Fabrication of chitosan microspheres for efficient adsorption of methyl orange☆
Linlin Zhai,Zhishan Bai*,Yong Zhu,Bingjie Wang,Wenqiang Luo
State Environmental Protection Key Laboratory of Environmental Risk Assessment and Control on Chemical Process,School of Mechanical and Engineering,East China University of Science and Technology,Shanghai 200237,China
1.Introduction
Dyes have become one of the most prominent contaminants contributing to wastewater pollution as a result of the rapid development of the textiles,leather and paper industries[1–3].These dye wastes are hazardous,toxic,non-biodegradable,and sometimes carcinogenic[4].It can cause headache,allergy and skin irritation,and even influence the normal functions of the liver.With respect to both number and production volume,azo dyes are the largest group of colorants,constituting 70% of all organic dyes produced in the world[5–7].Most of those dyes have a complex and stable molecular structure,but are difficult to be treated by conventional biological methods[3].Methylorange as an anionic dye,belongs to the azo dye group[2].The azo group of dyes contains nitrogen.Due to the existence of the--N=N--chromophore group and the aromatic structure,it is difficult to remove them from wastewater by chemical and biological degradation methods[8].Therefore,the development of dye-removal techniques for the industry has long been a challenge.
Several conventional methods such as biological treatment,coagulation,chemical oxidation,ozonation,membrane filtration,ion exchange methods and photo catalysis have been employed for the removal of dyes from wastewater[9–12].Although they have been widely used,these techniques suffer from several limitations including fairly large flow rates,producing a high-quality effluent that results in the formation of harmful substances,such as ozone and free radicals[1].Among these techniques,adsorption process has been found to be superior to other techniques.It is used to make natural or synthetic adsorbents for the physical and chemical adsorption of dyes in wastewater.The adsorption method is cheap,simple and effective to adapt.Its treatment period is short.Moreover,it causes no pollution to the environment and has been confirmed as one of the most promising technologies for removing dyes from waste waters[13–17].Therefore,adsorption can be considered as the most efficient technique for the removal of synthetic dyes because of the removal of complete dye molecules from aqueous effluents,while other methods destroy only the dye chromosphere leaving the harmful residues[18].
Various types of absorbents like silica,micro-particles,activated carbon and graphite have been proposed for the removal of dyes from aqueous solutions.Chitosan,a natural biosorbent material,can be considered as promising cost-effective adsorbents for the removal of hazardous anions in industrial wastewater.Chitosan is the second most abundantpolysaccharide in nature aftercellulose,is the only basic polysaccharide in nature and is non-toxic,bio-compatible,biodegradable and low cost[19].Chitosan is a type of natural polyaminosaccharide,has one primary amino and two free hydroxyl groups for each C6 unit and is synthesized from the deacetylation of chitin[20].It can be extracted from crustacean shell such as prawns,crabs,fungi,and insects.Combined with these special characteristics,chitosan can provide a promising and competitive alternative as green adsorbent for the adsorption of dyes to achieve water purification.Hence,the exploration and application of chitosan microsphere develop rapidly.Filipović-Grčić[21]adopted a spray-drying process to prepare chitosan microsphere in varying proportions of HPMC and chitosan with different molecular mass.Peng et al.[22]applied emulsion chemical cross-linking to synthesize a resveratrol–chitosan microsphere with smooth surface and continuous network,where vanillic aldehyde was used as a natural crosslink agent.According to Vakili et al.[23]and Gamage et al.[24],the biopolymer chitosan presented an excellent capability of removing the metal ion pollutant of Pb(II),Cu(II),Zn(II)and so on.Recently,chitosan composite has been proven to have better adsorption capacity and resistance to acidic environment.Chitosan microsphere adsorption materials possess numerous applications in process equipment such as heat exchangers and fixed bed reactors for wastewater treatment,with advantages including high mechanical strength and adaptability to operating conditions.Dong et al.[25]synthesized chitosan microparticles for methyl orange(methyl orange)adsorption on a micro fluidic platform coupled with a crosslinking approach.However,they did notdiscover the importance of mechanical strength of particles in industrial applications and change ofpH value after adsorption affected the adsorption capacity.In this work,high-performance monodispersed chitosan microspheres with high mechanical strength were prepared using a facile micro fluidic method combining chemical crosslinking methods,and further exploited for the adsorption of methylorange or detailed adsorption characterization studies.The microspheres have controllable size,morphology,monodispersity(coefficient of variation is 1.81%),high adsorption capacity(qm=207 mg·g−1)and the mechanical strength reaches 0.4 MPa.Methyl orange color's becoming shallow is in fluenced by the adsorption and change of pH.In addition,scanning electron microscopy(SEM),energy dispersive X-ray spectroscopy(EDS)and FTIR spectrum were used to compare the adsorption behavior of both dyes.The adsorption kinetics could be described well by the pseudo-second order model and the adsorption isotherm following the Langmuir equation.
2.Experimental
2.1.Materials
Chitosan(Aladdin Industrial Co.,Ltd.,Shanghai,China)was dissolved in acetic acid(Tianlian chemical Technology Co.,Ltd.,Shanghai,China)to prepare an aqueous solution.This serves as the dispersed phase in our experiments to form monodispersed droplets.An emulsi fier,Span 80(Reagent Co.,Ltd.,Shanghai,China),dissolved in n-octane(Ling Feng Chemical Co.,Ltd.,Shanghai,China)was used as the continuous phase.Glutaraldehyde(Ling Feng Chemical Co.,Ltd.,Shanghai,China)and Span 80 dissolved in n-octane was used as the solidification bath,and glutaraldehyde was used as a cross-linking reagent for solidification.Methyl orange(Reagent Co.,Ltd.,Shanghai,China),hydrochloric acid(HCl),Sodium hydroxide(NaOH),n-octanol,ethanol and other reagents utilized in this work were all of analytical grade.The stock solution with deionized distilled water was used throughout the experiments.
2.2.Preparation of monodispersed chitosan microspheres
However,allthese traditional methods have problems of no uniform size,uncontrollable component and bad stability.Micro fluidic technology can accurately control the number and size of emulsion droplets to internal.Polymerization conditions can be prepared by simple microparticles of various morphologies.These advantages have become the most promising methods of functional polymer preparation in the twenty- first century,receiving extensive attention.Some researchers have developed variouschitosan microspheres via micro fluidic method.Yang et al.[26]brought out monodispersed chitosan microsphere by chemical crosslinking.Xu et al.[27]exploited uniform and high monodispersed spherical particles through solvent extraction.In the past decade,micro fluidic methods have been developed as the novel approach for the controllable synthesis of monodispersed microspheres with different structures[20,28,29].In this paper,the micro fluidic flowfocusing chip was fabricated on three 50 mm×50 mm×1 mm PMMA plates by a Laser Microfabrication Machine(VLS2.30,Universal,America),which are,from top to bottom,the cover layer(containing three inlet orifices holes),the main layer(containing the flowfocusing junction)and the bottom layer(containing one outlet orifices hole).The three layers were sealed with hot-pressing bonding,thus forming the enclosed microchannel with a certain degree of strength and minimizing deformation and leakage.The top plate had three same holes of2 mmin diameter.Two holes were continuous phase inlets and the other hole was a dispersed phase inlet.The middle plate was carved with two orthogonal and absolute penetrable channels,which were 40 mm in length and 2 mm in width by laser engraving machine.At the cross aisle,channels shrank to 1 mm in width and 3 mm in length because of the fluid shear condition and the necessity of microsphere diameter limitation.The bottom plate contained a hole of 3 mm in diameter,which was larger than inlet holes in order to be beneficial for formed droplets out flowing.Single-channel syringe pumps(LSP02-1A,Longer,China)were used to pump the dispersed phase and a dual-channel syringe pump was used to pump the continuous phase.The pumps were connected to the chip through hoses.The continuous phase was pumped to sheardispersed phase atthe cross aisle to form spherical droplets.The formed droplets were collected in a container on the magnetic stirrer where they will be solidified through cross-linking.
In order to prepare chitosan microspheres,one musthave an aqueous solution with 2.0 wt%chitosan and 2.0 wt%acetic acid as the dispersed phase,while the continuous phase contains 2.0 wt%Span 80 and 98 wt%n-octane.The dispersed phase was injected into the middle channel and the continuous phase was injected into the side channels.When the volumetric ratio reaches to 5:65,the dispersed phase breaks into droplets by the shearing force of the continuous phase.These droplets are uniform.The microsphere flowed through the outletsteadily and uniformly into a beaker filled with solidification bath for crosslinking on a magnetic stirrer(Yuezhong,ZNCL-S-10D,China).The solidification bath was n-octane solution with 0.5 wt%glutaraldehyde and 2 wt%Span80 added in.The crosslinked chitosan droplets were taken into a break mixture containing n-octane and n-octanol solution.Then the prepared chitosan microspheres were transferred to Petri dishes and washed several times with ethanol and deionized water.Finally,clear microspheres were dried under 50°C in an oven(DHG-9023A,Heyi,China)for some hours,and then the dried microspheres are collected in glass jars.The formation process of microspheres was shown in Fig.1.
2.3.Analysis and characterization of microspheres
The microspheres were examined by an optical microscope(AZ100,Nikon,Japan)and an on-line CCD(C-FLED2,Nikon,Japan).Scanning electron microscopy(SEM)(S3400N,HITACHI,Japan)was used to analyze the surface morphology and detailed structures of microspheres.The FTIR spectrum(Nicolet 6700,Thermo Fisher,America)was used to obtain transitions of functional groups.The chemical composition of the prepared adsorbent before and after adsorption was analyzed by an Energy Dispersive Spectrometer(EDS)system.
Monodispersed particles were defined by coefficients of variation(CV).The size distribution of 300 dried microparticles was investigated to analyze their average diameter and coefficients of variation.The coefficient of variation values were calculated with the following equation.
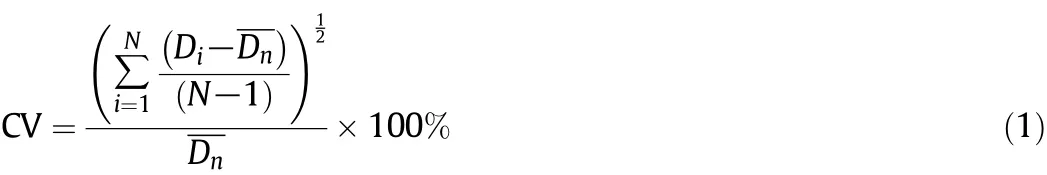

