Antiandrogenic activity of Calotropis procera latex in rats
Abdelgader Binyamen Abdelgader, Adil Salim Elsheikh
1College of Graduate Studies, University of Gezira, Sudan
2Department of Physiology, Faculty of Medicine, Najran University, Saudi Arabia
3Department of Applied Medical Sciences, Community College, Najran University, Saudi Arabia
1. Introduction
Herbal plants and treatment with herbs are worldwide popular as alternative for allopathic medicines and occupy a distinct position right from the primitive period to present day because of the expected serious side effects and the high cost of allopathic medicines. The Calotropis procera which belongs to the family Asclepiadaceae is well known for its traditional medicinal uses.This plant has many local names such as Calotropis procera, Sodom apple, Akund crown flower and Dead Sea fruit. Studies on the plant have revealed enormous diversity of medicinal uses in case of fevers, rheumatism, indigestion, cough, cold, eczema, asthma,elephantiasis, nausea, vomiting and diarrhea. The plant is used either as a whole, part of it or mixed with other plants’ materials to enhance the efficacy[1]. Calotropis procera produces various chemicals that are useful for various activities[2,3]. The plant contains alkaloids, sterols, flavonoids, cardiac glycosides, triterpenoids and usharin[4]. This plant has been advocated to cure varieties of diseases: leprosy, ulcers tumors, and piles[4]. Different extracts of the leaves of Calotropis procera have been claimed for diverse biological activities such as antidiarrheal[5], antioxidant[6-8], antipyretic[9],analgesic[10], antimicrobial[6,11,12], spasmolytic[13], schizonticidal[13],cytotoxic[14,15], hepatoprotective[16], hypoglycemic[17], anthelmintic[18],laxative, purgative, anti-inflammatory, diuretic[19] and abortifacient[20].The different parts of Calotropis procera and its latex are known to have antibacterial and wound healing properties[21,22] and anticancer,antifungal and insecticidal activity[23,24]. Although Caltropis procera has these favorable properties, the plant has been accused of varying toxic effects in animals[25-27]. Despite these claimed toxic effects,there are some trials to use the plant as feed stuff for fatting of sheep[28]. In Sudan, meat and milk producing animals especially the small ruminants as well as cattle are fed on dried leaves of Calotropis procera during drought and/or pasture scarcity. Thereafter,the human being is fed on animal products that presumably contain the plant’s residues. This situation necessitates that the effects of the plant on all physiological processes in animals must be investigated.Many toxicological studies on water and ethanolic extracts of the plant were conducted to elucidate its effects on mature mammals.However, researches on the effects Calotropis procera on immature animals are sparse or even absent, and if present they only investigated its toxicity. Therefore, it is necessary to conduct studies on Calotropis procera before using it as medicine or feed stuff. Thus,the current study was carried out to elucidate the possible positive and negative effects of Calotropis procera latex on pubertal traits of growing male Wistar rats.
2. Materials and methods
2.1. Experimental animals
Thirty males Wistar rats were kept with their dams, weaned at the age of 3 weeks and grouped into 5 groups, with 6 rats in each group.Each group was kept in a separate cage at 20 ℃-25 ℃ on a 12-hour light-dark cycle and offered commercial pellets and water ad libitum.
2.2. Preparation of Calotropis procera latex and calculating dose
Fresh latex of Calotropis procera was collected from the aerial parts of wildly growing plants in Najran city- Saudi Arabia. Thereafter, the latex was dried under a shade at ambient temperature and ground to small granules. Five milligrams of the dried latex were triturated in 10 mL distilled water (0.5 mg/mL) to obtain an aqueous suspension.This aqueous suspension was kept as stock solution. The dose of the aqueous suspension was calculated according to the equation of Nair & Jacob 2016. According to this equation the dose is calculated as follow:
V (mL)= [W (kg) )/ C (mg/mL)] ×D (mg/kg)
V: the volume of the dose (mL); W: the weight of the rat (kg);
C: concentration of the suspension (mg/mL); D: the dose (mg/kg).
