Low density lipoprotein in cryopreservation of semen
P. Perumal
Division of Animal Science, ICAR-Central Island Agricultural Research Institute, Port Blair, Andaman and Nicobar Islands-744 101, India
1. Introduction
Artificial insemination made a predominant contribution towards the improvement of genetic potential especially in dairy husbandry in which a single semen collection from a male is used to make pregnant of many females. Different processes of cryopreservation,cryostorage as well as transport of cryopreserved semen have reached a position of industry particularly for the milch animals due to wide use of cryopreserved semen, but still the semen cryopreservation method or process could not attain the status of reality or fully functional to develop a industry. This is mainly attributed to the truth that during cryopreservation and thawing,a large number of sperm cells are rendered incapable to fertilize the ovum. Various steps of cryopreservation induce physical,physiological, osmotic and bio-chemical stress on the sperm cell membrane and sperm structure, which may result in damages to spermatozoa followed by decreased post-thawed quality of spermatozoa[1] and fertility[2-4]. The conception rate in frozenthawed semen has been reported to be low in bovine species[2-5].Because sperm membranes have high content of unsaturated fatty acids and lack significant cytoplasmic component containing antioxidants, they are highly susceptible to lipid peroxidation by oxygen free radicals and hydrogen peroxide[2,3,6-13]. The first and foremost target in sperm cryopreservation is to reduce or minimize the lethal intra-cellular ice crystal formation as well as to reduce plasma & acrosomal membrane damage in different stage of cryopreservation with modification of the freezing protocol or inclusion of different additives or antioxidants in the semen extenders[2,3,10,11,14,15]. Different extenders have been analysed in an attempt to reduce the cellular damage or injury[16]. Therefore,it is clear that the interaction between the spermatozoa and surrounding medium is an importantly crucial and critical factor that determines the integrity and fertilizing ability of spermatozoa.Similarly, various additives were attempted to reduce the sperm damage or injury in cryopreservation or liquid storage of sperm in in-vitro[2,3,7,8,11,13,15]. Besides, in-vitro inclusion of additives or antioxidants[2,3,7,8,11,13,15] or cholesterol-loadedcyclodextrins[17] or heterogenous seminal plasma[18] or heterogenous follicular fluid[19]or herbal antioxidants[20] in the semen preservation extender, feeding of antioxidants in in-vivo to reduce cryodamage or cryoinjury in different livestock species[21,22] were studied to get improvement in the semen production and its quality. Moreover, various other factors such as season[11,23-26], vaccination[27-29], breed[3,4,23,24],pathological conditions such as cryptorchidism[30,31] & spontaneous erection and masturbation[32], method of cryopreservation of semen[33] and scrotal circumference & testicular parameters[34,35]significantly affect the semen production & its quality, freezability and fertility of sperm as well as the breeding bulls. Some studies were conducted with commercially available extenders for different species with varied success rates. But the researches are still undergoing to prepare or modify the extenders to improve the freezability and fertility of sperm for artificial breeding programme and one of the such methods is to replace the egg yolk(EY) with low density lipoprotein (LDL) extracted from EY of hen with standard method[36-39]. Therefore, the methodology was standardized to prepare a semen extender with extracted LDL from EY of hen instead of EY and utilized it in different species in semen cryopreservation. In this review, beneficial effects of LDL, harmful effects of the EY, mechanism of LDL function, composition of LDL,preparation of LDL based semen extender and a case study on semen preservation with different concentration of LDL in comparison with EY in mithun (bovine) species is reviewed.
2. General view on LDL in semen preservation
EY from hen has become a common component widely used in extenders for cryopreservation of semen for different species during the past six decades by the frozen semen bank centres or artificial insemination centres. The EY as well as the glycerol represents the important compounds of practically all the semen preservation media used for sperm preservation in liquid or cryopreserved form in different livestock species. It has been shown that EY helps against cold shock damages and improves sperm fertilizing ability in association with other substances[36-40]. There are various researches undergoing to replace the EY with the active components or sole responsible components in EY in semen preservation extenders instead of whole EY because of the detrimental effects of other components present in EY in semen preservation[41-43].Various compounds of EY have been studied to identify the most active ingredients or compounds responsible for the protective action[42,44,45], and reports indicated that the LDL fraction plays essential roles, which is chemically defined by Banaszak et al[46]and Kuksis[47] who suggested that it has highest protective ability for the sperm in semen preservation extender. The precise and accurate mechanism of cryoprotective EY is not known. EY has protected the sperm integrity, freezability and fertility in different ways due to the presence of LDL in EY[43,48-51]. In an attempt, the sterilized, purified, pasteurised, homogenous EY powder was used in the semen extender in place of whole EY to preserve the sperm in livestock species and it has improved the freezability and fertility[52].Similarly, in another study, effect of EY[53] and LDL[54] from various poultry species on the freezability and fertility was studied and indicated that there was significant variation among the poultry species and this may be due to variation in quantity and quality of EY and its LDL content. Pace and Graham[41] purified the EY and reported LDL has beneficial effects on protection against cold shock and cryodamage & cryoinjury in sperm preservation.
Later on, many researchers worked on purification of LDL from EY and studied on semen preservation, freezability and fertility in various animal species with different concentration of LDL,viz. bovine (8% LDL[50,55-59]), ovine (8% LDL[60]; 9% LDL[61]),bubaline (10% LDL[62]), canine (6 % LDL[63,64]), porcine (9%LDL([65-67]) & 12% LDL[68]), Agu pig (4%-6% LDL[69]), Iberian red deer (8% LDL[70]) and mithun (8% LDL[36-39]). Despite the significant benefits of EY on semen cryopreservation, it has many adverse effects on sperm preservation due to presence of high density lipoprotein (HDL) and other factors[41,71]. The EY, as an animal source, may represent a high potential microbiological risk and change the sperm chromatin structure, leading to poor freezability,viability and fertility[72]. It also contains high concentration of calcium ions which stimulate the premature acrosomal reaction as well as capacitation, which in turn results in poor fertilizing ability of sperm[43].
Moreover, the EY predominantly contains steroid hormone,progesterone as well as its precursor compounds which are responsible for the premature capacitation and cryoinjury/cryodamage during storage in liquid or frozen state. The detrimental effect of seminal plasma is largely attributed to presence of bovine seminal protein (BSP) which is in unbound form, stimulating continuous efflux of cholesterol and phospholipid from sperm plasma membrane, destabilizing the sperm and predisposing it towards cryodamage[73,74]. Similarly, Vishwanath et al[75] suggested that lipoproteins of EY compete with detrimental seminal plasma cationic peptides (<5 kDa) in binding to the sperm membrane and thus protect the sperm. Several EY compounds interfere with the laboratory biochemical assays and metabolic functional investigations[76] and also LDL has less interfered with the laboratory assays because the larger EY particles are removed during the process of LDL extraction whereas the EY particles in the extenders can interfere with laboratory assays. In flow cytometry method of sperm analysis,EY particles present in extender have similar properties of light scattering mechanism as sperm which in turn make it difficult to identify them. Similarly in computer assisted sperm analysis, the settings need to be adjusted in such a way as to avoid mistaking of an EY particle as a sperm head. These disadvantageous effects of EY can be overcome by inclusion of the purified, sterilized and homogenous LDL extracted from EY of hen in the semen extender in place of fresh EY. The LDL protects the sperm membrane by sequestration of BSP proteins in seminal plasma, thus preventing binding of BSP proteins on the sperm surface at ejaculation. Thus cholesterol and phospholipids efflux from the sperm membrane is minimized, minimizing membrane damage and cryoinjury[77]. On the other side, LDL forms a coat overlaying sperm membrane and protects from cold shock and cryoinjury of spermatozoa. And also,LDL alone attenuates the toxicity of glycerol in cryopreservation of semen.
3. Composition of EY, LDL and lipids of LDL
EY of hen consists of 33% proteins and 63% lipids as in dry matter basis. The fresh whole EY can be partitioned into 22% granules and 78% plasma. Granules consist of 16% HDL, 2% LDL and 4%phosvitin. In the EY plasma, the main compounds are LDL (66%)and livetins (10%)[78]. Another compound such as phosvitin is highly phosphorylated protein and has higher bactericidal as well as antioxidant properties[79]. Similarly, livetins are corresponded to the serum proteins and consist of albumin, immunoglobulin Y and α-2- glycoprotein[80]. LDL is made up of two-thirds of the total solids of EY and is positioned in the soluble portion of the EY and called as EY plasma and density of LDL is 0.982 g/mL. LDL are sphereshaped (17-60 nm diameters) with a liquid lipid core and is made up of cholesterol esters and triglycerides. One layer of phospholipids surrounds this core part. Some cholesterol and apoprotein are incorporated into the phospholipid layer[81]. Phospholipids have the vital roles in stability of LDL structure as well as function because of the presence of hydrophobic characteristics. It is possible to fractionate the LDLs into the lower and higher density lipoprotein[82].HDL was previously called as lipovitellin and was related with the phosvitins to form the compound granules. They consisted of 20%-25% lipids and 75%-80% proteins, of which the former contains 30% triglycerides, 5% cholesterol and 65% phospholipids[83,84]. The main phospholipids present in the whole EY are such as cardiolipin,phosphatidylinositol, phosphatidylethanolamine, phosphatidylserine,sphingo-myelin and phosphatidylcholine.
