in a counter-dose dependent order; this increase was however not significant in the animals treated with 0.20 mL/kg body weight.
3.6. Effect of ethanol infusion of Cissus populnea root on ACP activity
ACP levels were (6.16±0.10), (12.41±0.29) and (7.65±0.46) nM/min/mg protein respectively at the various doses administered when compared with the control which was (5.64±0.45) nM/min/mg protein. The highest increase observed was detected in the animals that received infusion concentration of 0.10 mL/kg body weight which was observed to be 12.41 nM/min/mg protein; 0.20 mL/kg body weight of ethanol infusion had 7.65 nM/min/mg protein while 0.05 mL/kg body weight of ethanol infusion had the lowest value which was 6.16 nM/min/mg protein. The level of testicular ACP was observed to increase significantly (P < 0.05) except 0.05 mL/kg body weight of ethanol infusion.
3.7. Effect of ethanol infusion of Cissus populnea root on ALP activity
ALP activity levels were (9.58±0.41), (4.06±0.31) and (6.28±0.19)nM/min/mg protein respectively at the various doses administered from control value of 2.59 nM/min/mg protein. The highest increase observed was detected in animals that received infusion concentration of 0.05 mL/kg body weight which was 9.58 nM/min/mg protein; animals that were administered 0.1 mL/kg body weight had 4.06 nM/min/mg protein which was the lowest observed value, while animals that received 0.20 mL/kg body weight had 6.28 nM/min/mg protein.There was an increase significantly (P < 0.05) when compared with the control.
3.8. Effect of ethanol infusion of Cissus populnea root on testicular glycogen
Testicular glycogen levels were (7.88±0.62), (6.67±0.47) and(3.81±0.57) mg/100 mg glucose at 0.05, 0.10 and 0.20 mL/kg body weight respectively when compared with the control which was(3.13±0.12) mg/100 mg glucose. The highest increase observed was detected in animals that received infusion concentration of 0.05 mL/kg body weight while the 0.20 mL/kg body weight had the lowest value.There was a significant increase (P < 0.05) across all treated groups except the 0.20 mL/kg body weight of the ethanol infusion of Cissus populnea root.
3.9. Effect of ethanol infusion of Cissus populnea root on testicular cholesterol
Testicular cholesterol levels were (2.29±0.21), (2.63±0.45) and(1.21±0.15) mmol/L at the various doses administered when compared with the control which was (0.77±0.01) mmol/L. The highest increase observed was detected in the animals that received infusion concentration of 0.10 mL/kg body weight which was 2.63 mmol/L, the 0.05 ml/kg body weight of ethanol infusion had 2.29 mmol/L, while the 0.20 mL/kg body weight of ethanol infusion had the lowest value which was observed to be 1.21 mmol/L. The effect of ethanol infusion of Cissus populnea root when compared with the control showed a significant increase in testicular cholesterol level.Figure 1 displayed the photomicrographs of the testes of rats administered with ethanol infusion of Cissus populnea root. The testes of control rats (Wistar rats that received distilled water only)displayed grossly normal tissue; testicular tissues of Wistar rats administered with 0.05 mL/kg infusion showed fairly distorted tissue with slight atrophy; experimental animals that received 0.10 mL/kg infusion indicated slightly distorted testicular tissue while Wistar rats that received 0.20 mL/kg infusion showed slightly hypertrophic testicular tissue.