2.3. Experimental design
This experiment is a one factorial design experiment to examine the effects of oral administration of Calotropis procera latex on pubertal traits of growing male Wistar rats. The pubertal traits tested were growth rate, pubertal body weight (BW), testicular and epididymae weights, semen properties, testicular histology and reproductive hormones. Three-week-old weaned 30 male Wistar rats were weighed and grouped randomly into 5 groups. Group A is the control group (n = 6); group B (n = 6); group C (n = 6); group D (n = 6) and the last group E (n = 6). A daily oral dose of the prepared suspension of Calotropis procera latex was given to the treatment rat groups (group B 5 mg/kg/day; group C 10 mg/kg/day; group D 15 mg/kg/day and group E 20 mg/kg/day). The control group (group A) was offered distilled water orally as placebo. The rats were weighed every day to adjust the dose and the treatment continued for 4 weeks. At the end of the treatment period pubertal BW was recorded and the weekly growth rate was calculated according to the following equation:
Growth rate % = (W2-W1)/ W1× 100
W1= body weight (g) at the previous week; W2= body weight (g) at the following week.
The blood samples collections, hormonal biochemical analysis,testicular weighing, tissues preparation and semen analysis were determined as following.
2.4. Blood samples collections, biochemical analysis,testicular weighing and tissues preparation
Then the rats were anesthetized with chloroform and sacrificed by cervical dislocation[30]. Blood samples were collected by cardiac puncture, left to coagulate at room temperature and then centrifuged at 3 000 rpm for 30 min to separate the serum. The sera were kept frozen at -30 ℃ till used for estimation of serum testosterone,follicle-stimulating hormone (FSH) and luteinizing hormone (LH).The enzyme-linked immuno-sorbent assay technique was used to estimate all hormones and commercially available enzyme-linked immuno-sorbent assay kit were purchased from Shanghai Crystal
Day Biotech Co., LTD. All hormones in question were determined as per the manufacturer’s instructions in the assay kits. The testes and epididymae were weighed and the relative testicular and epididymal weights were calculated as follows:
Relative organ weight (%)= [Absolute organ weight (g) / Body weight of rat on sacrifice day (g) ] ×100
The testes were fixed immediately with 10% formalin and then dehydrated through ascending grade of alcohol concentration started from 70%, 90% and 100%, respectively; subsequently all the tissues were cleared with xylene, then they were impregnated and embedded with paraffin wax. Sections of 5 µm thickness were cut by using an Accu Cut SRM Microtome form SAKURA, and then prepared using standard procedures for hematoxylin and eosin staining and examined under the light microscope.
2.5. Sperm counts, sperm motility and sperm morphology
Sperms of the right epididymae were counted by a modified method of Reeves and Rossow[31]. Briefly, the right cauda epididymis from each rat was weighed and minced with a scalpel blade in a petri dish which contains physiological saline solution(0.9% NaCl). The suspension was kept at 37 ℃ for 5 min to allow for the sperms to disperse in the medium. Thereafter, the sperm suspensions were gently mixed and sperm count was performed by hemocytometer under a Nikon microscope (Nikon Eclipse E600) at a final magnification of × 400 as described elsewhere[32]. The total number of sperms was calculated as per milliliter and converted as per 0.1 g weight. The percent of motile sperm was evaluated using the standard method mentioned elsewhere[33]. The cauda epididymae were squeezed with aid of 31-gauge needle into 2 mL of Tris buffer solution and were kept at 37 ℃. A slide was placed on phase contrast microscope and an aliquot of this solution was placed on the slide and percent of motile sperms was evaluated visually at a magnification of 400 ×. Motile sperms out of 200 sperms in each examined sperm suspension were counted and the means of 3 trails of the samples examined was used as the final motility score.
To determine the percentage of morphologically abnormal spermatozoa, the method described by Sonmez et al[34] was used.Briefly, a drop of Eosin stain was added to the sperm suspension,which was kept for 5 min, at 37 ℃. Then, a drop of sperm suspension was placed on a clean slide and gently spread to make a thin film. The film was air dried and the slides were then examined under a phase contrast microscope at 400 ×. A total of 200 sperm cells were examined on each slide and sperms with absent head,absent tail, bending tail, coiled tail, curved mid-piece, bending midpiece were scored. The abnormalities of spermatozoa were expressed as percentage.
2.6. Statistical analysis
Data were analyzed with analysis of variance (ANOVA) using SPSS-16.020 statistical package (Chicago, USA). The data were expressed as mean ± SD. The level of significance was set at P<0.05.