4. Mechanism of protection by LDL of EY
The low density protein is composed of 83%-89% of lipids and 11%-17% proteins. During the cryopreservation process, the bonding between protein and lipids is disturbed and the interaction among the proteins is increased. Ultimately, the composition of LDL has been disturbed during freezing and thawing process. The lipids of LDL are composed of 69% triglycerides, 26% phospholipids and 5% cholesterol. These triglycerides and phospholipids are released into the extender and form apoprotein gel on the sperm to protect from the harmful ice crystals. Moreover, the phospholipids are also released and form a protective coat over the surface of the sperm to protect from the cryoinjury or cryodamage[85]. It is broadly accepted that the protective compound in the EY is a phospholipid moiety of the LDL fraction[44,45,79]. Therefore, there are several mechanisms have been proposed that how the LDL decreases cryodamage to the sperm during the process of cooling and cryopreservation. One probable possibility is that the LDLs, especially the phospholipids which associate with the plasma membrane of sperm and thereby provide strong stabilization of membrane as well as sperm[86].The second possibility is that the phospholipids lost during the process of sperm processing and cryopreservation and are replaced by phospholipids present in the EY[51,87]. In another way, LDL is essential in the gelation process during the process of cryopreservation & thawing and this mainly occurred during the disruption of LDL structure and this process is favoured by the dehydration of spermatozoa especially during the process of the freezing-thawing[50,85]. The components of LDL have functions to penetrate the phospholipids as well as cholesterol into the plasma membrane of the sperm cell[88] and create the complex with proteins of seminal plasma leading to unavailability to function in the sperm plasma membrane[88,89]. But still, the functions of the components of lipid and protein in LDL during interactions with the membrane of spermatozoa have not been known[63]. However, Quinn et al[48]and Ricker et al[86] reported that the added phospholipids were not integrated into the sperm plasma membrane. The seminal plasma BSP proteins bind to choline phospholipids present on sperm plasma membrane at the time of ejaculation[90,91] and also bind with the other capacitation factors such as HDL and/or heparin[92-94] and the BSP proteins enhance sperm capacitation process induced by HDL as well as heparin[95,96]. Thus, BSP proteins are more beneficial for functions of sperm like acrosomal reaction and capacitation which in turn benefits fertilization.
But recent reports revealed that the LDLs of EY bind with detrimental BSP proteins[88] that bind with sperm membrane on ejaculation are responsible for the efflux of cholesterol and phospholipids from the sperm plasma membrane[97]. Efflux of lipid and cholesterol by BSP proteins is concentration and time dependence means more exposure and high concentration of BSP protein leads to higher release of lipids and cholesterol which in turn affect the sperm membrane biochemical integrity and reduce sperm viability and fertility. Therefore, the extender which contains either EY or LDL needs to be exposed to the sperm immediately after collection to minimize the effect of BSP proteins on sperm that present in the seminal plasma. Continuous exposure of spermatozoa to seminal plasma which contains BSP proteins has deleterious effect to the sperm plasma membrane and this may allow the sperm membrane very sensitive to sperm during process and the storage in liquid or cryopreserved states. Thus, BSP proteins in seminal plasma have the potential to act as both beneficial as well as detrimental weapons to the sperm depending on the exposure time and concentration in seminal plasma like two side knives. EY(contains LDL) or LDL extracted from EY has responsibility to bind with BSP protein at the time of extender preparation and prevents to bind on the sperm membrane and thus protects the sperm from the liberation of phospholipids and cholesterol of harmful effects of BSP.Bergeron and Manjunath[51] reported that any extender containing choline phosphates is able to protect the sperm during the process of cooling and cryopreservation. Even though the phosphatidylcholine in the LDL fraction is as the cryoprotective component, but the whole lipoprotein is required to minimize cryodamage, cryoinjury and improved cryoprotection[98]. This concept is supported by the truth that the liposomes that artificially prepared vesicles are made from dioleoylphosphatidylcholine, phosphatidylserine,phosphatidylcholine and combinations with cholesterol do not protect the sperm as well as the whole EY[98].
5. Harmful effects of EY in semen preservation
The freezing-thawing process exposes the spermatozoa to thermal shock, which in turn results in damage to the sperm plasma as well as acrosomal membrane[16]. Different extenders have been allowed to test in an attempt to minimize the cellular injury or damage.EY of hen has become a common component and widely used in extenders for cryopreservation of semen for different species.Most of the semen cryopreservation extenders contain EY at the level of 20% as a major source of lipoprotein to protect the sperm cells from cold shock and other cryo-damages[99]. Wide variability in the EY composition creates it difficult to analyze the beneficial effects of a particular key component in sperm cryopreservation as well as the components which are detrimental to sperm viability and fertility[100]. Moreover, it also changes the structure of sperm chromatin[101] which in turn leads to poor post-thaw motility,viability and fertility. Further, negative effects of whole EY on sperm viability as well as respiration have been contributed by the action of HDL, a larger molecule present within granules[41]. Furthermore, EY introduces a risk of microbial contamination, with the subsequent production of endotoxins capable of damaging the structure,function and fertilizing capacity of spermatozoa and deteriorates its quality[72]. Moreover, the use of EY in higher concentration may have deleterious effects as it affects the osmotic as well as the physiological integrity of the extender which is combined with toxicity (amino acid oxidase activity) of dead spermatozoa in turn resulting to lower post-thaw spermatozoal quality and fertility[99].EY based extender has higher concentration of calcium ions that might be responsible for the acrosomal reaction & damage to the sperm especially storage below 30 ℃ due to higher rate of Ca2+penetration. In EY, presence of unknown factors is responsible for destabilization of the sperm plasma membrane which in turn causes premature acrosomal reaction and capacitation[43].
6. Beneficial effects of LDL in semen preservation
EY functions against cold shock damages and improves fertilizing ability of sperm in association with other substances in the extender[40,102]. The components (granules) present in the EY are capable to reduce the metabolic exchanges of the sperm or minimize their forward progressive motility. Moreover, the composition of EY is very complex and consisted of many components which are not needed in sperm preservation, and even has harmful effects on sperm preservation. Therefore, it is needed to identify the necessary component which has significant effect in semen preservation as cold shock protector or absorber.
There are many investigation conducted to standardise the purification protocol to purify the component of the low density lipoprotein to chemically define the composition of semen preservation extender from complex to simple known extender[103].Therefore, the concept of isolation, purification and utilization of LDL instead of EY in the semen extender formulation has come into actions. The replacement of EY by LDL also benefits the post-thaw sperm viability and fertility by excluding the EY components such as minerals as well as granules. Tonieto et al[60] reported that the inclusion of LDL in the semen preservation extender has attenuated cytotoxic effect of glycerol in semen preservation. HDL supports the action of seminal plasma BSP proteins and the efflux of cholesterol and phospholipids[73]. LDL extender is simple one and not complex and known composition of chemical structure than the EY containing(20%) extender as it is more complex one which could explain the protective effect of LDLs, especially on the plasma membrane of spermatozoa. Cryopreservation technology is one known to have significant effect on the lipid biochemical organization & pattern and bio-chemical composition and structure of the spermatozoal membrane and plasma and acrosome[104]. It has been reported that LDL minimizes indirectly or/and directly these modification of sperm membrane and preserve intactness of sperm membranes[88].Manjunath et al[89] explained that the important and main mechanism of LDL which protects the spermatozoa is through sequestration of BSP proteins in seminal plasma by LDL of EY of extender. The major seminal plasma proteins of bull (BSP proteins: BSP-A1/A2,BSPA3 and BSP-30-kDa) bind to the sperm membrane surface at ejaculation and induce cholesterol and phospholipids efflux from the sperm membrane, leading to alteration of biochemical composition of the membrane structure. Since LDLs interact and bind especially to the BSP proteins[88], this would minimize the major proteins of BSP bind to sperm membrane which in turn prevent lipid &cholesterol efflux from the sperm membrane and would explain beneficial effects of the LDLs in semen preservation.
The semen preservation extender containing LDL protects the sperm in the following two ways. In the first method, there is an association between LDL fraction and BSP proteins that protects the sperm from disintegration by preventing the binding of BSP on surface of spermatozoa especially on sperm plasma membrane intrinsically. In the second method, the lipid released from LDL could associate or sediment with sperm plasma membrane which in turn preserves the intactness of the sperm plasma membrane in sperm cryopreservation. LDL has a very high capacity for BSP protein binding and the binding is specific, rapid, and stable even after semen freezing-thawing processes. It was reported that after frozen-thawed, the semen diluted with EY contained extender contains 80% lesser BSP proteins than in semen or sperms which are collected from fresh semen ejaculates[77]. EY contains LDL is the only one component of EY that binds specifically and rapidly with BSP proteins and thus the sequestration of BSP proteins by LDL might represent the major mechanism of sperm protection by EY in the extender[89]. Moreover, Manjunath et al[89] indicated that the beneficial function of LDL was not limited only to the direct attachment of LDL to the plasma membrane of sperm but also was involved in interaction between BSP proteins, LDL as well as the plasma membrane of sperm. Thus, LDL offers major protection by reducing the deleterious or detrimental effect of BSP proteins on plasma membranes and its integrity.
LDL is composed of about 12% proteins and 87% lipids. It is of spherical shape with a mean diameter of 35 nm[105], which is based on a triglyceride core surrounded by a film or coat of phospholipids and proteins[84]. During the process of freezing and thawing, the LDL is disrupted and cholesterol & phospholipid are liberated into the surrounding medium, which in turn form a protective coat or film over the surface of sperm plasma & acrosomal membranes[106].Further, Hu et al[85] reported that LDL is the component responsible for the process of gelation in the freezing-thawing. The disruption of the LDL structure is the first step of gelation process and this disruption in turn stimulates the spermatozoal dehydration caused by the freezing-thawing process. Moreover, Bergeron et al[88]reported that LDL could adhere to sperm cell membranes during the process of freezing-thawing and preserve the membrane integrity of sperm. Furthermore, it is clear that the dilution of semen to lowsperm number/dose results in a bull dependent reduction in the post thaw viability of bovine spermatozoa. It is possible that essential seminal plasma components are lacking in the higher dilution rates and in the freezing process[107]. The additions of LDL provide some compensatory protection against the detrimental effects of dilution and freezing in semen preservation.