Fig.1.The formation process of microspheres.
For mechanical strength test,we adopted a self-made method of using a strength test machine(HPA,Handpi,China)to measure twenty microspheres'strength values at the moment the microsphere fractured.Almost twenty uniform-sized microspheres were broken under the measuring instrument.The microspheres were observed under the microscope whether broken or not.After removing the maximum and minimum values,the average value was taken as the mechanical strength of the particles.
In desorption experiment,0.03 g of adsorbent was contacted with 60 ml of methyl orange solution with an initial concentration of 40 mg·L−1at pH 7.0.The absorbent with methyl orange was removed from the solution after reaching the adsorption equilibrium,quickly rinsed with deionized water and stirred for 4 h in 30 ml of 0.1 mol·L−1NaOH solutions.To test the reusability of the adsorbent,this adsorption/desorption cycle was repeated five times.The amount of regeneration methyl orange and the corresponding desorption rate of the material were analyzed and calculated in the same way as that in the adsorption experiments.Desorption efficiency(ηD)was calculated by using the following equation[30]:

where Cdis the amount of methyl orange desorbed(mg·L−1),Cais the amount of methyl orange adsorbed(mg·L−1).
2.4.Batch adsorption experiments
Batch adsorption experiments were carried out in an aqueous solution.A standard solution of methyl orange(C14H14N3NaO3S)(Reagent Co.,Ltd.,Shanghai,China)(1.0 g·L−1)was prepared for desired methyl orange concentrations.A certain amount of chitosanmicrosp heres were added to 60 ml of the dye solution with a specified concentration.The adsorption experiments were carried out at room temperature.The residual concentration after adsorption was determined from a constructed calibration curve by measuring the peak value using UV-1800.The calibration curves were measured at different pH conditions(pH=3,5,7,9,11)and were used separately to avoid any influence from different pH values.
Adsorption properties were investigated as an effect of contact time,pH and pre-soli dification time.In adsorption experiments,30 mg of the adsorbent dosage,40 mg·L−1initial concentration of methyl orange and pH 7 were used as the original parameters.The pH values of the solutions were adjusted by adding some volumes of 0.1 mol·L−1NaOH or HCl solution.The pH values were measured with a pH meter(3110-SET2,WTW,Germany).
Absorbance at certain interval(qt),the adsorption capacity(qe)and adsorption efficiency(η)was calculated according to the following equations:

where C0is the initial concentration of methyl orange(mg·L−1)and Ceis the equilibrium concentration of methyl orange,Ct(mg·L−1)is the instant concentration of methyl orange at a certain time t.V(ml)is the volume of solution and m(mg)is the adsorbent dosage.
3.Results and Discussion
3.1.Characterization of chitosan microspheres
To evaluate the textual structure of adsorbent surface,SEM micrographs of chitosan microspheres were shown in Fig.2a and b,which showed a homogeneous and reproducible spherical geometry and surface smoothness of particles.It can be clearly illustrated that a remarkable size uniformity and sphericity could be achieved for synthesized chitosan microspheres under micro fluidics,which is considerably superiorto that of microspheres synthesized using conventional methods including the reversed-phase suspension method[31,32].