3. Results
3.1. Effects of Calotropis procera on gross pubertal traits
As from Figure 1, all the gross pubertal traits were significantly(P<0.05) reduced by increasing the latex concentration in the oral dose. Also, the growth was significantly (P<0.05) retarded by increasing the latex dose beyond 5 mg/kg BW (Figure 1 A). Figure 1B showed the means of body weights of the rats at the end of the treatment period in group B, C, D, E. The rats’ body weights were significantly (P<0.05) decreased, compared to that of the control.The means of testicular weights and epididymal weights (Figure 1 C) and the mean percentages of the relative testicular weights as well as the mean percentages of the relative epididymal weights (Figure 1 D) of group B, C, D, E were significantly (P<0.05) decreased compared to those of the control.
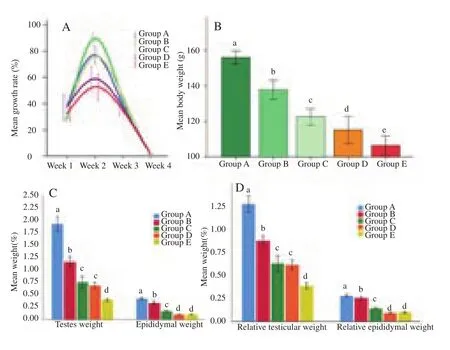
Figure 1. Effect of Calotropis latex on growth rate and gross pubertal traits of male Wistar rats.
3.2. Effects of Calotropis latex on pubertal rats’semen parameters
The pubertal rats’ semen motility and sperm counts were significantly (P<0.001) reduced (Figure 2) and the sperm abnormalities were significantly (P<0.001) increased (Figure 3)by increasing the concentration of Calotropis latex. These seminal changes happened in a dose dependent manner.
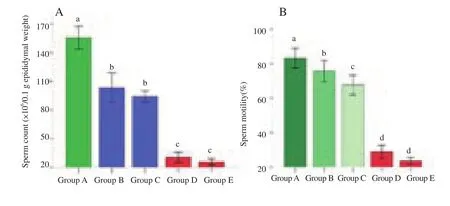
Figure 2. Effect of Calotropis latex on epididymal sperm count.
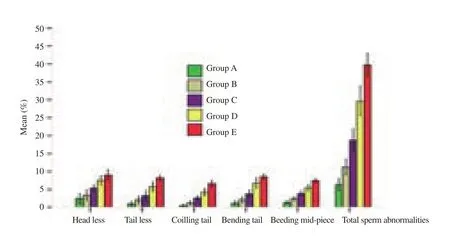
Figure 3. Effect of Calotropis latex on epididymal sperm normality.
3.3. Effects of Calotropis latex on male rats’reproductive hormones
As shown in Figure 4, high doses of Calotropis latex significantly(P<0.05) increased the serum levels of FSH in the treated male rats compared to that of the controls. In contrast, the serum concentrations of LH were significantly (P<0.05) decreased.Furthermore, the serum concentrations of testosterone of treated rats decreased significantly (P<0.05).