7. Preparation of LDL based extenders
In conventional method of semen extender preparation, 20% EY is used. In LDL based extender, 8% (w/v dry weight) LDL is used. The 20% EY contains 68% LDL (13.6 gm) and on dry matter percentage is approximately 60% (8.16 gm). The calculation indicates that 20%EY contains 8% LDL on dry matter basis. The preparation of LDL based clarified extenders is similar to conventional method of EY based extenders where bigger size EY particles are removed in LDL based extender. The EY is either centrifuged at 600 ×g for 10 min and only the top most supernatant is used to prepare the semen freezing extender[108] or the Tris glycerol extender containing 20% EY is centrifuged at 50 000 × g for 2 h and the top most supernatant is used in the cryopreservation extender[76]. In preparation of LDL extenders, the extraction of low density fraction is done from the sterilized EY from the hen. This can be done with several different methods all that have in common phenomenon that the yolk components separation is by centrifugation technology. The recent method was explained by Moussa et al[50] and considered as four basically steps: a) the yolk granules are separated from the yolk plasma and the granules are allowed to soluble in sodium chloride[109] and the granules are removed after centrifugation as the pellet at the bottom[50]. b) The crucial precipitation of the livetins in the remaining EY plasma can be performed with ammonium sulphate[50]. The livetins are removed after centrifugation as the pellet. c) Frequent dialysis of the remaining supernatant (LDLs)against distilled water to remove the ammonium sulfate and finalize the purification[50]. d) The centrifugation of the dialysate to collect the LDL fraction as the creamy white floating top LDL rich layer[50].After this stepwise extraction, the LDL extender is prepared with the basic components of the conventional EY extenders. The extender for bovine semen differs by containing 8% (w/v) LDL on dry matter basis instead of 20% (v/v) whole EY[50].
8. Effect of LDL in different species
Many investigators made attempts to find out the components in the EY responsible for cryoprotection of sperm, but Pace and Graham[41] first identified and purified the components (LDL) from the EY by using the method of ultracentrifugation and assessed in the cryopreservation and proved that LDL has cryoprotective action on sperm cryopreservation. Later on, Foulkes[45] and Moussa et al[50]confirmed that LDLs have the cryoprotective action in the EY. In recent times, many investigators has reported that instead of whole EY, LDL extracted from EY has been utilized in semen extender and has improved the freezability and fertility in many different species at different concentration viz. bovine (8% LDL[50,55-59]), ovine(8% LDL[60]; 9% LDL[61]), bubaline (10% LDL[62]), canine (6 %LDL[63,64], porcine (9% LDL([65-67]) & 12% LDL[68]), Agu pig(4%-6% LDL[69]), Iberian red deer (8% LDL[70]) and mithun (8%LDL[36-39]) (Table 1).
9. LDL in semen preservation with reference to mithun species
9.1. Post thaw motility
In mithun bulls, semen extender containing 8% LDL (w/v dry mater basis) had significantly higher sperm motility than 20%EY[36-39,50,57-59,77] (P<0.05). The 8% LDL contained extender has supported for the best protection to sperm acrosomal integrity and forward progressive motility due to the possible action on direct repair or exchange of plasma & acrosomal membrane cholesterol and/or phospholipids or by the LDL contained extender has less progesterone molecules or its precursor than in EY because of the effect of filtration process by the membrane of dialysis in the procedure to extract the LDL from EY of hen. Besides, it is also reported that sperm forward progressive motility is dependent upon partiality, the membrane integrity as well as transport. Inclusion of LDL in the extender, more than optimum (8%) results lowered sperm motility as the increased level of LDL in the extender above 8% may lead to alteration in the osmotic pressure and physiological status of the extender and may lead to deleterious effects to spermatozoa during cryopreservation. Diminished sperm metabolism and reduced post thaw sperm motility have been reported for both ram[43]and bull sperm[71] frozen in diluents with EY. Further, negative deleterious effects of fresh whole EY on sperm viability as well as motility have been contributed by the direct action of HDL[41,73].The significantly higher cryoprotection effect is possible in LDL contained extender because the presence of detrimental substances like HDL, progesterone, microbial substances, etc. are not present in the LDL based extender as compared to EY based extender. Further,it has been reported the composition of EY is differentiable based on the nutrition and breeds of the chickens[44]. EY of hen is considered to protect sperm, and also known that the substances present in EY inhibit respiration of sperm as well as the motility[42].
9.2. Livability
LDL at 8% in semen preservation extender has significantly higher sperm livability than 20% EY[36-39,50,57-59,77] (P<0.05). In mithun,LDL at 10% or more may cause alteration in the physiological osmolarity of the extender and lead to poor sperm viability, and extender containing whole EY leads to higher level of high density lipoprotein, steroid hormone especially progesterone and higher level of calcium ions, which causes deleterious effects on sperm viability. Similarly, variable concentration of LDL has improved on sperm viability in different species: bovine (8% LDL[50,55,56-59]),ovine (8% LDL[60]; 9% LDL[61]), bubaline (10% LDL[62]), canine (6% LDL[63,64]), porcine (9%[65-67]-12% LDL[68]), Agu pig (4%-6%LDL[69]), Iberian red deer (8% LDL[70]) and mithun (8% LDL[36-39]). The variation in different species may be due to the composition of phospholipids and cholesterol of sperm plasma membrane and ratio of chemicals in the membrane among the species and also degrees of susceptibility to cold shock or freezing protocols.
9.3. Total sperm abnormality
In mithun species, 8% LDL treated semen extender has significantly lesser percentage of total sperm abnormality than inextender contains 20% EY[36-39]). 8% LDL is more suitable for mithun (bovine) semen preservation than 10% LDL or 20% EY.The LDL is composed of about 12% proteins and 87% lipids, it is also of spherical in shape with a average diameter of 35 nm[105] and the triglyceride core is surrounded by a coat of phospholipids and proteins[84], therefore 8% LDL contained optimum amount of lipids as well as protein than 10% LDL. Further, EY as such contains high level of HDL, steroid molecules and Ca2+, which results in premature capacitation as well as premature acrosomal reaction and causes deleterious effect on the normality of sperm in EY treated group. During freeze-thawing process, 8% LDL is disrupted and optimum concentration of phospholipid is liberated into the semen preservation extender, which could form a protective coat over the surface of sperm membranes[106]. In cattle, as 8% LDL was more suitable and has lesser abnormality than 20% EY as reported in other bovine species[50,57-59,77]. However, the effect of LDL concentration on total sperm abnormality in semen extender is species-specific[60,62,63,68].

Table 1Comparison between LDL and EY (20%) on semen quality parameters in different domestic livestock species.
9.4. Acrosomal integrity
Detachment of acrosome or loss of acrosomal intactness may result into decreased adenosine triphosphate and loss of intracellular enzymes and proteins from spermatozoa. In spite of loss of acrosomal intactness, the spermatozoa may be highly motile but not fertile. Therefore, the acrosomal integrity is always considered as a major part of evaluation of quality of spermatozoa or semen[110].8% LDL treated semen extender has higher number of sperms with acrosomal intactness than in extender contains 20% EY in post thaw stage of semen preservation[36-39]. Acrosomal integrity of spermatozoa has been preserved by LDL in the following two different path ways. In the first method, there is an association between LDL fraction & BSP proteins that protects the sperm from disintegration by preventing the binding of BSP on surface of spermatozoa especially on sperm plasma membrane. In the second method, the lipid released from LDL could associate or sediment with the sperm plasma membrane which in turn preserves the intactness of the plasma membrane of sperm during sperm cryopreservation. LDL has a very high capacity for BSP protein binding and the binding is specific, rapid and stable even after semen freezing-thawing processes[77]. Therefore, LDL offers higher protection to maintain the acrosomal integrity. Sperms that undergoe acrosome reaction spontaneously after semen ejaculation and/or following freezing and thawing are not have capability to bind with the zona pellucida and therefore are not able to fertilize the oocyte[111]. Witte and Schäfer-Somi[112] stated that EY prevents a significant higher capacitated spermatozoa. Extender made up of 8% LDL supported the best protection of acrosome intactness of sperm, by the repair and/or exchange of membrane of acrosome phospholipids and cholesterol or the LDL contained extender have less progesterone than in extender containing EY due to the dialysis membrane as it has filtration effect in the process of preparation of LDL or both the mechanisms mentioned above.
9.5. Plasma membrane integrity
The integrity of the sperm plasma membrane, motility and acrosomal integrity of spermatozoa are essential for the fertilizing capacity of spermatozoa. A hypo-osmotic swelling test can help to assess the functional as well as physiological status of the membrane potentiality[113]. 8% LDL added semen has significantly higher percent of spermatozoa with plasma membrane integrity than 20% EY at pre-freeze and post thaw stage[36-39]. More number of spermatozoa with intact acrosome is observed in 8%LDL treated semen extender[50,57-59,77]. Cryopreservation can have effect on organization of lipid and sperm plasma membrane chemical composition[104]. The LDL contained extender is simple and the chemical composition is less complex than the EY extender which has strong protective action on plasma membrane of sperm subjected to ultralow preservation[56]. LDL indirectly or directly minimizes the modifications of sperm membrane during the sperm freezing-thawing process and this is mainly responsible for the sperm protective action[88]. The lipid and cholesterol from LDL fraction could associate with the plasma membrane of sperm and the intactness of the sperm plasma membrane in sperm preservation has been preserved. The extender containing LDL possibly protects sperm plasma membrane integrity in the following methods.LDL fraction associates with BSP proteins, and that combination protects the sperm from disintegration by preventing the binding of BSP on surface of spermatozoa especially on sperm plasma membrane. The LDL releases the phospholipid that could associate or sediment or form a coat over the sperm plasma membrane which in turn preserves the intactness of the plasma membrane of sperm during sperm cryopreservation. LDL has a very high affinity for BSP protein to bind and the binding is specific, rapid and stable in cryopreserved semen. It was reported that after frozen-thawed, the semen diluted with EY contained extender containing almost 80%lesser BSP proteins than semen or sperm from fresh ejaculates[77].EY contains LDL is the only one component that binds specifically and rapidly with BSP proteins and thus the sequestration of BSP proteins by LDP might represent the major mechanism of sperm protection by EY in the extender[89]. Moreover, Manjunath et al[89]indicated the beneficial effect of LDL was not limited only to the binding of LDL directly to the plasma membrane of sperm but also was involved in interaction between the BSP proteins, LDL as well as the sperm plasma membrane. Therefore, LDL offers major protection by reducing the detrimental or deleterious effect of seminal plasma proteins on sperm membranes and its integrity.