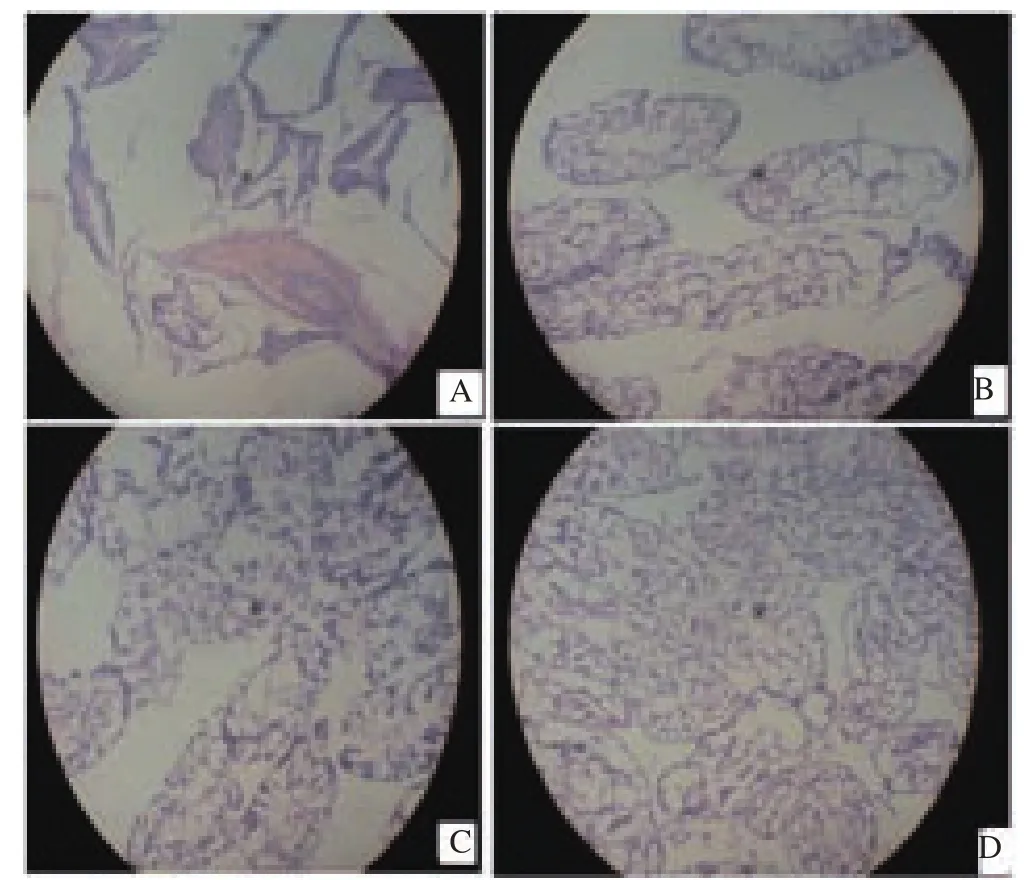
Figure 1. Micrographs of testes of male Wistar rats administered with aqueous infusion of Cissus populnea root.
4. Discussion
This present study displays in the experimental rats an overall elevation in the testes-body weight ratio after 14-day oral administration of the ethanol infusion of Cissus populnea root. This significant increase observed in the testes-body weight may be as a result of increased synthesis of testosterone and androgen since testes is an androgendependent organ[18,19]. The study also reveals a direct relationship between testosterone level and testes-body weight. This finding corroborates the observation of Morakinyo et al[20].
Also, it was noted that the administration of the ethanol infusion of Cissus populnea root to experimental animals for 14 days increased significantly the serum testosterone concentration compared with the control. Testosterone secretion is stimulated by LH and its role is to enhance growth and secretory activity of the testes[21]. The increase in testosterone level observed in this study corroborates those of Etuk and Muhammad[5], where they worked on the fertility enhancing effects of aqueous stem bark extract of Lophira lanceolata in male Sprague dawley rats; however, this result contradicts the result of Morakinyo et al[22], where there was an observed reduction in the level of testosterone in arsenite-treated rats.
Administration of ethanol infusion of Cissus populnea root in this study to experimental animals increased significantly the level of serum LH. LH is responsible for stimulating testosterone production in leydig cells which subsequently stimulates spermatogenesis by acting on the sertoli cells and peritubular cells of the seminiferous tubules[21]. The result observed in this current study however refutes those of Yakubu[19], where significant decrease in the level of serum LH level was detected after a 60-day oral administration of a crude alkaloid extract from Chromolaena odorata leaves to male rats in his study on spermatogenic and hormonal indices of male rats.