Fig.2.SEMgraphs of(a)some prepared chitosan microspheres,(b)single chitosan microspheres,(c)size distribution and(d)mechanicalstrength with drying time and solidifying time of chitosan microspheres.
The particle diameterdistribution range was obtained by average diameteranalysis software,which demonstrated the high monodispersity in as-synthesized materials(coefficient of variation is 1.81%)and an average diameter around 440 μm(Fig.2c).This could be attributable to micro fluidic technology with accurate control of size and morphology,which is considerably lower to that of microspheres synthesized using the methods such as stirring and membrane methods.

Fig.3.The FTIR spectra of(a)chitosan powder,(b)crosslinked chitosan microspheres and(c)crosslinking process.
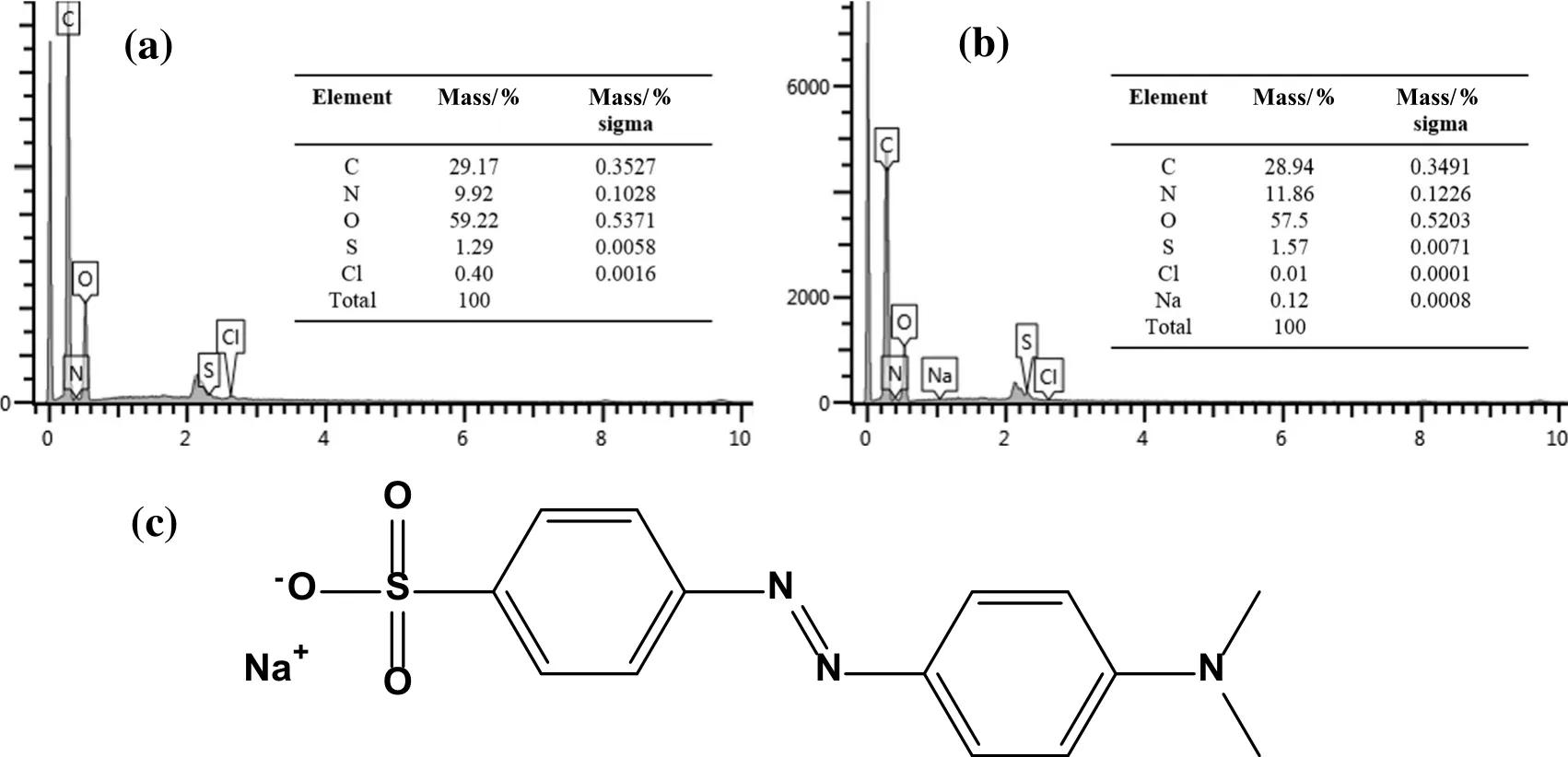
Fig.4.The EDS spectra of(a)chitosan,(b)adsorbed chitosan,(c)the chemical structure of methyl orange.
Successful absorbents also need sufficient mechanical strength in addition to other characteristics,so it is necessary to protect the absorbent from breaking when resisting various forces in the application process.In order to get a high mechanical strength microsphere,we took the method of drying and solidifying to prepare it.Fig.2d shows the influence of drying time and solidifying time on mechanical strength.The result shows that microspheres contained enough water at the firststage ofdrying that liquid microspheres were unable to resist external pressure.Before the drying 4 h,mechanical strength improved with the drying time increasing,while water decreased gradually giving microspheres flexibility and the ability to oppose deformation.This was the reason for the high mechanical strength after drying for 4 h at this stage.When water evaporated completely,solid microspheres become so brittle that,to a certain extent,they are directly broken by forces and without plastic deformation.Mechanical strength reduced to a low level again at the drying time(12 h),which was different from the first stage absolutely.So the chitosan microsphere drying time(4 h)was chosen in the experiments.The surface of microspheres becomes more relatively compact with the increase of solidifying time.Mechanical strength improved with the drying time increasing.It is because of this that the water in the microspheres was extracted out of the spheres gradually.When the solidifying time reached 80 min,mechanical strength almost changes a little.This represents that almost all water was discharged.Too much solidifying time affects the properties of particles;therefore,we used chitosan micro spheres,which were solidifying for 30 min in our experiments.
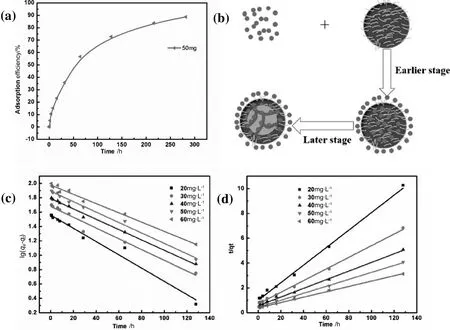
Fig.5.(a)The influence of contact time,(b)The process of methyl orange adsorption and kinetic models for methyl orange adsorption for different initial methyl orange concentration:(c)Pseudo- first-order model,(d)pseudo-second-order model.
FTIR spectroscopy was used to identify the functional groups that are responsible for adsorption.FTIR spectral data of chitosan microspheres was obtained in the range of 500–4000 cm−1,and data was shown in Fig.3.Spectralanalysis results were discussed asfollows:in FTIRspectra various peaks can be observed.The peak at3447 cm−1for the spectrum of pristine chitosan was ascribed to intermolecular hydrogen bonds and the overlap between O--H and N--H stretching vibrations[33–36].The characteristic peak at 2925.5 cm−1,2875.4 cm−1and 2854.1 cm−1was attributed to the C--H stretching of--CH2and--CH3groups[37].The peak appearing at 1110 cm−1corresponded to the stretching vibration of C--O in chitosan molecule on C-3 and C-6 positions[38].In the FTIR spectrum of chitosan powder,1602.6 cm−1was the characteristic absorption peak of amides[39].Fig.3b shows the FTIR spectrum of cross-linked chitosan microspheres.Crosslinking,based on the Schiffbase reaction between--NH2 of chitosan and--CHO of glutaraldehyde for the generation of C=N,was so important that chitosan microspheres would have the ability to resist various forces and adapt to strong acidic situations[40].These microspheres,free fromcrosslinking,were brittle and breakable,which resulted in unavailable practical application.However,more crosslinking would lead to more consumption of amino.Amino is the efficient functional group of adsorbed methyl orange dye,and its reduction will give rise to the decrease of adsorption capacity.Therefore,suitable crosslinking degrees should be controlled for the comprehensive consideration of mechanical properties and adsorption capacity.Besides,some shift in wave numbers from 1602.6 cm−1to 1654.7 cm−1.These significant changes were related to C=O stretching vibration of acetylgroup and the stretching vibration ofamine group in spectrum b[41].Itsuggested thatcrosslinking had occurred successfully based on Schiff-base reaction between--CHO of glutaraldehyde and NH2of chitosan[42].
In order to confirm the existence of certain elements in chitosan microspheres,EDS analysis was used to characterize the particles before and after methyl orange adsorption.The EDS spectrum of chitosan was shown in Fig.4a,and it was observed that the elements of C,N,O,S,and Cl existed in chitosan particles.The total quantity of all elements was counted as 100%.After adsorption,the EDS spectrum and the mass percentage of each element were illustrated in Fig.4b.Sodium elements appeared.The content was as high as 0.12%(wt%)and the mass percentage was calculated to be 0.08%.The chemical structure of methyl orange was shown in Fig.4c.The formula of methyl orange contains sodium element,which demonstrated that methyl orange wassuccessfully adsorbed onto the chitosan particles.
3.2.Adsorption kinetics
The contact time is an important parameter,which influences the adsorption process.This investigation was accomplished with 40 mg·L−1as initial concentration of methyl orange dye and 50 mg chitosan particles.As shown in Fig.5a,it can be seen that the adsorption capacity of methyl orange increased with the prolongation of contact time until the adsorption equilibrium time(200 h).The methyl orange uptake increased rapidly within the first 25 h,and,gradually,adsorption increased almost 70%.Almost 88%methyl orange can be removed, finally reaching equilibrium.The relatively fast and efficient adsorption process can be attributed to the abundance of active sites on the adsorbents[43].After the contact time equilibrium,we can see that there is no additional enhancement in adsorption capacity.Fig.5b shows the typical adsorption kinetic curve.Adsorption action developed quickly at the earlier stages,according to the slope of the curve,and then became flat gradually.This could explain that adsorption acted on the microsphere surface at first,and the process of methyl orange arriving at the microsphere surface from solution happened quickly.Adsorption sites on chitosan microspheres provided a place for methyl orange to land.Then,methyl orange in filtrated into the interior of the microsphere from the surface,which was slower than the previous stage.The number of channels and pores in microsphere decides the in filtration rate as well as adsorption rate on the later stage,provided that the initial concentration and the temperature of solution are fixed so pored microsphere can reach adsorption equilibrium quickly.