9.6. Nuclear integrity
Spermatozoa in 8% LDL treated semen extender have significantly higher percent of nuclear intactness than in 20% EY treated semen extender[36-39]. The use of LDL instead of pure EY has improved cell protection in terms of protection of DNA integrity at 8%concentrations. In addition of LDL was optimized and 8% LDL was considered the most convenient for the bovine species, due to the increase in DNA damage observed at higher concentrations[50].Moussa et al[50] reported that higher concentration of LDL resulted in an increase in osmotic pressure, because of salt precipitation procedure or the effect of LDL aggregation. EY is a well known to protect the DNA/nucleus and the effect is due to of its LDL as DNA & the membrane stabilizers has been widely reported in different species of livestock in preservation of semen[36-39,57].Previous works in mammals have shown a more beneficial effect of the use of the LDL fraction instead of pure EY since it is not only as a membrane protector but also in the reduction of DNA /nuclear damage[36-39,57]. Moussa et al[50] developed a new extraction methodology for LDL from EY of hen and tested it in bull sperm cryopreservation at concentrations between 2.5% and 20%, showing that the best motility and preservability were achieved at between 5% and 10% and than 20% EY was used. Hu et al[85] compared the use of 9% LDL and 20% EY in boar sperm and obtained significant improvements in motility, DNA damage reduction and plasma membrane & acrosome integrity with the LDL extract in the former than the later.
9.7.Cervical mucus penetration test
In mithun species, 8% LDL treated semen extender has significantly(P<0.05) higher vanguard distance travelled by sperm in the estrus bovine cervical mucus than 20% EY treated extender[36-39]. This is because of improved the motility, viability, plasma membrane,acrosomal membrane, nuclear integrity and mitochondrial membrane potential through different mechanisms like formation of protection coat on the sperm membrane, by preventing the binding of BSP on surface of spermatozoa, reducing steroid concentration, HDL,microbiological risk, aromatic amino oxidase enzyme activity, lesser Ca2+infusion, lessening the glycerol toxicity, filtration of unwanted or unknown substances that detrimental to the sperm, supplying of cholesterol & phospholipids to the membrane, and minimizing the lipid peroxidation (LPO) production & leakage of intra-cellular enzymes and protection of antioxidant profiles in the extender prepared with LDL.
9.8. Computer assisted sperm analysis
In mithun species, 8% LDL have significantly (P<0.05) higher motility and velocity parameters than 20% EY treated extender at post thaw stage of semen preservation[36-39] (Figure 1), as the LDL has improved the motility, viability, plasma membrane, acrosomal membrane and nuclear integrity through formation of protection coat on the sperm membrane and by preventing the binding of BSP
on surface of spermatozoa intrinsically. Thus it ultimately improved the motility and velocity parameters of sperm in LDL treated semen. Further, 8% LDL added semen samples had higher motility and velocity parameters than 10% LDL added or 20% EY as 10%LDL may lead to detrimental effects on the osmotic pressure of the extender and may be harmful to spermatozoa, leading to a decrease in motility and velocity parameters at post thaw stage of semen preservation[36-39].
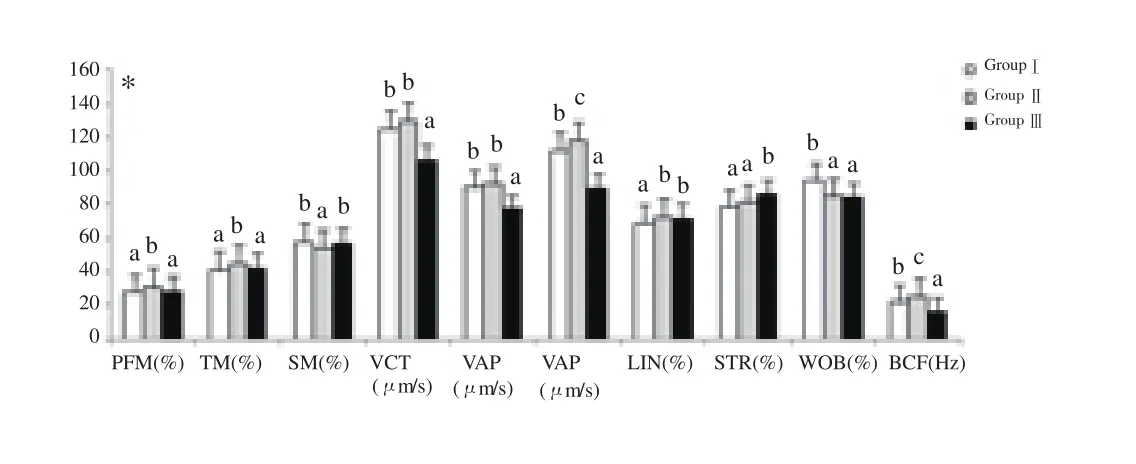
Figure 1. Effect of LDL on motility and velocity parameters in post thawed mithun semen.
9.9. Biochemical attributes
9.9.1. Leakage of intra-cellular enzymes
In mithun species, 8% LDL treated semen has significantly lower leakage of intra-cellular enzymes such as aspartate aminotransferase (AST), alanine aminotransferase (ALT) and lactic acid dehydrogenase (LDH) than in 10% LDL and 20% EY treated extender in post thawed semen[36-39]. Higher activity of AST and ALT at post thaw seminal plasma clearly indicated that much of the enzyme leaked out in the extracellular fluid following deep freezing of semen due to structural damage and increased cell membrane permeability (AST: 236 vs. 280, ALT: 44 vs. 51, LDH: 726 vs. 829 for 8% LDL vs. 20% EY). The LDL protects acrosomal membrane through which in turn protects the intracellular enzymes[77].Therefore, LDL offers protection to acrosomal enzyme in the acrosome of sperm. The 8% LDL contained extender has provided higher protection to acrosomal intactness and its enzyme through the repair and/or exchange of phospholipids of acrosomal membrane or minimized the concentration of progesterone in LDL treated extender than EY based extender due to filtration dialysis membrane effect in the process of LDL based extender preparation. But 10%LDL has lower intact acrosome and higher intracellular enzyme release as the increase concentration of LDL in the extender above optimum (8%) leads to alteration of the osmolarity of the extender.This may be harmful to spermatozoa, which leads to decrease in percent of spermatozoa with intact acrosomal membrane and its contents after freeze thaw. The analysis of LDH activity revealed that 8% LDL treated semen has lower LDH activity than 20% EY treated group in post thawed semen.
9.9.2.Antioxidant profiles
Analysis of antioxidant levels revealed that 8% LDL treated semen has significantly higher value in glutathione (GSH), glutathione peroxidase (GSHPx), glutathione reductase (GSHRx), catalase(CAT), and total antioxidant capacity (TAC) than 20% EY treated group in post thawed semen (GSH: 14 vs. 9, GSHPx: 3.76 vs. 2.12,GSHRx: 47 vs. 36, CAT: 0.83 vs. 0.51, TCA: 160 vs. 119 for 8%LDL vs. 20% EY)[36-39]. Production of the LPO is a physiological phenomenon and the oxygen metabolites are capable to adversely modify the functions of cell and finally lead to endanger the life of the cell[114]. Further, it has been proposed that EY containing extenders may increase the effects of hydrogen peroxide[115].During processing and preservation, the reactive oxygen species(ROS) deteriorates the semen quality as well as the spermatozoa has been damaged[16]. The antioxidant group comprising GSH,GSHPx, GSHRx, CAT, superoxide dismutase (SOD) and TAC has been explained as the important protection functioning mechanisms go towards the LPO and are essential in maintaining the sperm viability and motility[116-118]. SOD is also another essential group of the antioxidant system. Potential function of the CAT antioxidant is to control the ROS in sperm and decide all the parameters that determine semen quality[119]; however, little or no CAT is available in most of the mammalian semen[120]. GSH is another antioxidant that has important function to protect as intra-cellularily on LPO as it functions as important cofactor in GSH peroxidase which catalyses for minimizing of toxic H2O2[118]. GSHPx is also to minimize the hydrogen peroxidation[121]. However, matured sperm discards the most of the cytoplasmic antioxidants in the last states of spermatogenesis and the sperm lose protective defense-enzymes[122].Thus, the spermatozoa are especially easily susceptible to damage due to oxidative damage, mainly during and after cryopreservation along with loss of membrane intactness, decreased total motility,impaired sperm cell function and the fertilizing ability[123]. The antioxidant activities of GSHRx, CAT, GSHPx, TAC and GSH were increased significantly with the LDL containing extender. 8%LDL included extender significantly improved freezability of bull semen. It could be inferred that LDL containing extender has given very good results in protection of sperm against ROS by constantly having good quality and quantity of SOD, CAT, GSHPx, GSHRx,GSH and TAC concentration as compared to 20% EY containing extender. These reports suggested that the deleterious compounds included in EY are not present and extender containing LDL has enhanced/protected the activities of SOD, CAT, GSHPx, GSHRx,GSH and TAC and enhanced quality of frozen semen in bull.