In addition, the FSH level was observed in this study to increase in the experimental animals administered with the infusion. FSH facilitates the testosterone passage via sertoli-sertoli junctional complexes by acting on sertoli cells, leading to the androgen-binding protein production[3]. This result is in agreement with the findings of Akdogan et al[23]. In their report, on the effects of peppermint tea on plasma testosterone, LH and FSH in rats, there was an increase in plasma FSH level during their study.
Following the 14-day administration of ethanol infusion of Cissus populnea root to experimental animals, testicular ACP level was observed to increase significantly. ACP is an enzyme in an acidic medium which hydrolysis orthophosphoric acid esters[24]. Its gene is up-regulated and down-regulated by androgens and estrogens respectively[25]. The result of this current study however disagrees with those of El-kashoury[26], where there was significant decrease in testicular ACP level previously administered with profenofos.
An elevation in level of ALP was noted in this present study following a 14-day administration of ethanol infusion of Cissus populnea root. ALP has previously been associated with the secretion of gonadotropins and also intracellular and extracellular transportation of metabolites during steroidogenesis[19,27]. The result of this current study is in contrast with Sadik[28], where there was significant reduction in ALP level following the administration of cadmium to adult male wistar rats for 4 weeks while investigating the effect of zinc and diallyl sulphide on testicular steroidogenesis in cadmium-treated male rats.
There was significant increase in testicular protein level after the administration of ethanol infusion of Cissus populnea root to male rats for 14 days. This may be as a result of the stimulation of growth proteins and ribonucleic acid synthesis[29,24]. The increase in testicular total protein observed agrees with the result of Joshi et al[30], where testicular protein content in rats was observed to increase significantly following their 30-day exposure to chlorpyrifos.
Testicular glycogen level was observed to increase significantly in this study after the administration of the infusion. Testicular glycogen has been associated with a supply of energy for testicular cells and tissue, and an increase in glycogen level may also enhance synthesis of protein in spermatogenic cells due to its dependence on glucose for energy supply[19]. This observation corroborates with the findings of the testicular protein level from this current study. The observation in this study however negates the report of Yakubu et al[31].
The level of testicular cholesterol increased significantly following the 14-day administration of the infusion. Cholesterol is the main precursor of steroid hormones like testosterone and it is also associated with normal testicular function[32]. The significant increase in testicular cholesterol level observed may imply increased testicular function in the male rats. This result is in line with those of Yakubu and Afolayan[33], where there was significant increase in testicular cholesterol level of male wistar rats administered with aqueous extract of Bulbine natalensis stem as a result of enhanced anabolic and androgenic activities of the plant.
From this current study, it was observed that administration of ethanol infusion of Cissus populnea root to male Wistar rats for 14 days at 0.05 mL/kg body weight of the infusion increased significantly the concentration of serum testosterone, luteinising and FSH, as well as testicular ALP , protein, glycogen and cholesterol.However, there was gradual reduction in the level of these testicular function indices overall which may be as a result of an increase in ethanol which has previously been reported to be detrimental to male reproductive health[34,35]. Therefore, there is a need to be cautious about the intake of ethanol infusion of Cissus populnea root when the dosage exceeds 0.05 mL/kg body weight.
The result of the histopathology from this study shows a gradual deterioration of the testicular tissue from slight distortion to hypertrophy in animals administered with ethanol infusion of Cissus populnea root after 14 days oral administration. This may be as a result of the ethanol used as the solvent for preparing this infusion as ethanol has adverse effect on the reproductive system[34,35].
The ethanol infusion of Cissus populnea root at 0.05 mL/kg body weight administered to experimental animals in this current study resulted in increased level of testicular function indices and serum hormones of the animals after 14 days oral administration. This implies that Cissus populnea root at this dosage has the potential to boost the secretion of testosterone, the gonadotropins as well as other testicular function indices. However, there may a need to be cautious about dosages exceeding this as concentrations of ethanol infusion of Cissus populnea higher than 0.05 mL/kg body weight was found to gradually deplete the level of reproductive indices assayed for in this current study.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The authors would like to appreciate Landmark University for the Laboratory facilities provided for this research. The authors also would like to thank the chief technologist Mr. MA Ajibade of Biochemistry Laboratory, Landmark University for the technical assistance rendered during the course of this research.