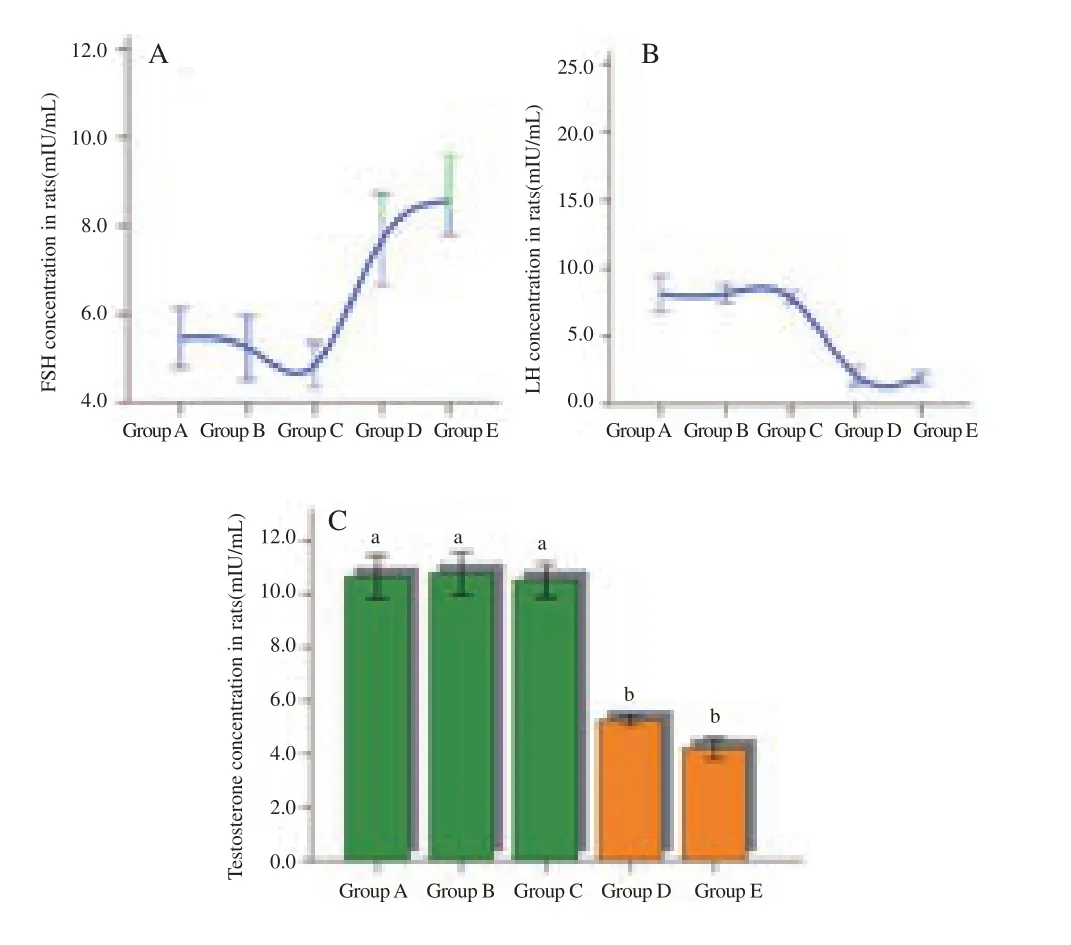
Figure 4. Effect of different doses of Calotropis latex on reproductive hormones of pubertal male rats.
3.4. Effects of different doses of Calotropis latex on pubertal male rats’ testicular histology
Figure 5 showed H&E stained histological sections of testes of rats dosed with different concentration of Calotropis latex. The seminiferous tubules (STs) of the control rats were completely differentiated with obvious layers of spermatogenic epithelium,wide lumen and were of normal shape. The STs had clear basement membranes and their lumens contain mature sperms.The spermatogenic cells including spermatogonia, primary spermatocytes, early spermatids, late spermatids and sertoli cells were obvious, abundant and healthy. In the treatment groups,when the concentration of Calotropis latex was increased, the STs histology was distorted. When the concentration of the latex reached 10 mg/kg BW (Figure 5 C), the spermatogenic epithelial layers were remarkably reduced and the spermatogenesis was significantly affected. The lumens of some of the STs contain masses of degenerated cells. More severe reduction in STs epithelium and spermatogenesis was witnessed when Calotropis latex was increased to 15 or 20 mg/kg BW. (Figure 5 D & C).

Figure 5. Light photomicrographs of rat’s testes parenchyma.
4. Dsiscussion
The results of this study showed that oral dosing of immature male Wistar rats with doses ≥10 mg/kg BW of Calotropis procera latex reduced their growth rate, pubertal and consequently the testicular and epididymal weights were reduced. Additionally, the pubertal testicular histology and semen traits of treated rats swere abnormal.Contrarily to these deleterious effects of high doses of Calotropis latex, a dose of 5 mg/kg BW weight improved rats’ growth rate.