9.9.3. Total cholesterol
Cholesterol is known to be one of the major components of seminal plasma[124]. Several studies have been reported that cholesterol influx reduces spontaneous acrosome reaction[125]. However, its efflux mimics the capacitation and acrosome reaction[96]. Total cholesterol content of spermatozoa in 8% LDL added semen had significantly higher total cholesterol than 20% EY treated group in post thawed semen (Total cholesterol: 13 vs. 8.45 for 8% LDL vs. 20% EY)[36-39].Chemical composition of LDL based extender is simple than the EY based extender, which could explain the protective effect of LDL on the integrity of plasma membrane of spermatozoa. Cryopreservation is known to have deleterious effect on the chemical composition and lipid organization of the plasma membrane of spermatozoa[104]. It has the assumption that LDL has indirectly or directly reduced these modifications of sperm membrane[88]. Manjunath et al[89] properly described that the main mechanism to protect the spermatozoa is through the sequestration of BSP proteins in seminal plasma by using LDL. The bull seminal plasma contains major proteins such as BSP proteins, and the BSP-A1/A2, BSPA3 & BSP-30-kDa bind to the sperm surface at ejaculation and stimulate cholesterol and phospholipids efflux from the sperm membrane. Since LDL interacts specifically with BSP proteins[88], this would reduce the binding of the majority of proteins of seminal plasma of bovine to the spermatozoa and would prevent phospholipid and cholesterol efflux from the sperm membrane, which could explain its beneficial effect.During freeze-thawing process, LDL is disintegrated and phospholipid is liberated into the medium, which could form a protective coat over the surface of sperm plasma membranes[106]. Hu et al[85] reported that LDL is responsible for the process of gelation in the freezing-thawing process. The disruption of the LDL structure is the first step of gelation process and this disruption favours the spermatozoa for dehydration and is caused by the freezing-thawing process. It was reported that LDL could adhere to sperm cell plasma membranes during the process of freezing-thawing and preserve the integrity of membranes of the sperm[88].
9.9.4. LPO / Malondialdehyde (MDA) production
LPO is measured in the form of estimation of malondialdehyde in the semen and this can cause rabid irreversible loss of motility, changes in metabolism and loss of intra-cellular sperm constituents[126].Loss of membrane integrity correlates with LPO levels based on malondialdehyde concentration, as malondialdehyde is a marker of lipid peroxidation[127]. During cryopreservation or liquid preservation, the sperm cells are exposed to cold shock or cold damage with atmospheric oxygen, which in turn increases the susceptibility to lipid peroxidation due to higher production of ROS. Thus, it stimulates ageing of sperm thereby reduces the life span of sperm and affects the semen quality with poor success rate. The LPO occurs in seminal plasma and spermatozoa and can effectively be measured by MDA concentration. MDA production was lower in 8% LDL based extender than in 20% EY based extender in post thawed semen (LPO: 11 vs.15 for 8% LDL vs. 20% EY)[36-39]. LPO level is more due to aromatic amino oxidase enzyme activity in dead sperms, and the increased number of dead sperms may be one of the important attributing factor for increased levels of LPO[128,129] besides oxidative damage to the sperms. A number of studies have been reported on membrane lipid peroxidation as one of the major causes of defective sperm function both in fresh[130] as well as after semen cryopreservation[131]. Unsaturated fatty acids,which are predominant in sperm membranes, are susceptible to peroxidation[132] and the consequences are numerous ranging from membrane damage, inhibition of respiration and leakage of intracellular enzymes to loss of integrity and death of the sperm[133].Slaweta et al[134] reported that LPO in bull sperm increases after cryopreservation. Moreover, Trinchero et al[135] reported that frozen thawed bull sperm are more easily peroxidized than fresh sperm.Induction of membrane leakiness also occurs under conditions that lead to sperm phospholipid peroxidation[136,137], suggesting that cryopreservation might directly or indirectly causes membrane damage by enhancing LPO and a lethal damage. The loss of phospholipids in cryopreserved samples occurs at a more rapid rate as compared to fresh samples and follows the pattern expected for LPO[138].
10. Zona binding assay
Zona binding assay revealed significantly (P<0.05) higher binding percent (BP) and binding index (BI) in 8% LDL based extender than in 20% EY based extender in cryopreserved semen[36-39].This was because of the LDL has improved the motility, viability,plasma membrane, acrosomal membrane & nuclear integrity through formation of protection coat on the sperm membrane and by preventing the binding of BSP on surface of spermatozoa[50,58].Thus, it ultimately improved the BP and BI parameters of sperm in LDL treated semen. Moreover, 8% LDL treated semen samples had significantly higher BP and BI value than 10% LDL, as 10%LDL leads to alteration in osmotic pressure or physiological status of the extender and may be harmful to spermatozoa, which leads to decrease in motility and velocity parameters of spermatozoa.Further, EY present in standard tris citrate extender in control group has higher level of high density lipoprotein, steroid molecules and high level of Ca2+, which causes adverse effects on sperm structure& functions as well as velocity parameters resulting in poor BP and BI[50,58]. It was reported that cleavage rate was higher in LDL extender, and the blastocyst formation and pregnancy rates were not different from sperm cryopreserved in chicken yolk extender[55].
11. Conclusion
Semen collection, preservation and artificial insemination play important roles in genetic improvement and improvement of production potential in livestock husbandry. More than half of the sperms die on frozen thawing process in cryopreservation of semen and this is due to various processing and preservation causes. Of which, one such main cause is cold shock or cryoinjury especially during the cryopreservation process and this is reduced by various methods or modification of methods of cryopreservation or inclusion of different additives in the semen preservation extender. The one such method is to find out the active substances present in the EY responsible for cold shock protection instead use of EY as such.Finally, the key component was identified and purified as LDL has the cryoprotective effect for mammalian sperm preservation in ultralow temperature state. The EY contains other substances, like HDL, steroid molecules, microorganisms, high content of calcium ions, etc. which are responsible for cryo capacitation and premature acrosomal reaction, lead to poor vitality & motility, and in turn affect the fertility of the sperm. Therefore, there was increased demand on replacement of the EY with the key substances (LDL) in the semen extender. Different researchers in different countries tried to extract the LDL from the EY of hen and utilize it in semen preservation instead of use of EY and finally succeeded. The level of LDL is varied in different species and this may be due to the composition and concentration of phospholipids, cholesterol and its proportion in the sperm membranes. For instance, in bovine, the optimum dose of LDL was standardized as 8% (w/v) on dry matter basis. This is equal to 20% EY as used in conventional semen extender. This was described as the 20% EY contains 68% LDL (13.6 g) and on dry matter basis, it is about 60% (8.16 gm). This assumption indicates 20% EY contains 8% LDL on dry matter basis. The LDL protects the sperm by various mechanisms to maintain the integrity of sperm membrane, which in turn increases the motility, viability and ultimately the fertility. But investigations still need to be carried out to find out the exact roles of apoproteins and lipids of LDL and to indentify and isolate the detrimental substances present in the whole EY.
Conflict of interest statement
The author declares that he has no conflict of interest.
[1] Ozkavukcu S, Erdemli E, Isik A, Oztuna D, Karahuseyinoglu S. Effects of cryopreservation on sperm parameters and ultrastructural morphology of human spermatozoa. J Assist Reprod Genet 2008; 25(8): 403-411.
[2] Perumal P. Cryopreservation of bovine semen with some additives for augmenting fertility[M.V.Sc. thesis]. Bhubaneswar, Orissa, India: Orissa University of Agriculture and Technology; 2008.
[3] Perumal P, Selvaraju S, Selvakumar S, Barik AK, Mohanty DN, Das S,et al. Effect of pre-freeze addition of cysteine hydrochloride and reduced glutathione in semen of crossbred Jersey bulls on sperm parameters and conception rates. Reprod Domest Anim 2011; 46(4): 636-641.
[4] Perumal P, Selvaraju S, Barik AK, Mohanty DN, Das S, Mishra PC. Role of reduced glutathione in improving post-thaw seminal parameters in poor freezable Jersey crossbred bull semen. Indian J Anim Sci 2011; 81(8):807-810.
[5] Waberski D. Boar seminal plasma and fertility. Reprod Domest Anim 2007;31(1): 87-90.
[6] Perumal P, Selvaraju S, Barik AK, Mohanty DN, Das S, Mishra PC, et al.Reduced glutathione and cysteine hydrochloride on sperm motility and velocity parameters of poor crossbred bull semen. Int J Bio-Resour Stress Manag 2012; 3(2): 145-151.
[7] Perumal P, Vupru K, Rajkhowa C. Effect of addition of reduced glutathione on the liquid storage (5 ℃) of mithun (Bos frontalis) semen.Indian J Anim Sci 2013; 83(10): 1024-1028.
[8] Perumal P, Vupru K, Rajkhowa C. Effect of addition of trehalose on the liquid storage (5 ℃) of mithun (Bos frontalis) semen. Indian J Anim Res 2015; 49(6): 837-846.
[9] Perumal P, Chamuah JK, Rajkhowa C. Effect of catalase on the liquid storage (5 ℃) of mithun (Bos frontalis) semen. Asian Pac J Reprod 2013;2(3): 209-214.
[10] Perumal P, Vupru K, Rajkhowa C. Effect of addition of cysteine hydrochloride on the liquid storage (5 ℃) of mithun (Bos frontalis)semen. Indian Vet J 2014; 91(02): 76-78.
[11] Perumal P, Chamuah JK, Nahak AK, Rajkhowa C. Effect of melatonin on the liquid storage (5 ℃) of semen with retrospective study of calving rate at different season in mithun (Bos frontalis). Asian Pac J Reprod 2015;4(1): 1-12.
[12] Perumal P, Srivastava SK, Ghosh SK, Baruah KK, Bag S, Rajoriya JS, et al. Low density lipoproteins as additive improves quality parameters and biomarkers of oxidative stress following cryopreservation of mithun (Bos frontalis) spermatozoa. Reprod Domest Anim 2016; 51: 708-716.