[1] Murdakai T, Buraimoh AA, Kwanashie HO. Histological observations of the testis of Wister rats following the oral administration of cotecxin(dihydroartemisinin). Int J Anim Vet Adv 2011; 3(5): 402-406.
[2] Weinbauer GF, Luetjens CM, Simoni M, Nieschlag E. Andrologymale reproductive health and dysfunction. In: Nieschlag E, Behre HM,Nieschlag S, editors. Physiology of testicular function. Berlin: Springer-Verlag; 2010, p. 11-60.
[3] D’Cruz SC, Vaithinathan S, Jubendradass R, Mathur PP. Effects of plants and plants products on the testis. Asian J Androl 2010; 12: 466-479.
[4] Lembe DM, Koloko BL, Bend EF, Domkam J, Oundoum PC, Njila MN,et al. Fertility enhancing effects of aqueous extract of Rauvolfia vomitoria on reproductive functions of male rats. J Exp Integr Med 2014; 4(1): 43-49.
[5] Etuk EU, Muhammad AA. Fertility enhancing effects of aqueous stem bark extract of Lophira lanceolata in male Sprague Dawley rats. Int J Plant Physiol Biochem 2009; 1(1): 001-004.
[6] Woode E, Alhassan A, Abaidoo CS. Effect of ethanolic fruit extract of Xylopia aethiopica on reproductive function of male rats. Int J Pharm Biomed Res 2011; 2(3): 161-165.
[7] Yakubu MT, Afolayan AJ. Effect of aqueous extract of Bulbine natalensis(Baker) stem on the sexual behaviour of male rats. Int J Androl 2009;32(6): 629-636.
[8] Burkill HM. The useful plants of West Tropical Africa. Richmond, United Kingdom: Royal Botanic Gardens, Kew; 2000, p. 296-297.
[9] Irvine FR. Woody plants of Ghana. London: Oxford University Press;1961, p. 486-487.
[10] Moody JO, Ojo OO, Deyemo AA, Olumese PE, Ogundipe OO. Antisickling potential of a Nigerian herbal formula (ajawaron HF) and the major plant component (Cissus populnea L. CPK). J Med Plant 2003;17(10): 1173-1176.
[11] Osibote E, Noah N, Sadik O, McGee D, Ogunlesi M. Electrochemical sensors, MTT and immunofluorescence assays for monitoring the proliferation effects of Cissus populnea extracts on Sertoli cells. J Endocrinol 2011; 9: 65.
[12] Gornall AC, Bardawill CJ, & David MM. Determination of serum protein by means of biuret reaction. J Biol Chem 1949; 177: 751-756.
[13] Fredrickson DS, Levy RI, Lees R.S. Fat transport in lipoproteins-An integrated approach to mechanisms and disorders. N Engl J Med 1967;276:148-156.
[14] Kemp A, Adrienne J, Heijningen K. A colorimetric micro-method for the determination of glycogen in tissues. Biochem J 1954, 56: 646-648.
[15] Warren L. The thiobarbituric acid assay of sialic acids. J Biol Chem 1959,234: 1971-1975.
[16] Wright PJ, Leathwood PD, Plummer DT. Enzymes in rat urine: Alkaline phosphatase. Enzymologia 1972, 42: 317-327.
[17] Wright PJ, Leathwood PD, Plummer DT. Enzymes in rat urine: Acid phosphatase. Enzymologia 1972, 42: 459-462.
[18] Klinefelter GR, Hess RA. Toxicology of the male excurrent ducts and accessory sex glands. In: Korach KS, editor. Reproduction and developmental toxicology. New York: Marcel Dekker Inc.; 1998, p. 553-591.
[19] Yakubu MT. Effect of a 60-day oral gavage of a crude alkaloid extract from Chromolaena odorata leaves on hormonal and spermatogenic indices of male rats. J Androl 2012; 33(6): 1199-1207.
[20] Morakinyo AO, Adeniyi OS, Arikawe AP. Effects of Zingiber officinale on reproductive functions in the male rat. African J Biom Res 2008; 11:329-334.