Fig.6.Freundlich and Langmuir adsorption isotherm for methyl orange adsorption.
Two kinetic models such as pseudo- first-order and second-order were used to investigate the adsorption process of methyl orange onto chitosan particles.The pseudo- first-order equation assumes the adsorption is of one adsorbate molecule onto an active site of the adsorbent surface.Whereas,in the pseudo-second order model,one adsorbate molecule is adsorbate onto two active sites.Through adsorption kinetics,the mechanism for methyl orange adsorption rate and the adsorption time can be determined.
Pseudo- first-order kinetics can be represented as follows:

where qeand qtare the amounts of adsorbed methyl orange at equilibrium and at time t,respectively.k1is the rate constant.
Pseudo-second-order model can be expressed as:

where qtand qeare the amount of adsorbed methyl orange attime t and atequilibrium,respectively.k2is the pseudo-second-orderrate constant and h0=k2qe2is the initial adsorption rate of methyl orange.
The kinetic parameters such as adsorption capacity,rate constant and correlation coefficient(R2)were determined from linear plots of lg(qe−qt)versus t for the pseudo- first-order model and t/qtversus t for the pseudo-second-order model.Aftercomparing the correlation coefficient values of pseudo- first-order and pseudo-second-order(shown in Fig.7),it is clear that the adsorption behavior of methyl orange onto chitosan microspheres follows pseudo-second-order kinetics(R2>0.99)rather than first-order.The R2value for the pseudo-secondorder model was higher than the pseudo- first-order model.Additionally,the experimental qevalues(qe,exp)are closer to the calculated values(qe2,cal)obtained from the pseudo-second-order kinetics.Hence,these factors suggest that the process of methyl orange adsorption is chemical adsorption,due to the influence of chemical bonding along with electrons transferring in a significant degree.It also can be summarized that the rate-limiting step may be a chemical adsorption,and intraparticle diffusion is also involved in the process[44].

Table 1 Isotherm parameters for methyl orange adsorption.

Table 2 Comparison of adsorption capacity(mg·g−1)for methyl orange onto chitosan particles with other adsorbent
3.3.Adsorption isotherms
Adsorption isotherms are function expression that correlates the interaction mechanism of solution and adsorbent at room temperature.Two well-known adsorption isotherm models(Langmuir and Freundlich isotherms)were used to fit the equilibrium data obtained from experiments performed with different initial methyl orange concentrations.Langmuir theory assumes that adsorption takes place at specific homogeneous sites on the surface of the adsorbent.Once a dye molecule occupies a site,no further adsorption can take place at that site.The rate of adsorption to the surface should be proportional to the concentration in the solution and the specific surface area.The Langmuir equation is expressed as:

where Ceis the equilibrium concentration(mg L−1);qeis the amount of dye adsorption atequilibrium(mg·g−1),and qmis the maximumamount of monolayer adsorption capacity of adsorbent.KLis the Langmuir adsorption equilibrium constant(L·mg−1).Furthermore,a dimensionless constant called equilibrium parameter(RL)is commonly used to predict whether an adsorption system is favorable or unfavorable:

The value of RLindicates the adsorption process to be irreversible(RL=0),favorable(0<RL<1),linear(RL=1)or unfavorable(RL>1)[45].
Comparatively,the Freundlich is an empirical equation to describe multilayer adsorption on the surface heterogeneity of the adsorbent.It demonstrated that the amount of dye being adsorbed onto a given concentration is not constant.The Freundlich model is:

where Ceis the equilibriumconcentration(mg·L−1),qeis the amountof dye adsorption at equilibrium(mg·g−1),and KFand n are Freundlich constants.n is related to the adsorption intensity distribution,and KFindicates the adsorption capacity of the adsorbent.Adsorption data plotted based on Langmuir and Freundlich isotherm are shown in Fig.6.
The calculated parameters of Langmuir and Freundlich isotherms along with R2are listed in Table 1.The data shows that the experimental adsorption data have an excellent fit with the Langmuir model with R2=0.999,as compared to less fitted Freundlich model(R2=0.908).The Langmuir equation is based on the theory of adsorption as a monolayer,and adsorbent surface is uniform and smooth with no attractions existing between absorbed methyl orange molecules.The results confirm that the adsorption is monolayer.Once a methyl orange molecule occupies homogeneous sites within the adsorbent surface,the adsorption is completed.Consequently,a monolayerofmethylorange is formed[46].The whole surface has identical adsorption activity,and the adsorbed methyl orange does not interact or compete with each other.From Langmuir adsorption isotherms,the maximum adsorption capacities of the chitosan microspheres for methyl orange were estimated to be 207 mg·g−1.These are significantly higher than other adsorption materials(Table 2).
3.4.Adsorption experiment studies
3.4.1.Effect of solidifying time
The microspheres were prepared with a different solidifying time.The color of chitosan microspheres changed after solidification,while unsolidified chitosan microspheres were fragile and easy to polymerize together.After solidification,the mechanical properties were enhanced.The chain structure of chitosan molecule previously turned into a network structure for a crosslinking reaction,but longer solidification time indicated deeper level of crosslinking reaction and more consummation of amino.Besides,crosslinking also gave rise to an increase in the rigidity of the structural conformation of chitosan,which reduced the malleability of the polymer chain and impeded ion diffusion.Therefore,this shed lighton a crucial relation between solidification time and adsorption capacity based on the principle of amino chelating metal ions and crosslinking reaction.The surface of microspheres becomes relatively more compact with the increase of solidifying time.It is because of this that the water in the microspheres was extracted out of the spheres gradually,while the glutaraldehyde in the solidification bath diffused into the spheres to crosslink with chitosan[20].When the microspheres were placed in the accepted phase with the octanol,the unreacted glutaraldehyde was extracted by octanol,and then the microspheres were cleaned by acetone,ethyl alcohol and deionized water some times.Fig.7(a)shows the adsorption capacity of the microspheres with different solidifying time.The adsorption capacity reaches the maximum at the solidifying time 30 min.Because the reaction between chitosan and glutaraldehyde consumes--NH2,the adsorption capacity decreases by increasing the solidifying time.Ithas been proven that the crosslinking between chitosan and glutaraldehyde can decrease the adsorption capacity.Although the--NH2in microspheres solidified in 20 min,more solidified in 30 min.There were more water droplets in the microspheres,which was disadvantageous for adsorption and machinery strength.Then the machinery strength of microspheres with a different solidifying time was studied.The machinery strength of the microspheres is reinforced by increasing the solidifying time.Therefore,considering the adsorption and mechanical performance,30 min was the best solidifying time for microspheres.
Fig.7.Effect of(a)solidification time,(b)adsorbent dosage on methyl orange adsorption capacity.
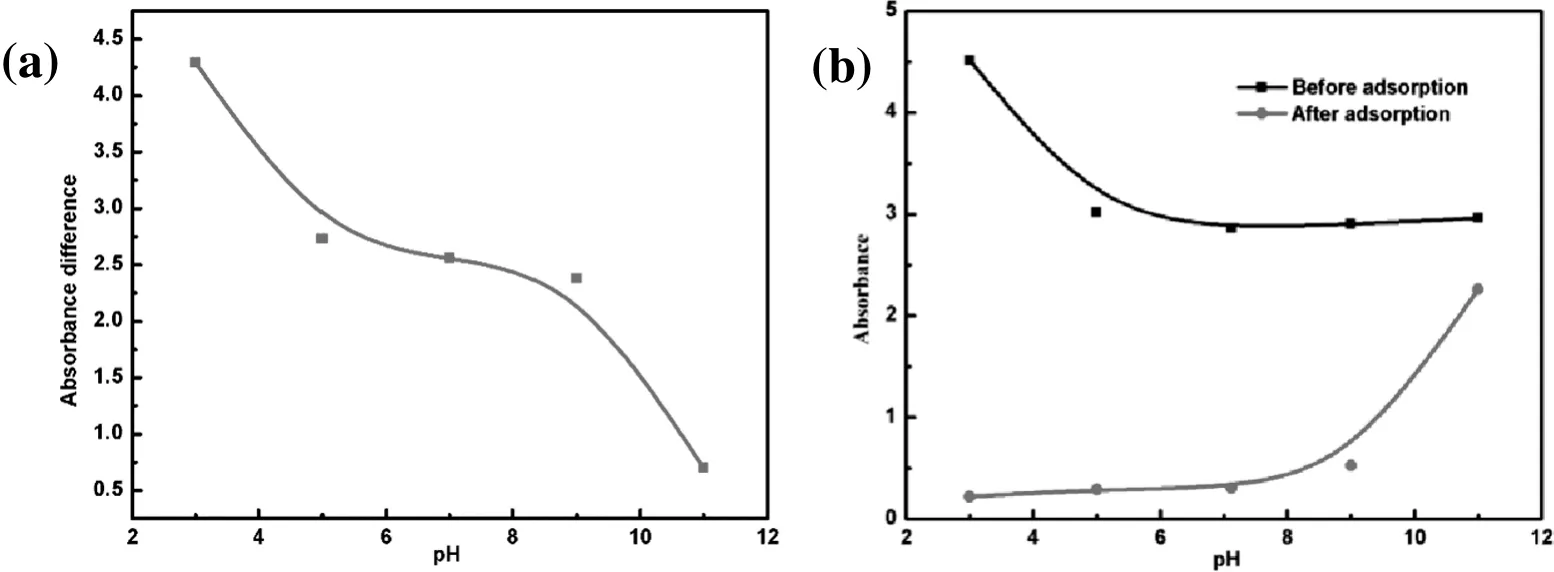
Fig.8.(a)The absorbance and(b)absorbance difference at different pH.
3.4.2.Effect of adsorbent dosage
Fig.7(b)illustrates the effect of the chitosan microsphere dosage on adsorption efficiency of methyl orange solution.Methyl orange adsorption efficiency increased with a slight increase of adsorbent dosage.Surface area obviously increases with an increase in the adsorbent dosage,and then the available sites obviously increase,which consequently result in the increase of the amount of adsorbed dye.
3.4.3.Effect of pH
Solution pH is one of the vital factors in adsorption studies.It influences the surface charge of the adsorbent and the solubility of dyes,and controls the adsorption efficiency and capacity.When pH is too low,the phenomenon of molecularaggregation appears in the water solution,forming a large number of aggregates.This makes the solution become turbid.Considering this,a series of adsorption experiments were conducted at different initial pH values(from 3.1 to 11).To avoid the pH effect on the determination of methyl orange concentration,the UV calibration curves at different pH values(3.1,5.0,6.9,9.0,and 11.0).The obtained results are shown in Fig.10a.It may be seen that the maximum absorption wavelength is different under the condition of acid and alkali.After adsorption,assume that the pH value of methyl orange solution remains unchanged.The relationship between pH and absorbance value before and after adsorption was shown in Fig.8b.Through Fig.8b,the fitting curve was obtained between pH and absorbance difference(Fig.8a),and the fitting degree(R2)reached to 0.9993.The fitting formula can be expressed as:

The absorbance difference at pH=3.0,5.0,7.0,9.0,11.0 was obtained from the formula.The absorbance difference under different pH was brought into the corresponding standard curve,and the adsorption capacity under different pH conditions was calculated.The calculated adsorption capacity wascompared with the experimental value and found that their values were almostequal.The resultconcluded that the fitting formula can be used to calculate the adsorption capacity of chitosan microspheres under different pH conditions.
After adsorption,the color of the methyl orange solution changes from deep to shallow.The obtained results are shown in Fig.9a.It may be seen,that the percentage removals of methyl orange decreases with increase in pH,and adsorption capacity decreases from 77 to 15 mg·g−1by increasing the pH value.Therefore,at pH 3.1 the adsorbent has a maximum positive charge,and the methyl orange molecule has a maximum negative charge.This will contribute to the high adsorption capacity[43].Furthermore,as the pH increases,the number of positive charges decreases,and negatively charged sites increase on the adsorbent.The alkaline solution can also result from the presence of excessive hydroxyl ions,which competed with methyl orange molecules for adsorption sites.Additionally,we measured the pH of methyl orange solution in the process of the experiment.It can be found that the pHvalue of methyl orange solution is changed compared to the preceding adsorption(as shown in Fig.9b).pH value increases under the condition of acid,which was reduced under the condition of alkaline.After adjusting pH to the initial value,under acidic condition,the color of methyl orange solution became darker and the absorbance value increased.This result confirms that the methyl orange color becomes shallow not only through the association with the adsorption of chitosan,but also the change of pH value.pH value increasing to about 6.5 at given initial solution is acidic,while the pH value of alkaline solution has been decreasing to about6.5.Methyl orange has a different color in a different pH range.Methylorange is red when pH is less than 3.1,orange at a 3.1–4.4 range,and yellow when pH is greater than 4.4.The pKa value of methyl orange was 3.45.Considering this in the present study,we prepared the initial solution of pH from 2.0 to 11 for adsorption,while measuring the pH and adsorption capacity.These results indicated that when the solution of pH<4.4,color change of methyl orange was effected by not only adsorbent adsorption,but also pH value.While when the 4.4<pH<9.0,the color of methyl orange was effected by adsorbent adsorption.The change of pH value had almost no influence.
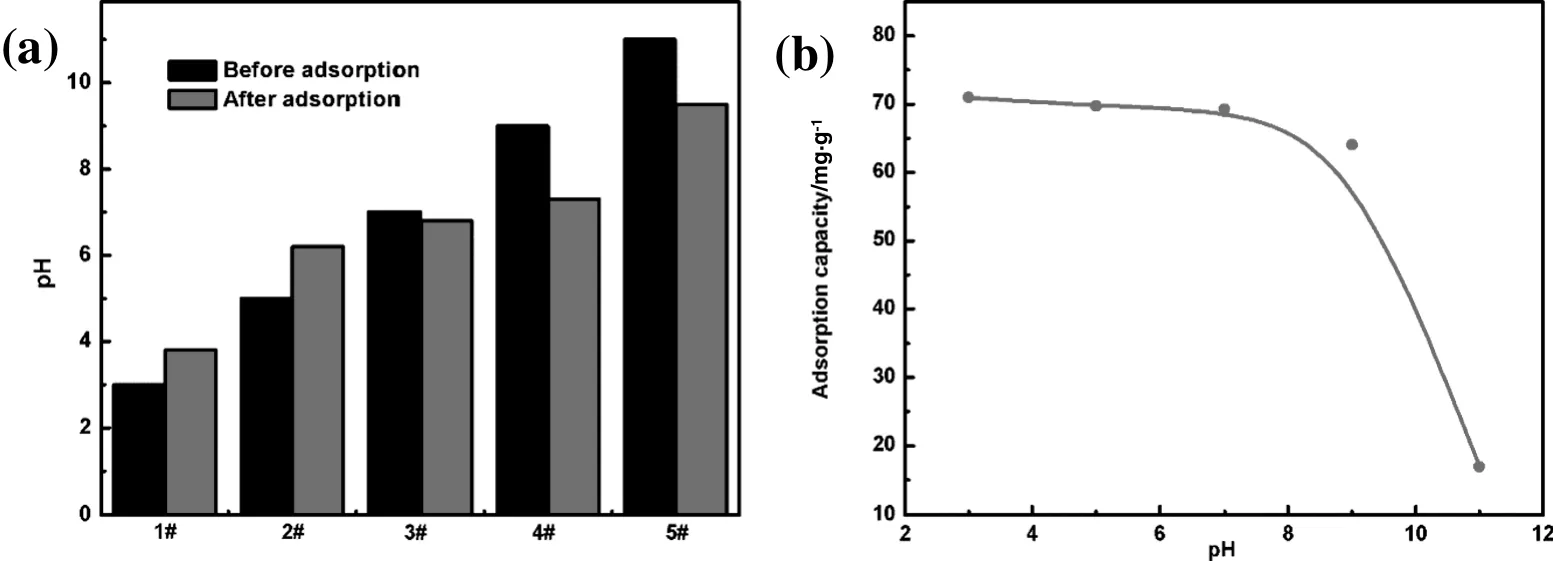
Fig.9.(a)Effect of pH on methyl orange adsorption capacity,(b)the change of pH.