[13] Perumal P, Savino N, Sangma CTR, Khan MH, Ezung E, Chang S, et al. Seasonal effect on physiological, reproductive and fertility profiles in breeding mithun bulls. Asian Pac J Reprod 2017; 6(6): 268-278.
[14] Perumal P, Vupru K, Rajkhowa C. Effect of addition of taurine on the liquid storage (5 ℃) of mithun (Bos frontalis) semen. Vet Med Int 2013;2013: 165348.
[15] Perumal P. Effect of superoxide dismutase on the liquid storage (5 ℃) of mithun (Bos frontalis) semen. J Anim 2014; 2014: 821954.
[16] Celeghini ECC, Arruda RP, Andrade AFC, Nascimento J, Raphael CF,Rodrigues PHM. Effects that bovine sperm cryopreservation using two different extenders has on sperm membranes and chromatin. Anim Reprod Sci 2007; 100: 1-13.
[17] Rajoriya JS, Prasad JK, Ramteke SS, Perumal P, Ghosh SK, Singh M, et al. Enriching membrane cholesterol improves stability and cryosurvival of buffalo spermatozoa. Anim Reprod Sci 2016; 164: 72-81.
[18] Perumal P, Bora B, Veeraselvam M, Rajoriya JS, Nazar S, Srivastava N, et al. Effect of heterogenous cattle bull seminal plasma on seminal parameters of mithun (Bos frontalis). Indian J Anim Sci 2015; 85(4): 361-363.
[19] Perumal P, Brijesh Kumar, Balamurugan TC, Srivastava N. Effect of in vitro incubation of buffalo (Bubalus bubalis) follicular fluid on physico morphological attributes of mithun (Bos frontalis) spermatozoa. Indian J Anim Sci (accepted) 2017; Manuscript ID: 46209.
[20] Perumal P, Rajkhowa C. Effect of addition of Pomegranate (Punica granatum) juice on the liquid storage (5 ℃) of mithun (Bos frontalis)semen. Indian J Anim Res 2015; 49(4): 470-473.
[21] Jayaganthan P, Perumal P, Balamurugan TC, Verma RP, Singh LP,Pattanaik AK, et al. Effects of Tinospora cordifolia supplementation on semen quality and hormonal profile of ram. Anim Reprod Sci 2013;140(1): 47-53.
[22] Jayaganthan P, Perumal P, Balamurugan TC, Verma RP. Effect of Tinospora cordifolia supplementation on sexual behaviuor and semen production of rams. Indian J Anim Res 2015; 49(1): 135-137.
[23] Rajoriya JS, Prasad JK, Ghosh SK, Perumal P, Anuj Kumar, Kaushal S, et al. Effects of seasons on enzymatic changes and cholesterol efflux in relation to freezability in Tharparkar bull semen. Asian Pac J Reprod 2013; 2(4): 280-288.
[24] Rajoriya JS, Prasad JK, Ghosh SK, Perumal P, Anuj Kumar, Kaushal S,et al. Studies on effect of different seasons on expression of HSP 70 and HSP 90 gene in sperm of Tharparkar bull semen. Asian Pac J Reprod 2014; 3(3): 192-199.
[25] Baruah KK, Mondal, M, Dhali A, Bora B, Perumal P, Rajkhowa C. Effect of season on quality of mithun (Bos frontalis) semen. Indian J Anim Sci 2014; 85(5): 475-476.
[26] Rajoriya JS, Chauhan M, Prasad JK, Ghosh SK, Singh M, Kumar A, et al. Enzymatic profile of Tharparkar bull semen in winter and summer season. Int J Bio-Resour Stress Manag 2016; 7(6): 1356-1360.
[27] Perumal P, Khate K, Rajkhowa C. Effect of foot and mouth disease vaccination on seminal and biochemical profiles of mithun (Bos frontalis)semen. Asian Pac J Reprod 2013; 2(3): 209-214.
[28] Perumal P, Khate K, Rajkhowa C. Effect of foot and mouth disease vaccination on seminal and biochemical profiles of mithun (Bos frontalis)semen. Asian Pac J Reprod 2013; 2(3): 209-214.
[29] Perumal P. Effect of foot and mouth disease vaccination on semen production in mithun (Bos frontalis). J Res 2014; 1: 935.
[30] Perumal P. Effect of unilateral cryptorchidism on seminal parameters of mithun (Bos frontalis). Indian Vet J 2013; 91(9): 43-46.
[31] Perumal P, Chang S, Sangma CTR, Khate K, Saminathan M. Unilateral cryptorchidism on mobility and velocity parameters in mithun sperm. J Exper Biol Agric Sci 2016; 4(suppl 3): S116-S122.
[32] Perumal P, Vupru K, Khate K, Veeraselvam M, Verma AK, Nahak AK, et al. Spontaneous erection and masturbation in mithun (Bos frontalis) bulls.Int J Bio-Resour Stress Manag 2013; 4(4): 645-647.
[33] Khan MH, Sinha P, Perumal P, Hazarika SB. Cryopreservation of Mithun semen: Comparative study of conventional vs controlled freezing. Indian J Anim Sci 2017; 87(6): 728-730.
[34] Perumal P, Rajkhowa C. Scrotal circumference and its relationship with testicular growth, age and semen production in mithun (Bos frontalis)bulls. Indian J Anim Sci 2013; 83(10): 1074-1077.
[35] Perumal P, Savino N, Sangma CTR, Chang S, Sangtam TZT, Khan MH, et al. Effect of season and age on scrotal circumference, testicular parameters and endocrinological profiles in mithun bulls. Theriogenology 2017; 98: 23-29.
[36] Perumal P. Studies on effect of low density lipoprotein on freezability and fertilizing ability of spermatozoa in mithun (Bos frontalis) bulls[Ph.D. thesis]. Izatnagar, Bareilly, Uttar Pradesh, India: ICAR-Indian Veterinary Research Institute, Deemed University, 2014.
[37] Perumal P, Srivastava SK, Ghosh SK, Baruah KK, Khan MH, Rajoriya JS, et al. Effect of low density lipoprotein in liquid preservation (5 ℃) of mithun semen. Indian J Anim Sci 2016; 86(4): 427-430.
[38] Perumal P, Srivastava SK, Ghosh SK, Baruah KK, Bag S, Rajoriya JS, et al. Low density lipoproteins as additive improves quality parameters and biomarkers of oxidative stress following cryopreservation of mithun (Bos frontalis) spermatozoa. Reprod Domest Anim 2016; 51: 708-716.
[39] Perumal P, Srivastava SK, Baruah KK, Rajoriya JS, Srivastava N.Low density lipoprotein on poor quality mithun (Bos frontalis) semen preservation. Indian J Anim Res 2016; 51(3): 576-581.
[40] Burris C, Webb G. Effects of egg yolk source on the cryopreservation of stallion semen. J Equine Vet Sci 2009; 29: 336-337.
[41] Pace MM, Graham EF. Components in egg yolk which protect bovine spermatozoa during freezing. J Anim Sci 1974; 39: 1144-1149.
[42] Moustacas VS, Zaffalon FG, Lagares MA, Loaiza-Eccheverri AM,Varago FC, Neves MM, et al. Natural, but not lyophilized, low density lypoproteins were an acceptable alternative to egg yolk for cryopreservation of ram semen. Theriogenology 2011; 75: 300-307.
[43] Kulaksız R, Çebi Ç, Akçay E, Da kın A. The protective effect of egg yolk from different avian species during the cryopreservation of Karayaka ram semen. Small Rumin Res 2010; 88: 12-15.
[44] Watson PF. The protection of ram and bull spermatozoa by the low density lipoprotein fraction of egg yolk during storage at 5 ℃ and deepfreezing. J Ther Biol 1976; 1: 137-141.
[45] Foulkes JA. The separation of lipoproteins from egg yolk and their effect on the motility and integrity of bovine spermatozoa. J Reprod Fertil 1977;49: 277-284.
[46] Banaszak L, Sharrock W, Timmins P. Structure and function of a lipoprotein: Lipovitellin. Annu Rev Biophys Chem 1991; 20: 221-246.
[47]Kuksis A. Yolk lipids. Biochim Biophys Acta 1992; 1124: 205-222.
[48] Quinn PJ, Chow PY, White IG. Evidence that phospholipid protects spermatozoa from cold shock at a plasma membrane site. J Reprod Fertil 1980; 60: 403-407.
[49] Babiak I, Glogowski J, Luczynski MJ, Luczynski M, Demianowicz W.The effect of egg yolk, low density lipoproteins, methylxanthines and fertilization diluent on cryopreservation efficiency of northern pike (Esox lucius) spermatozoa. Theriogenology 1999; 52: 473-479.
[50] Moussa M, Martinet V, Trimeche A, Tainturier D, Anton M. Low density lipoproteins extracted from hen egg yolk by an easy method:Cryoprotective effect on frozen-thawed bull semen. Theriogenology 2002;57: 1695-1706.
[51] Bergeron A, Manjunath P. New insights towards understanding the mechanisms of sperm protection by egg yolk and milk. Mol Reprod Dev 2006; 73: 1338-1344.
[52] Perumal P, Chang S, Ezung E, Khan MH, Vupru K, Khate K. Effect of egg yolk powder on cryopreservation of mithun semen. Indian J Anim Sci 2018; 88(1): 37-39.
[53] Singh M, Ramteke SS, Ghosh SK, Prasad JK, Rajoriya JS. Efficacy of egg yolk from three avian species on semen freezability of Tharparkar bull. Indian J Anim Reprod 2013; 34(2): 25-28.
[54] Rauch A. Cryopreservation of bovine semen in egg yolk based extenders[M.Sc. Thesis]. Saskatoon, Canada: University of Saskatchewan; 2013.
[55] Amirat L, Tainturier D, Jeanneau L, Thorin C, Gerard O, Courtens JL,et al. Bull semen in vitro fertility after cryopreservation using egg yolk LDL: A comparison with optidyl, a commercial egg yolk extender.Theriogenology 2004; 61: 895-907.