The abnormal pubertal traits that result from dosing rats with high concentrations of Calotropis latex are probably due to the effects of toxic components of Calotropis procera particularly the cardiac glycosides (calotropin), anthraqunions, flavonoids, alkaloids,tanines and phenol glycosides[9]. Similar findings were reported by Srivastava et al[35] who employed Calotropis roosts’ extract; and El-Shahat et al[36] who evaluated the effects of ethanolic crude extract of Calotropis procera leaves. These authors have reported reductions in the total BW and the weights of vital organs (liver, kidney, and testis). The positive effect of the low dose of Calotropis latex on growth rate of rats agrees with the finding of Madruga et al[28].who reported that feeding low concentrations of Calotropis procera hay improved the growth and quality of meat in growing lambs.Contrarily to these finding, Neto et al[37] have reported that the extract of dried leaves of Calotropis procera has no effect on rat BW;however, they reported that the extract of dried leaves of Calotropis procera has beneficial effect on certain parts of the rats’ body especially the epididymal adipose tissue and soleus and extensor longus digitorium muscles. Neto et al have concluded that Calotropis procera leaves extract has reduced the blood glucose and improved the metabolic status of the rats thus they explained why Calotropis procera leaves extract has a beneficial effect on certain tissue masses,while it has no effect on the total BW of the rats. Additionally,Neto et al have reported that the Calotropis procera extract caused a decrease in protein catabolism through increasing glucose uptake.The difference between the findings of the current study and that of Neto et al is probably due to the differences in the part of Calotropis procera employed, dose given, age of rats employed and/or the environment. In the current study, Calotropis procera latex was given to immature rats, while Neto et al offered extract of the dried leaves of Calotropis procera to mature rats.
Degenerative changes were observed in testicular structure of the testes of rats treated with the latex of Calotropis procera and consequently reduced testicular and epididymal weights were observed. Similar finding were reported by Akinloye et al[38] who investigated the effect of aqueous extract of Calotropis procera latex on the BW and reproductive organs weights of male Wistar rats. In this study, most of the testes of treated rats examined have narrow interstitial spaces with a reduced number of leydig cells.The degenerative changes and the reduction in the testicular weights could be attributed to the decrement in the number of leydig cells that are responsible for synthesis and secretion of testosterone, which is known to maintain normal testicular architecture. In accordance with the findings of this study, Akinloye et al[39] have observed varying degrees of testicular lesions as well as severe reduction in STs diameters in rats treated with aqueous extract of Calotropis procera latex. These lesions, coupled with the reduced number of leydig cells and the consequent expected reduction in testosterone production,probably may result in a reduced sperm production or even aspermia when the dose of Calotropis procera latex is increased. These observations explain why Calotropis procera have been reported as possible antifertility candidate[40,41]. The pattern of cellular damage and hypoplasia of the STs observed in this study is consistent with the effects of different endocrine disrupting chemicals that were reported to have similar effects on testis histology and functions[42-46]. Therefore, Calotropis procera latex could be a possible member of the endocrine disrupting chemicals that could adversely affect the endocrine glands and consequently the male reproduction. Whether the latex of Calotropis procera works on endocrine glands or directly on the testis remain to be elucidated.
In the current study, increment in sperm abnormalities, decrement in sperm count and motility were observed in male rats treated with latex of Calotropis procera. These abnormalities in sperm quality could be due to multi-factors; however, the possible candidates that could induce these changes are the cardiac glycosides that are found in the latex extract of Calotropis procera[47]. Additionally, these abnormalities in quality and count of sperms could be attributed to the low level of testosterone that followed the decrement in Leydig cells. The reduction in sperm counts and motilities recorded here are in accordance with the findings of Shafey et al[48] who reported a similar phenomenon in mature rats treated with extract of latex of Calotropis procera.
The disturbances of reproductive hormones of rats treated with the latex of Calotropis procera can be explained by 3 theories: 1)The latex of the Calotropis procera has affected the hypothalamopituitary-gonadal axis especially the LH releasing hormone of the hypothalamus leading to LH reduction and consequently reduced the serum level of testosterone; meanwhile the serum level of FSH is elevated. 2) The latex of Calotropis procera affected the pituitary gland and/or the hypothalamus resulting in a reduced serum level of LH and consequently a reduced testosterone level. 3) Latex of Calotropis procera intoxicated the Leydig and/or Sertoli cells of the testes and impaired the synthesis and secretion of testosterone.Contrarily to the finding of this study, Shafey et al[48] found that the ethanol extract of Calotropis procera has no effect on serum testosterone level of mature male rats although they recorded a mild reduction. This discrepancy is presumably due to the differences of the plants’ parts used and/ or the rats’ age, since in the current study immature rats were employed. The findings of Toson et al[36]agree with the results of the current study where they reported that ethanolic extract of the leaves of the plant reduced the concentrations of testosterone, LH, epididymal sperms count, percent of motile sperms, percent of live sperms besides an increment in the percent of abnormal sperms. However, they reported a reduced FSH that in contradiction with the findings of this study, where the FSH level is found high. Sharma and Jacob[42] reported functional sterility without loss of libido and sexual behavior in male mice treated with an intramuscular aqueous of ethanolic extracts of the plant’s flowers. This finding presumably contradicts the findings of this study since libido is controlled by testosterone hormone, which is reduced by Calotropis procera latex in this study; however, this study did not investigate rats’ libido. The discrepancy between the study of Sharma & Jacob and this study seems to be due to species difference and the different parts of the plant used. It is concluded that latex of Calotropis procera has anti-androgenic activity and/or an endocrine disrupting effect. Furthermore, the latex of Calotropis procera presumably contains substances that if extracted, identified and proven save can be employed to regulate and control male reproductive traits and behaviors.