Fig.10.(a)Desorption of methyl orange,(b)reabsorption of methyl orange.
To reduce the cost of dye adsorption,it is desirable to study the material regeneration capability to provide indication for extracting dyes from the liquid phase.The desorption experiments were performed with different NaOH concentrations.Fig.10(a)shows that the desorption efficiency decreased with the increase of NaOH concentrations.The maximum percentage recovery of methyl orange was 0.1 mol·L−1NaOH solution,and is,therefore,suitable for use as a desorption agent.The adsorption–desorption experiment was repeated up to five times and the results are illustrated in Fig.10(b).The adsorption capacity decreased from 78 mg·g−1to 70 mg·g−1after 5 adsorption–desorption cycles.These small decreases are due to part of the residual dye adsorbed on the surface of adsorbent leading to a decline in adsorption capacity.The results showed that the chitosan adsorbent could be used repeatedly in the methyl orange adsorption experiment due to a small loss in the total adsorption capacity.
4.Conclusions
Mono dispersed chitos an micro spheres with uniform size(coefficient of variation is 1.81%),controllable morphology,nice renew ability and component and high adsorption capacity were prepared successfully for adsorption of methyl orange.The structural characterization was done by SEM,FTIR and EDS techniques.The adsorbents showed good adsorption capacity,giving maximum uptakes of 207 mg·g−1.In addition,adsorption performances were optimized through investigating the influences of adsorption conditions.The effect of pH revealed that the adsorption process was independent of pH,and the change of a pH value after adsorption indicated that adsorption capacity was affected through the associated role of chitosan nature and pH variation.The adsorption kinetics could be described well by the pseudo-second order model.The adsorption isotherm was fitted well with the Langmuir equation.In overall,the chitosan material developed in this study could be effectively utilized as a functional adsorbent for methyl orange.
[1]V.K.Gupta,R.Kumar,A.Nayak,T.A.Saleh,M.A.Barakat,Adsorptive removal of dyes from aqueous solution onto carbon nanotubes:A review,Adv.Colloid Interf.Sci.193-194(2013)24–34.
[2]S.A.Hassanzadeh-Tabrizi,M.M.Motlagh,S.Salahshour,Synthesis of ZnO/CuO nanocomposite immobilized on γ-Al2O3and application for removal of methyl orange,Appl.Surf.Sci.384(2016)237–243.
[3]H.Y.Zhu,R.Jiang,J.Yao,F.Q.Fu,J.B.Li,Novel ZnO/Zn–Cr hydrotalcite-like anionic clay as a high-performance and recyclable material for efficient photocatalytic removal of organic dye under simulated solar irradiation,Res.Chem.Intermed.42(2015)1–14.
[4]P.Sharma,H.Kaur,M.Sharma,V.Sahore,A review on applicability of naturally available adsorbents for the removal of hazardous dyes from aqueous waste,Environ.Monit.Assess.183(2011)151–195.
[5]T.A.Gadallah,S.Kato,S.Satokawa,T.Kojima,Treatment of synthetic dyes wastewater utilizing a magnetically separable photocatalyst(TiO2/SiO2/Fe3O4):Parametric and kinetic studies,Desalination 244(2009)1–11.
[6]H.Y.Zhu,R.Jiang,Y.Q.Fu,J.H.Jiang,L.Xiao,G.M.Zeng,Preparation,characterization and dye adsorption properties of γ-Fe2O3/SiO2/chitosan composite,Appl.Surf.Sci.258(2011)1337–1344.
[7]E.Brillas,C.A.Martínez-Huitle,Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods.An updated review,Appl.Catal.B Environ.87(2009)105–145.
[8]S.Hosseini,M.A.Khan,M.R.Malekbala,W.Cheah,T.S.Y.Choong,Carbon coated monolith,a mesoporous material for the removal of methyl orange from aqueous phase:Adsorption and desorption studies,Chem.Eng.J.171(2011)1124–1131.
[9]F.Foroughi,S.A.Hassanzadeh-Tabrizi,J.Amighian,A.Saffar-Teluri,A designed magnetic CoFe2O4–hydroxyapatite core–shell nanocomposite for Zn(II)removal with high efficiency,Ceram.Int.41(2015)6844–6850.
[10]M.L.Yola,T.Eren,N.Atar,S.Wang,Adsorptive and photocatalytic removalofreactive dyes by silver nanoparticle-colemanite ore waste,Chem.Eng.J.242(2014)333–340.
[11]J.A.González,M.E.Villanueva,L.L.Piehl,G.J.Copello,Development of a chitin/graphene oxide hybrid composite for the removal of pollutant dyes:Adsorption and desorption study,Chem.Eng.J.280(2015)41–48.
[12]K.S.Sudheer,Enhancement of visible light photocatalytic activity of CdO modified ZnO nanohybrid particles,J.Photochem.Photobiol.B Biol.142(2014)1–7.
[13]M.Gui,L.E.Ormsbee,D.Bhattacharyya,Reactive functionalized membranes for polychlorinated biphenyl degradation,Ind.Eng.Chem.Res.52(2013)10430–10440.
[14]H.P.Chao,C.K.Lee,L.C.Juang,Y.L.Han,Sorption of organic compounds,oxyanions,and heavy metal ions on surfactant modified titanate nanotubes,Ind.Eng.Chem.Res.52(2013)9843–9850.
[15]I.Khosravi,M.Yazdanbakhsh,M.Eftekhar,Z.Haddadi,Fabrication of nano Delafossite LiCo0.5Fe0.5O2as the new adsorbent in efficient removal of reactive blue 5 from aqueous solutions,Mater.Res.Bull.48(2013)2213–2219.
[16]M.Yazdanbakhsh,I.Khosravi,E.K.Goharshadi,A.Yousse fi,Fabrication of nanospinel ZnCr2O4using sol-gel method and its application on removal of azo dye from aqueous solution,J.Hazard.Mater.184(2010)684–689.
[17]K.Y.Shin,J.Y.Hong,J.Jang,Heavy metal ion adsorption behavior in nitrogen-doped magnetic carbon nanoparticles:isotherms and kinetic study,J.Hazard.Mater.190(2011)36–44.
[18]B.Bi,L.Xu,B.Xu,X.Liu,Heteropoly blue-intercalated layered double hydroxides for cationic dye removal from aqueous media,Appl.Clay Sci.54(2011)242–247.
[19]S.K.Shukla,A.K.Mishra,O.A.Arotiba,B.B.Mamba,Chitosan-based nanomaterials:A state-of-the-art review,Int.J.Biol.Macromol.59(2013)46–58.
[20]H.Zhao,J.Xu,W.Lan,T.Wang,G.Luo,Micro fluidic production of porous chitosan/silica hybrid microspheres and its Cu(II)adsorption performance,Chem.Eng.J.229(2013)82–89.
[21]J.Filipović-Grčić,B.Perissutti,M.Moneghini,D.Voinovich,A.Martinac,I.Jalšenjak,Spray-dried carbamazepine-loaded chitosan and HPMC microspheres:Preparation and characterisation,J.Pharm.Pharmacol.55(2003)921–931.