[56] Vera-Munoz O, Amirat-Briand L, Diaz T, Vasquez L, Schmidt E,Desherces S, et al. Effect of semen dilution to low-sperm number per dose on motility and functionality of cryopreserved bovine spermatozoa using low-density lipoproteins (LDL) extender: Comparison to Triladyl®and Bioxcell®. Theriogenology 2009; 71: 895-900.
[57] Hu JH, Li QW, Zan LS, Jiang ZL, An JH, Wang LQ, et al. The cryoprotective effect of low-density lipoproteins in extenders on bull spermatozoa following freezing-thawing. Anim Reprod Sci 2010; 117: 11-17.
[58] Hu JH, Jiang ZL, Lv RK, Li QW, Zhang SS, Zan LS, et al. The advantages of low-density lipoproteins in the cryopreservation of bull semen. Cryobiology 2011; 62: 83-87.
[59] Amirat-Briand L, Bencharif D, Vera-Munoz O, Pineau S, Thorin C,Destrumelle S, et al. Factors affecting the quality of cryopreserved buffalo (Bubalus bubalis) bull spermatozoa. Reprod Domest Anim 2010;44: 552-569.
[60] Tonieto RA, Goularte KL, Gastal GDA, Schiavon RS, Deschamps JC, Lucia (Jr.) T. Cryoprotectant effect of trehalose and low-density lipoprotein in extenders for frozen ram semen. Small Rumin Res 2010;93: 206-209.
[61] Hong W, Ni L, Tian D, Lu Y, Deng C. Effect of LDL on cryopreservation of sheep spermatozoa. Anim Husbandry Feed Sci 2008; 4: 29-31.
[62] Akhter S, Ansari MS, Rakha BA, Andrabi SMH, Khalid M, Ullah N.Effect of low density lipoproteins in extender on freezability and fertility of buffalo (Bubalus bubalis) bull semen. Theriogenology 2011; 76: 759-764.
[63] Bencharif D, Amirat L, Anton M, Schmitt E, Desherces S, Delhomme G, et al. The advantages of LDL (low density lipoproteins) in the cryopreservation of canine semen. Theriogenology 2008; 70: 1478-1488.
[64] Bencharif D, Amirat L, Pascal O, Anton M, Schmitt E, Desherces S, et al. The advantages of combining low-density lipoproteins with glutamine for cryopreservation of canine semen. Reprod Domest Anim 2010; 45:189-200.
[65] Jiang ZL, Li QW, Hu JH, Li WY, Zhao HW, Zhang SS. Improvement of the quality of boar cryopreservation semen by supplementing with low density lipoprotein in diluents. Cryobiology 2007; 54: 301-304.
[66] Jiang ZL, Li QW, Li WY, Hu JH, Zhao HW, Zhang SS. Effect of low density lipoprotein on DNA integrity of freezing-thawing boar sperm by neutral comet assay. Anim Reprod Sci 2007; 99: 401-407.
[67] Hu JH, Li QW, Jiang ZL, Li WY. Effects of different extenders on DNA integrity of boar spermatozoa following freezing-thawing. Cryobiology 2008; 57: 257-262.
[68] Pérez-Cerezales S, Martínez-Páramo S, Beirão J, Herráez MP.Evaluation of DNA damage as a quality marker for rainbow trout sperm cryopreservation and use of LDL as cryoprotectant. Theriogenology 2010;74: 282-289.
[69] Yamauchi S, Nakamura S, Lay KM, Azuma T, Yakabi T, Muto N, et al.Characteristics of Okinawan native Agu pig spermatozoa after addition of low-density lipoprotein to freezing extender. J Reprod Dev 2009; 55:558-565.
[70] Martinez-Pastor F, Martinez F, Alvarez M, Maroto-Morales A, Garcia-Alvarez O, Soler AJ, et al. Cryopreservation of Iberian red deer (Cervus elaphus hispanicus) spermatozoa obtained by electroejaculation.Theriogenology 2009; 71: 628-638.
[71] Pace MM, Graham EF. The release of glutamic oxaloacetic transaminase from bovine spermatozoa as a test method of assessing semen quality and fertility. Biol Reprod 1970; 3: 140-146.
[72] Akhter S, Ansari MS, Andrabi SMH, Ullah N, Qayyum M. Effect of antibiotics in extender on bacterial and spermatozoal quality of cooled buffalo (Bubalus bubalis) bull semen. Reprod Domest Anim 2008; 43: 272-278.
[73] Therien I, Moreau R, Manjunath P. Bovine seminal plasma phospholipidbinding proteins stimulate phospholipid efflux from epididymal sperm.Biol Reprod 1999; 61: 590-598.
[74] Srivastava N, Srivastava SK, Ghosh SK, Prasad JK, Amit Kumar, Tripathi RP, et al. Effect of sequestration of PDC-109 protein on freezabilty and fertilizing ability of crossbred bull spermatozoa during cryopreservation.Anim Reprod Sci 2012; 131: 54-62.
[75] Vishwanath R, Shannon P, Curson B. Cationic extracts of egg yolk and their effects on motility, survival and fertilizing ability of bull sperm.Anim Reprod Sci 1992; 29: 185-194.
[76] Wall RJ, Foote RH. Fertility of bull sperm frozen and stored in clarified egg yolk-tris-glycerol extender. J Dairy Sci 1999; 82(4): 817-821.
[77] Nauc V, Manjunath P. Radioimmunoassay for bull seminal plasma proteins (BSP-AI/-A2, BSP-A3, and BSP-30-kDa), and their quantification in seminal plasma and sperm. Biol Reprod 2000; 63: 1058-1066.
[78] Anton M. Composition and structure of hen egg yolk. In: Huopalahti R,López-Fandiño R, Anton M, Schade R, editors. Bioactive egg compounds.Berlin/Heidelberg: Springer-Verlag; 2007, p. 1-6.
[79] Anton M, Nau F, Nys Y. Bioactive egg components and their potential uses. Worlds Poult Sci J 2006; 62: 429-438.
[80] Schade R, Chacana PA. Livetin fractions (IgY). In: Huopalahti R, López-Fandiño R, Anton M, Schade R, editors. Bioactive egg compounds. Berlin/Heidelberg: Springer Verlag, 2007; p. 25-32.
[81] Anton M. High-density lipoproteins (HDL) or lipovitellin fraction. In:Huopalahti R, López-Fandiño R, Anton M, Schade R, editors. Bioactive egg compounds. Berlin/Heidelberg: Springer- Verlag; 2007, p. 13-16.
[82] Dong QX, Rodenburg SE, Hill D, VandeVoort CA. The role of lowdensity lipoprotein (LDL) and high-density lipoprotein (HDL) in comparison with whole egg yolk for sperm cryopreservation in rhesus monkeys. Asian J Androl 2011; 13: 459-464.
[83] Anton M. Low-density lipoproteins (LDL) or lipovitellenin fraction. In:Huopalahti R, López-Fandiño R, Anton M, Schade R, editors. Bioactive egg compounds. Berlin/Heidelberg: Springer-Verlag; 2007, p. 7-12.
[84] Anton M, Castellani O, Guérin-Dubiard C. Phosvitin. In: Huopalahti R,López-Fandiño R, Anton M, Schade R, editors. Bioactive egg compounds.Berlin/Heidelberg: Springer-Verlag; 2007, p. 17-24.
[85] Hu JH, Li QW, Li G, Chen XY, Yang H, Zhang SS, et al. The cryoprotective effect on frozen-thawed boar semen of egg yolk low density lipoproteins. Asian-Australian J Anim Sci 2006; 19: 486-494.
[86] Ricker JV, Linfor JJ, Delfino WJ, Kysar P, Scholtz EL, Tablin F.Equine sperm membrane phase behaviour: The effects of lipid-based cryoprotectants. Biol Reprod 2006; 74: 359-365.
[87] Maldjian A, Pizzi F, Gliozzi T, Cerolini S, Penny P, Noble R. Changes in sperm quality and lipid composition during cryopreservation of boar semen. Theriogenology 2005; 63: 411-421.
[88] Bergeron A, Crête MH, Brindle Y, Manjunath P. Low-density lipoprotein fraction from hen’s egg yolk decreases the binding of the major protein of bovine seminal plasma to sperm and prevents lipid efflux from the sperm membrane. Biol Reprod 2004; 70: 708-717.
[89] Manjunath P, Nauc V, Bergeron A, Menard M. Major proteins of bovine seminal plasma bind to the low-density lipoprotein fraction of hen’s egg yolk. Biol Reprod 2002; 67: 1250-1258.
[90] Desnoyers L, Manjunath P. Major proteins of bovine seminal plasma exhibit novel interactions with phospholipids. J Biol Chem 1992; 267:10149-10155
[91] Manjunath P, Chandonnet L, Leblond E, Desnoyers L. Major proteins of bovine seminal vesicles bind to spermatozoa [published erratum appears in Biol Reprod 1994: 50(4): 977]. Biol Reprod 1994; 50(1): 27-37.
[92] Manjunath P, Baillargeon L, Marcel YL, Seidah NG, Chretien M,Chapdelaine A. Diversity of novel proteins of gonadal fluids. In:McKerns KW, Chretien M, editors. Molecular biology of brain and endocrine systems. New York: Plenum Press; 1988, p. 259-273.
[93] Manjunath P, Marcel YL, Uma J, Seidah NG, Chretien M, Chapdelaine A. A polipoprotein A-1 binds to a family of bovine seminal plasma proteins. J Biol Chem 1989; 264: 16853-16857.
[94] Chandonnet L, Roberts KD, Chapdelaine A, Manjunath P. Identification of heparin-binding proteins in bovine seminal plasma. Mol Reprod Dev 1990; 26: 313-318.