Conflict of interest statement
The author declares that there is no conflict of interest.
[1] Silva MCC, da Silva AB, Teixeira FM, de Sousa PCP, Rondon RMM,Honrio JER, et al. Therapeutic and biological activities of Calotropis procera (Ait.) R. Br Asian Pac J Trop Med 2010; 3(4): 332-336.
[2] Sheth F. Range of seasonal phytochemical variations in Calotropis procera(Ait.) R. Br Int J Med Arom Plants 2011; 1(2): 180-183.
[3] Begum N, Sharma B, Pandey RS. Calotropis procera and Annona squamosa: Potential alternatives to chemical pesticides. J Appl Sci Tech 2013;3(2): 254-267.
[4] Suresh P, Suresh E, Kalavathy S. Review on a potential herb Calotropis gigantea (L.) R. Br Sch Acad J Pharm 2013; 2(2): 135-143.
[5] Gutiérrez SP, Angel M, Sánchez Z, González CP. Antidiarrhoeal activity of different plants used in traditional medicine. African J Biotechnol 2007;6(25): 2988-2994.
[6] Yesmin N, Uddin S, Mubassara S, Akond MA. Antioxidant and antibacterial activities of Calotropis procera Linn. Am Eur J Agric Env Sci 2008; 4(5): 550-553.
[7] Joshi R, Sharma A, Jat B. Analysis of antioxidant activity in extract of Calotropis procera (Ait.) R. Br J Appl Biosci 2009; 17: 899-903.
[8] Murti Y, Yogi B, Pathak D. In-vitro antioxidant activity of column chromatographic elutes of different extracts of Calotropis procera (giant milkweed) leaves. J Pharm Res 2011; 4(10): 34-36.
[9] Mossa JS, Tariq MA, Mohsin AM, Ageel MA, Al-Yahya MS, Al-Said S,et al. Pharmacological studies on aerial parts of Calotropis procera. Am J Chin Med 1991; 19(3-4): 223-231.
[10] Dewan S, Sangraula H, Kumar V. Preliminary studies on the analgesic activity of latex of Calotropris procera. J Ethnopharmacol 2000; 73(1-2): 307-311.
[11] Derald E, Edwin S, Pandlan S, Damodaran S, Jain P, Patel J.Antimicrobial activity of leaves of Lantana camara Linn and Calotropis procera R. Br Indian Drugs 2008; 45(9): 736-737.
[12] Kareem SO, Akpan I, Ojo OP. Antimicrobial activities of Calotropis procera on selected pathogenic microorganisms. Afr J Biomed Res 2008; 11: 105-110.
[13] Iwalewa E, Elujoba A, Bankole O. In vitro spasmolytic effect of aqueous extract of Calotropis procera on Guinea-pig trachea smooth muscle chain.Fitoterapia 2005; 76(2): 250-253.
[14] Verma S, Singh S, Mathur A. In vitro cytotoxicity of Calotropis procera and Trigonella foenum-graecum against human cancer cell lines. J Chem Pharm Res 2010; 2(4): 861-865.
[15] Sundaram S, Verma S, Dwivedi P. In-vitro cytotoxic activity of Indian medicinal plants used traditionally to treat cancer. Asian J Pharm Clin Res 2011; 4: 27-29.
[16] Olaleye M, Akindahunsi A, Ewemade E. Hepatoprotective activity of leafextract of Calotropis procera against acetaminophen-and hepatitis virusinduced liver damage in rats. Niger J Nat Prod Med 2008; 12: 214-220.