[22]H.Peng,H.Xiong,J.Li,M.Xie,Y.Liu,C.Bai,L.Chen,Vanillin cross-linked chitosan microspheres for controlled release of resveratrol,Food Chem.121(2010)23–28.
[23]M.Vakili,M.Rafatullah,B.Salamatinia,A.Z.Abdullah,M.H.Ibrahim,K.B.Tan,Z.Gholami,P.Amouzgar,Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater:A review,Carbohydr.Polym.113(2014)115–130.
[24]A.Gamage,F.Shahidi,Use of chitosan for the removal of metal ion contaminants and proteins from water,Food Chem.104(2007)989–996.
[25]Z.Dong,H.Xu,Z.Bai,H.Wang,L.Zhang,X.Luo,Z.Tang,R.Luque,J.Xuan,Micro fluidic synthesis of high-performance monodispersed chitosan microparticles for methyl orange adsorption,RSC Adv.5(2015)78352–78360.
[26]C.H.Yang,K.S.Huang,P.W.Lin,Y.C.Lin,Using a cross- flow micro fluidic chip and external crosslinking reaction for monodisperse TPP-chitosan microparticles,Sensors Actuators B Chem.124(2007)510–516.
[27]J.H.Xu,S.W.Li,C.Tostado,W.J.Lan,G.S.Luo,Preparation of monodispersed chitosan microspheres and in situ encapsulation of BSA in a co-axial micro fluidic device,Biomed.Microdevices 11(2009)243–249.
[28]J.H.Xu,S.W.Li,J.Tan,G.S.Luo,Correlations of Droplet Formation in T-junction Micro fluidic Devices:From Squeezing to Dripping,Pantheon Books,2008.
[29]W.J.Lan,S.W.Li,J.H.Xu,G.S.Luo,Rapid measurement of fluid viscosity using coflowing in a co-axial micro fluidic device,Micro fluid.Nano fluid.8(2010)687–693.
[30]M.V.Subbaiah,D.S.Kim,Adsorption of methyl orange from aqueous solution by aminated pumpkin seed powder:kinetics,isotherms,and thermodynamic studies,Ecotoxicol.Environ.Saf.128(2016)109–117.
[31]Z.Zhou,F.Jiang,T.C.Lee,T.Yue,Two-step preparation of nano-scaled magnetic chitosan particles using Triton X-100 reversed-phase water-in-oil microemulsion system,J.Alloys Compd.581(2013)843–848.
[32]Y.G.Ko,S.S.Shin,U.S.Choi,S.P.Yong,J.W.Woo,Gelation of chitin and chitosan dispersed suspensions under electric field:Effect of degree of deacetylation,ACS Appl.Mater.Interfaces 3(2011)1289.
[33]N.S.Kumar,M.Suguna,M.V.Subbaiah,A.S.Reddy,N.P.Kumar,A.Krishnaiah,Adsorption of phenolic compounds from aqueous solutions onto chitosan-coated perlite beads as biosorbent,Ind.Eng.Chem.Res.49(2010)9238–9247.
[34]T.V.Toledo,Preparation and evaluation ofmagnetic chitosan particles modified with ethylenediamine and Fe(III)for the removal of Cr(VI)from aqueous solutions,Quim.Nova 37(2014)327–329.
[35]S.Peng,H.Meng,Y.Ouyang,J.Chang,Nanoporous magnetic cellulose–chitosan composite microspheres:preparation,characterization,and application for Cu(II)adsorption,Ind.Eng.Chem.Res.53(2014)2106–2113.
[36]V.Nair,A.Panigrahy,R.Vinu,Development of novel chitosan–lignin composites for adsorption of dyes and metal ions from wastewater,Chem.Eng.J.254(2014)491–502.
[37]S.Zhang,Y.Zhou,W.Nie,L.Song,T.Zhang,Preparation of uniform magnetic chitosan microcapsules and their application in adsorbing copper ion(II)and chromium ion(III),Ind.Eng.Chem.Res.51(2012)14099–14106.
[38]X.Tang,D.Niu,C.Bi,B.Shen,Hg2+adsorption from a low-concentration aqueous solution on chitosan beads modified by combining polyamination with Hg2+-imprinted technologies,Ind.Eng.Chem.Res.52(2013)13120–13127.
[39]S.Saravanan,S.Nethala,S.Pattnaik,A.Tripathi,A.Moorthi,N.Selvamurugan,Preparation,characterization and antimicrobial activity of a bio-composite scaffold containing chitosan/nano-hydroxyapatite/nano-silver for bone tissue engineering,Int.J.Biol.Macromol.49(2011)188–193.
[40]H.Ren,Z.Gao,D.Wu,J.Jiang,Y.Sun,C.Luo,Efficient Pb(II)removal using sodium alginate–carboxymethyl cellulose gel beads:preparation,characterization,and adsorption mechanism,Carbohydr.Polym.137(2016)402–409.
[41]O.Duman,S.Tunç,T.G.Polat,B.K.Bozoğlan,Synthesis of magnetic oxidized multiwalled carbon nanotube-κ-carrageenan-Fe3O4nanocomposite adsorbent and its application in cationic Methylene Blue dye adsorption,Carbohydr.Polym.147(2016)79–88.
[42]M.M.El-Zawahry,F.Abdelghaffar,R.A.Abdelghaffar,A.G.Hassabo,Equilibrium and kinetic models on the adsorption of Reactive Black 5 from aqueous solution using Eichhornia crassipes/chitosan composite,Carbohydr.Polym.136(2015)507–515.
[43]S.Duan,R.Tang,Z.Xue,X.Zhang,Y.Zhao,W.Zhang,J.Zhang,B.Wang,S.Zeng,D.Sun,Effective removal of Pb(II)using magnetic Co0.6Fe2.4O4micro-particles as the adsorbent:synthesis and study on the kinetic and thermodynamic behaviors for its adsorption,Colloids Surf.A Physicochem.Eng.Asp.469(2015)211–223.
[44]H.Tajizadegan,O.Torabi,A.Heidary,M.H.Golabgir,A.Jamshidi,Study of methyl orange adsorption properties on ZnO–Al2O3nanocomposite adsorbent particles,Desalin.Water Treat.57(2015)1–11.
[45]M.Costa,Effective removal of Cu(II)ions from aqueous solution by aminofunctionalized magnetic nanoparticles,J.Hazard.Mater.184(2010)392–399.
[46]J.Fan,Z.Zhao,W.Liu,Y.Xue,Y.Shu,Solvothermal synthesis of different phase N–TiO2and their kinetics,isotherm and thermodynamic studies on the adsorption of methyl orange,J.Colloid Interface Sci.470(2016)229–236.
[47]M.Shariati-Rad,M.Irandoust,S.Amri,M.Feyzi,F.Ja'Fari,Magnetic solid phase adsorption,preconcentration and determination of methyl orange in water samples using silica coated magnetic nanoparticles and central composite design,Int.Nano Lett.4(2014)91–101.
 Chinese Journal of Chemical Engineering2018年3期
Chinese Journal of Chemical Engineering2018年3期
- Chinese Journal of Chemical Engineering的其它文章
- Numerical investigation on flow and heat transfer characteristics of corrugated tubes with non-uniform corrugation in turbulent flow
- Investigations on pool boiling critical heat flux,transient characteristics and bonding strength of heater wire with aqua based reduced graphene oxide nano fluids
- Heavy metals adsorption by banana peels micro-powder:Equilibrium modeling by non-linear models
- Potential aspect of rice husk biomass in Australia for nanocrystalline cellulose production
- Fouling evaluation on membrane distillation used for reducing solvent in polyphenol rich propolis extract
- Investigation on a vertical radial flow adsorber designed by a novel parallel connection method☆