[21] Parandin R, Yousof N, Ghorbani R. The enhancing effects of alcoholic extract of Nigella sativa seed on fertility potential, plasma gonadotropins and testosterone in male rats. Iran J Reprod Med 2012; 10(4): 355-362.
[22] Morakinyo AO, Achema PU, Adegoke OA. Effect of Zingiber officinale(ginger) on sodium arsenite-induced reproductive toxicity in male rats.Afr J Biom Res 2010; 13: 39-45.
[23] Akdogan M, Ozguner M, Kocak A, Oncu M, Cicek E. Effects of peppermint teas on plasma testosterone, follicle stimulating hormone,and luteinizing hormone levels and testicular tissue in rats. Urology 2004;64: 394-398.
[24] Dutta AL, Sahu CR. Emblica officinalis Garten fruits extract ameliorates reproductive injury and oxidative testicular toxicity induced by chlorpyrifos in male rats. Springer Plus 2013; 2: 541.
[25] Yousef GM, Diamandis M, Jung K, Eleftherios P. Gene that is highly expressed in the testes. Genomics 2001; 74(3): 385-395.
[26] EL-Kashoury AA. Influence of subchronic exposure of profenofos on biochemical markers and microelements in testicular tissue of rats. Mars Land Press J Am 2009; 5(1): 19-28.
[27] Latchoumycandane C, Gupta SK, Mathur PP. Inhibitory effects of hypothyroidism on the testicular functions of postnatal rats. Biomed Lett 1997; 56: 171-177.
[28] Sadik NAH. Effects of diallyl sulphide and zinc on testicular steroidogenesis in cadmium-treated male rats. J Biochem Mol Toxicol 2008; 22(5): 345-353.
[29] Puga FR, Rudrigues FR, Lark MA. Effect of dimethoate and propoxur on the metabolism of IB-RS2 cells and their susceptibility to foot and mouth disease. Sau Paulo, Brazil: Arquivos do Institu Biologico; 1974, p. 141-144.
[30] Joshi SC, Mathur R, Gulati N. Testicular toxicity of chlorpyrifos (an organophosphate pesticide) in albino rat. Toxicol Ind Health 2007; 23:439-444.
[31] Yakubu MT, Akanji MA, Oladiji AT. Evaluation of antiandrogenic potential of aqueous extract of Chromolaena odoratum (L.) K. R. leaves in male rats. Andrologia 2007; 39: 235-243.
[32] Nurudeen QO, Ajiboye TO. Aqueous root extract of Lecaniodiscus cupanioides restores the alterations in testicular parameters of sexually impaired male rats. Asian Pac J Reprod 2012; 1(2): 120-124.
[33] Yakubu MT, Afolayan AJ. Anabolic and androgenic activities of Bulbine natalensis stem in male Wistar rats. Pharm Biol 2010; 48(5): 568-576.
[34] Emmanuele MA, Emmanuele NV. Alcohol’s effects on male reproduction. Alcohol Health Res World 1998; 22(3): 195-201.
[35] La Vignera S, Condorelli RA, Balercia G, Vicari E, Calogero A. Does Alcohol have any effect on male reproductive function? A review of literature. Asian J Androl 2013; 15(2): 221-222.
 Asian Pacific Journal of Reproduction2018年3期
Asian Pacific Journal of Reproduction2018年3期
- Asian Pacific Journal of Reproduction的其它文章
- Missed estradiol determination resulting in oocyte retrieval and embryo development following controlled ovarian hyperstimulation at early pregnancy: Case report
- Role of preputial washing in reducing microbial load and improving bovine semen quality
- Effect of different concentration of fish oil in skim milk-egg yolk extenders on postthawed semen qualities of Kalang swamp buffalo bull
- Prediction of protein structure of novel protein (116 kDa) from human sperm membrane
- Antiandrogenic activity of Calotropis procera latex in rats
- Improvement of cortical granules migration and in vitro embryo production of vitrified bovine oocyte by 9-cis retinoic acid