[17] Rahmatullah M, Sultan S, Taher, Tanzila T, Lucky S-S, Chowdhury MH,et al. Effect of Cuscuta reflexa stem and Calotropis procera leaf extracts on glucose tolerance. Afr J Tradit Complement Altern Med 2010; 7(2): 109-112.
[18] Murti Y, Sharma S, Mishra P. In vitro antheliminthic activity of Calotropis procera (Ait.) R. Br Leaves 2015; 8(6): 6-8.
[19] Iqbal Z, Lateef M, Jabbar A, Muhammad G, Khan M. Anthelmintic activ ity of Calotropis procera (Ait.) Ait F. flowers in sheep. J Ethnopharmacol 2005;102(2): 256-261.
[20] James A, Magbagbeola O, Saibu G, Oloyo A. Possible biochemical mechanism of action of petroleum-ether extract of Calotropis procera leavesnism (Asclepiadaceae) in gravid pregnant spraguedawley rats. Fed Am Soc Exp Biol J 2009; 23: 879.
[21] Lima-Filho J, Patriota J, Silva A, Filho N, Oliveira R, Alencar N.Proteins from latex of Calotropis procera prevent septic shock due to lethal infection by Salmonella enterica serovar Typhimurium. J Ethnophar Macol 2010; 129: 327-334.
[22] Laitiff A, Teoh S, Das S. Wound healing in diabetes mellitus: Traditional treatment modalities. Clin Ter 2010; 161(4): 359-364.
[23] Hassan S, Bilbis F, Ladan M, Umar R, Dangoggo S, Saidu Y, et al.Evaluation of antifungal activity and phytochemi- cal analysis of leaves,roots and stem bark extracts of Calotropis procera (Asclepiadaceae). Pak J Biol Sci 2006; 9(14): 2624-2629.
[24] Ahmed U, Zuhua S, Bashier N, Muafi H, Hao ZP, Gao YL. Evaluation of insecticidal potentialities of aqueous extracts from Calotropis procera(Ait.) against Henosepilachna elaterii Rossi. J Appl Sci 2006; 6: 2466-2470.
[25] Singhal A, Kumar VL. Effect of aqueous suspension of dried latex of Calotropis procera on hepatorenal functions in rat. J Ethnopharmacol 2009; 122: 172-174.
[26] Vadlapudi V, Naidu CK. In vitro bioactivity of indian medicinal plant Calotropis procera (Ait). J Glob Pharm Technol 2010; 2: 43-45.
[27] Mahmoud OM, Adam SEI, Tartour G. The effects of Calotropis procera on small ruminants. ii. Effects of administration of the latex to sheep and goats. J Comp Pathol 1979; 89: 251-263.
[28] Madruga MS, Costa RG, Silva AM, Marques AVMS, Cavalcanti RN,Narain N, et al. Effect of silk flower hay (Calotropis procera Sw) feeding on the physical and chemical quality of longissimus dorsi muscle of Santa Inez lambs. Meat Sci 2008; 78(4): 469-474.
[29] Nair AB, Jacob SA. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 2016; 7: 27-31.
[30] Elsheikh AS, Takahashi Y, Hishinuma M, Sayed M, Nour M, Kanagawa H. Effect of encapsulation on development of mouse pronuclear stage embryos in vitro. Anim Reprod Sci 1997; 48: 317-324.
[31] Reeves PG, Rossow KL. Zinc deficiency affects the activity and protein concentration of angiotensin-converting enzyme in rat testes. Exp Biol Med 1993; 203: 336-342.
[32] Eleawa SM, Alkhateeb MA, Alhashem FH, Bin-Jaliah I, Sakr HF,Elrefaey HM, et al. Resveratrol reverses cadmium chloride-induced testicular damage and subfertility by downregulating p53 and Bax and upregulating gonadotropins and Bcl-2 gene expression. J Reprod Dev 2014; 60(2): 115-127.
[33] Bearden H, Fuquay J, Willard ST. Applied animal reproduction. 5th ed.Bearden HJ, Fuquay JWE, editors. New Jersey: Prentice Hall, Upper Saddle River; 2000, 168-182.
[34] Sonmez M, Turk G, Yuce A. The effect of ascorbic acid supplementation on sperm quality, lipid peroxidation and testosterone levels of male Wistar rats. Theriogenology 2005; 63: 2063-2072.
[35] Srivastava SR, Keshri G, Bhargavan B, Singh C, Singh MM. Pregnancy interceptive activity of the roots of Calotropis gigantea Linn. in rats.Contraception 2007; 75(4): 318-322.