[95] Therien I, Bleau G, Manjunath P. Phosphatidylcholine-binding proteins of bovine seminal plasma modulate capacitation of spermatozoa by heparin. Biol Reprod 1995; 52: 1372-1379.
[96] Therien I, Souberyrand S, Manjunath P. Major proteins of bovine seminal plasma modulate sperm capacitation by high-density lipoprotein. Biol Reprod 1997; 57: 1080-1088.
[97] Therien I, Moreau R, Manjunath P. Major proteins of bovine seminal plasma and high-density lipoprotein induce cholesterol efflux from epididymal sperm. Biol Reprod 1999; 59: 768-776.
[98] Vishwanath R, Shannon P. Storage of bovine semen in liquid and frozen state. Anim Reprod Sci 2000; 62: 23-53.
[99] Andrabi SMH, Ansari MS, Ullah N, Anwar M, Mehmood A, Akhter S. Duck egg yolk in extender improves the freezability of buffalo bull spermatozoa. Anim Reprod Sci 2008; 104: 427-433.
[100] Muller-Schlosser F, Aires V, Hinsch E, Hinsch KD. Evaluation of the quality of a new generation of egg yolk free semen diluters for cryopreservation of bovine semen. In: the 34th Conference on Physiology and Pathology of Reproduction; Giessen, 2001.
[101] Gil J, Lundeheim N, Soderquist L, Rodriguez-Martinez H. Influence of extender, temperature, and addition of glycerol on post-thaw sperm parameters in ram semen. Theriogenology 2003; 59: 1241-1255.
[102] Luster SM. Cryopreservation of bovine and caprine oocytes by vitrification [dissertation]. USA: Louisiana State University and Agricultural and Mechanical College, Urbana-Champaign; 2004.
[103] Ali Al Ahmad MZ, Chatagnon G, Amirat L, Moussa M, Tainturier D,Anton M, et al. Use of glutamine and low density lipoproteins isolated from egg yolk to improve buck semen freezing. Reprod Domest Anim 2008; 43: 429-436.
[104] Amann RP, Pickett BW. Principles of cryopreservation and a review of cryopreservation of stallion spermatozoa. Equine Vet Sci 1987; 7: 145-173.
[105] Anton M, Martinet V, Dalgalarrondo M, Beaumal V, David-Briand E,Rabesona H. Chemical and structural characterization of low-density lipoproteins purified from hen egg yolk. Food Chem 2003; 83: 175-183.
[106] Cookson AD, Thomas AN, Foulkes JA. Immunochemical investigation of the interaction of egg yolk lipoproteins with bovine spermatozoa. J Reprod Fertil 1984; 70: 599-604.
[107] Garner DL, Thomas CA, Gravance CG, Marshall CE, De Janette JM,Allen CH. Seminal plasma addition attenuates the dilution effect in bovine sperm. Theriogenology 2001; 56: 31-40.
[108] Vidament M, Ecot P, Noue P, Bourgeois C, Magistrini M, Palmer E.Centrifugation and addition of glycerol at 22 ℃ instead of 4 ℃ improve post-thaw motility and fertility of stallion spermatozoa. Theriogenology 2001; 54: 907-919.
[109] De Meulenaer B, Huyghebaert A. Isolation and purification of chicken egg yolk immunoglobulins: A review. Food Agric Immunol 2010; 13:275-288.
[110] Kumar N, Verma RP, Singh LP, Varshney VP, Dass SS. Effect of different levels and sources of zinc supplementation on quantitative and qualitative semen attributes and serum testosterone level in crossbred cattle (Bos indicus × Bos taurus) bulls. Reprod Nutr Dev 2006; 46: 663-675.
[111] Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill J, editors.Physiology of reproduction. New York: Raven Press; 1994, p. 189-317.
[112] Witte TS, Schäfer-Somi S. Involvement of cholesterol, calcium and progesterone in the induction of capacitation and acrosome reaction of mammalian spermatozoa. Anim Reprod Sci 2007; 102: 181-193.
[113] Kumar A, Saxena A, Verma AK, Perumal P. Hypo osmotic swelling test on buffalo (Bubalus bubalis) semen. Buffal Bull 2014; 33(1): 111-114.
[114] Prathalingam NS, Holt WV, Revell SG, Mirczuk S, Fleck RA, Watson PF. Impact of antifreeze proteins and antifreeze glycoproteins on bovine sperm during freeze-thaw. Theriogenology 2006; 66: 1894-1900.
[115] Bilodeau JF, Blanchette S, Cormier N, Sirard MA. Reactive oxygen species-mediated loss of bovine sperm motility in egg yolk Tris extender: Protection by pyruvate, metal chelators and bovine liver or oviductal fluid catalase. Theriogenology 2002; 57: 1105-1122.
[116] Aitken RJ, Baker MA. Oxidative stress and male reproductive biology.Reprod Fertil Dev 2004; 16: 581-588.
[117] Gadea J, Sellés E, Marco MA, Coy P, Matás C, Romar R, et al.Decrease in glutathione content in boar sperm after cryopreservation:Effect of the addition of reduced glutathione to the freezing and thawing extenders. Theriogenology 2004; 62(3-4): 690-701.
[118] Bilodeau JF, Blanchette S, Sirad MA. Thiols prevent H2O2mediated loss of sperm motility in cryopreserved semen. Theriogenology 2000;51: 337.
[119] Griveau JF, Le Lannou D. Reactive oxygen species and human spermatozoa: Physiology and pathology. Int J Androl 1997; 20: 61-69.
[120] Holland MK, Alvarez JG, Storey BT. Production of superoxide and activity of superoxide dismutase in rabbit epididymal spermatozoa. Biol Reprod 1982; 27: 1109-1118.
[121] Gadea J, Gumbao D, Canovas S, Garcýa-Vazquez FA, Grullon L A,Gardon JC. Supplementation of the dilution medium after thawing with reduced glutathi one improves function and the in vitro fertilizing ability of frozen-thawed bull spermatozoa. Int J Androl 2008; 31: 40-49.
[122] Baarends WM, van der Laan R, Groottegoed JA. DNA repair mechanisms and Gametogenesis. Reproduction 2001; 121: 31-39.
[123] Maxwell WMC, Watson PF. Recent progress in the preservation of ram semen. Anim Reprod Sci 1996; 42: 55-65.
[124] Zarintash JR, Cross LN. Unsterified cholesterol content of human sperm regulates the response of the acrosome to the agonist, progesterone. Biol Reprod 1996; 55: 19-24.
[125] Davis BK. Interaction of lipids with the plasma membrane of sperm cells. I. The antifertilization action of cholesterol. Arch Androl 1980; 5:249-254.
[126] Jones R, Mann T, Sherins RJ. Peroxidative breakdown of phospholipids in human spermatozoa; spermicidal effects of fatty acids peroxides and protective action of seminal plasma. Fertil Steril 1979; 31: 531-537.
[127] Reubal WT, Tappel AL. Damage to proteins, enzymes and amino acids by peroxidizing lipids. Arch Biochem Biophys 1996; 113: 5-8.
[128] Saleh R A, Agarwal A. Oxidative stress and male infertility: from research bench to clinical practice. J Androl 2002; 23(6): 737-752.
[129] Upreti GC, Jensen K, Mundat R, Duganzich DN, Vishwanath R, Smith JF. Studies on aromatic amino acid oxidase activity in ram spermatozoa:Role of pyruvate as an antioxidant. Anim Reprod Sci 1998; 51: 275-287.
[130] Aitken RJ. Mechanisms and prevention of lipid peroxidation in human spermatozoa. In: Fenichel P, Parinaud J, editors. Human sperm acrosome reaction. Montrouge, France: John Libby Eurotext; 1995, p. 339-353.
[ 131 ]Salamon S, Maxwell WMC. Frozen storage of ram semen I.processing, freezing, thawing and fertility after cervical insemination.Anim Reprod Sci 1995; 37: 185-249.
[132] Halliwell B, Gutterridge J. Lipid peroxidation, oxygen radicals, cell damage and antioxidant therapy. Lancet 1984; 1: 1396-1398.
[134] Slaweta R, Wasowicz W, Laskowska T. Selenium content, glutathione peroxidase activity, and lipid peroxide level in fresh bull semen and its relationship to motility of spermatozoa after freezing-thawing. Zentralbl Veterinarmed 1998; 35: 455-460.
[135] Trinchero GD, Affranchino MA, Schang LM, Beconi MT. Antioxidant effect of bovine spermatozoa on lipid peroxidation. Com Biol 1990; 8:339-350.
[136] Alvarez JG, Storey BT. Assessment of cell damage caused by spontaneous lipid peroxidation in rabbit spermatozoa. Biol Reprod 1984;30: 323-331.
[137] Alvarez JG, Touchstone JC, Blasco L, Storey BT. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa superoxide dismutase as major enzyme protectant against oxygen toxicity. J Androl 1987; 8: 338-348.
[138] Alvarez JG, Storey BT. Evidence for increased lipid peroxidative damage and loss of superoxide dismutase activity as a mode of sublethal cryodamage to human sperm during cryopreservation. J Androl 1992; 13: 232-240.
 Asian Pacific Journal of Reproduction2018年3期
Asian Pacific Journal of Reproduction2018年3期
- Asian Pacific Journal of Reproduction的其它文章
- Missed estradiol determination resulting in oocyte retrieval and embryo development following controlled ovarian hyperstimulation at early pregnancy: Case report
- Role of preputial washing in reducing microbial load and improving bovine semen quality
- Effect of different concentration of fish oil in skim milk-egg yolk extenders on postthawed semen qualities of Kalang swamp buffalo bull
- Prediction of protein structure of novel protein (116 kDa) from human sperm membrane
- Antiandrogenic activity of Calotropis procera latex in rats
- Improvement of cortical granules migration and in vitro embryo production of vitrified bovine oocyte by 9-cis retinoic acid