[36] Toson El-Shahat A, Habib Salem A, Saad Entsar A, Harraz Nahla H,Saad EA. Toxic and anti-fertility effects of Alocasia macrorrhiza and Calotropis procera ethanolic extracts on male mice. Int J Biochem Phot 2014; 195(195): 328-338.
[37] Neto MCL, de Vasconcelos CFB, Thij-an VN, Caldas G FR, Araujo AV, Costa-Silva J, et al. Evaluation of antihyperglycaemic activity of Calotropis procera leaves extract on streptozotocin-induced diabetes in Wistar rats. Brazilian J Pharmacogn 2013; 23(6): 913-919.
[38] Akinloye A, Abatan M, Onwuka S, Oke B. Growth-suppressing effect of Calotropis procera (Giant Milkweed) on body weight and the male reproductive organs of Wistar rats. Trop Vet 20(3): 132-138.
[39] Akinloye AK, Abatan MO, Alaka OO, Oke BO. Histomorphometric and histopathological studies on the effect of Calotropis procera (Giant Milkweed) on the male reproductive organs of Wistar rats. Afr J Biomed Res 2002; 5: 57-61.
[40] Malhi BS, Trivedi VP. Vegetable antifertility drugs of India. Q J Crude Drug Res 1972; 12(3):1922-1928.
[41] Sharma N, Jacob D. Inhibition of fertility and functional alteration in the genital organs of male Swiss Albino mouse after administration of Calotropis procera flower extract. Pharm Biol 2001; 39(6): 403-407.
[42] Elsheikh AS, Fadul TF, Aboagla EME, Rahim Gameel AA. Effects of potassium bromate on male rat growth and testicular histology. Asian Pac J Reprod 2016; 5(5): 376-380.
[43] Ahmed SI, Ali TO, Elsheikh AS. Ultra-structure of testes of rats born to dams treated with hydroxy-progesterone hexanoate. Asian Pacific J Reprod 2016; 5(6): 510-513.
[44] Atef M, N’ounssef SAH, Ramaddan A, Nawito MF, El-Sayed MK,El-Rahman HA. Influence of phoxim on testicular and seminal vesicle organs, testosterone and cholinesterase level and its tissue residues in male rats. Dtsch-Tierarztl wochenschrift 1995; 102(8): 301-305.
[45] Piyachaturawat P, Timinkul A, Chuncharunee A, Suksamrarn A. Growth suppressing effect of Curcuma comosa extract on male reproductive organs in immature rats. Pharm Biol 1998; 36(1): 44-49.
[46] Köhler-Samouilidis G, Papaioannou N, Kotsaki-Kovatsi VP, Vadarakis A.Effect of estradiol valerate on the male reproductive organs and various semen parameters in rats. Berl Munch Tierarztl Wochenschr 1998; 111(1):1-5.
[47] Al-Robai AA, Abo-Khatwa AN, Jamal ZA. Toxocological studies on the latex of the usher plant Calotropis procera (Ait.) in Saudi Arabia.V-seasonal variation of total cardiac glycosides in the usher plant latex and in various tissues of the usher hopper, poekilocerous bufonfius Klug.Ara Gulf J Sci 1997; 1(16): 129-144.
[48] Shafey E, Seliem MME, El-Mahrouky F, Gabr WM, Kandil RA. Some physiological and biochemical effects of Oshar extract and abamectin biocide on male albino rats. J Am Sci 2011; 7(12): 254-261.
 Asian Pacific Journal of Reproduction2018年3期
Asian Pacific Journal of Reproduction2018年3期
- Asian Pacific Journal of Reproduction的其它文章
- Missed estradiol determination resulting in oocyte retrieval and embryo development following controlled ovarian hyperstimulation at early pregnancy: Case report
- Role of preputial washing in reducing microbial load and improving bovine semen quality
- Effect of different concentration of fish oil in skim milk-egg yolk extenders on postthawed semen qualities of Kalang swamp buffalo bull
- Prediction of protein structure of novel protein (116 kDa) from human sperm membrane
- Improvement of cortical granules migration and in vitro embryo production of vitrified bovine oocyte by 9-cis retinoic acid
- Effect of alcohol infusion of Cissus populnea root on testicular function and serum hormone of male Wistar rats
