Effects of nitrogen doping on surface-enhanced Raman scattering(SERS)performance of bicrystalline TiO2 nano fibres☆
Haijuan Zhang ,Rong An ,Xinghong Ji,Yihui Dong ,Fan Pan ,Chang Liu ,*,Xiaohua Lu
1 College of Chemistry and Chemical Engineering,State Key Laboratory of Materials-Oriented Chemical Engineering,Nanjing Tech University,Nanjing 210009,China
2 Herbert Gleiter Institute of Nanoscience,Nanjing University of Science and Technology,No.200,Xiaolingwei,Nanjing 210094,China
1.Introduction
Surface-enhanced Raman scattering(SERS)is a novel spectroscopic technology that acquires the advantages of fast measurement,low detection limit and nondestructive analysis[1–3].Since the discovery of SERS in 1974,it became an effective toolin multidisciplinary research fields,such as photonics,biochemistry,biomedical engineering,electrochemistry,surface science,and life science[4,5].The SERS is primarily governed by two mechanisms,electromagnetic(EM)enhancement and chemical enhancement(CE).The EM enhancement is induced by local electric field that is generated from the collective oscillations of surface plasmons in metallic particles[6].And the CE is mostly related to the charge transfer between chemisorbed molecules and the substrates.The mostly used SERS-active substrates are nobel metal nanoparticles,such as Au and Ag,which have unique features of localized surface plasmon resonance[7],and can result in excellent activity and good sensitivity as a SERS substrate.However,the applications of noble metal substrates are largely limited by their shortcomings such as low stability,poor biocompatibility,high-cost[8].Recently,researchers have sought other alternative materials as SERS substrates.SERS from molecules on semiconductor has attracted more attentions.
Semiconductors such as ZnO,TiO2,and WO3are attracting more interest in recent years due to their unique optical,chemical,electrical,and catalytic properties[9–11].These semiconductors provide an alternative option to replace traditional SERS and found applications in biochemistry[12],materials science[13],and surface science[14,15].Among the various semiconductors,TiO2has attracted considerable attention due to its high chemical stability,photocatalytic activity,economy as well as biocompatibility[16,17].However,like other semiconductor SERS substrates,the enhancement factor of TiO2is still much lower than that of metal substrates due to its wide band gap.The wide band gap limits the separation of photogenerated charges,thereby reducing charge transfer efficiency[18–20].Most SERS performance with visible laser excitation on semiconductor nanoparticles is induced by charge transfer transitions between the semiconductor and molecules,which is based on the CE mechanism[21].In orderto improve the charge transfer between TiO2and adsorbents,many scientists have focused on ion-doping of TiO2by introducing several sub-band-gap energy levels.Yang et al.[22]reported that doped metal ions(Fe3+,Co2+,Ni2+)can embed abundant doping levels in TiO2band gap,which can promote the charge transferprocess and improve SERS effects.Xue et al.[23]found that Mn2+doped TiO2nanoparticles exhibit a high SERS intensity of the 4-MBA molecules,which increases by a factor of six as compared to the pure TiO2nanoparticles.Nevertheless,TiO2doped with metal elements has poor photostability and often leads to the aggregation of recombination centers,as wellas the decrease of its biocompatibility[24,25].Recently,doping TiO2with non-metal elements such as nitrogen,carbon, fluorine or sulfur has become the most accepted approach for the enhancement of TiO2photoactivity[26–28].Among these non-metal elements,the simplest and most feasible approach seems to be nitrogen doping,which is usually deemed to be the most effective approach for introducing the doping energy levels of TiO2and can induce high separation efficiency of photoinduced charge[29].
In this work,bicrystalline anatase/TiO2(B)nano fibers were fabricated via ion exchange and calcination of precursor K2Ti2O5.The unique bicrystalline phase in anatase/TiO2(B)could help to stabilize the charge transfer process[30],and thus expect to enhance the Raman scattering performance.In order to increase the SERS activity,bicrystalline TiO2nano fibres were further doped with nitrogen element and used as the substrate material.The SERS performance was characterized and comparatively investigated with the spectra of 4-MBA adsorbed on nitrogen-doped and undoped bicrystalline TiO2nano fibres.Calcination temperature(500,600 and 700°C)was adjusted to control the nitrogen doping level.Meanwhile,the effect of nitrogen doping level on the Raman scattering property is studied.The mechanism of nitrogen doping on the SERS enhancement is also discussed in this work.
2.Experimental
2.1.Materials
Hydrous titanium dioxide(TiO2·n H2O)was obtained from Nanjing oil chemicals Co.,Ltd.,China.Potassium carbonate(K2CO3)was obtained from Shanghai chemical reagent factory.4-Mercaptobenzoic acid(4-MBA)was purchased from Aldrich,USA.All the other chemicals were of analytical grade and used without further purification.Triply distilled water was used in our experiment.
2.2.Synthesis of bicrystalline TiO2 nano fibres
The fabrication processes for TiO2and N-TiO2in this work are similar to that described in our previous papers[31].The synthetic procedure was brie fly described as follows.First,TiO2·n H2Owas uniformly blended with K2CO3by ballmilling,the mixture was dried at90°C in the oven for 10 h,then sintered at810°Cfor 2 h to produce potassium dititanates.The molarratio of TiO2/K2Owas fixed at1.9.Subsequently,the sintered product was soaked in distilled water for 7 days.The as-prepared hydration product was suspended in a certain amount of vigorously stirred HCl solution(pH=1)until potassium ion was completely removed.Then the product was separated by filtration,washed with distilled water,and dried in oven at 60°C to obtain H2Ti2O5.Finally,the dried H2Ti2O5sample was calcined in air for 2 h at different temperatures of 500,600 and 700°C.The calcined TiO2nano fibers were named as T500,T600,and T700,referring to the different sintering temperatures 500,600,and 700°C,respectively.For preparing nitrogen doped TiO2,same heating profile was used while keeping the calcination process in ammonia gas environment.The products were named NT500,NT600 and NT700 after heat treatment.
2.3.Adsorption of probing molecules
Samples for Raman spectroscopic studies were prepared as follows:20 mg of TiO2or N-TiO2substrates was dispersed in 10 ml of 4-MBA(1 × 10−3mol·L−1)ethanol solution,and the mixture was stirred for 3 h.Then,the precipitate was centrifuged and rinsed with purified water for two times.Samples were then subject to vacuum dry at 40°C for 2 h.Afterwards,N-TiO2or TiO2nano fibers modified by 4-MBA were obtained.
2.4.Characterization
The crystal structures of the products were confirmed by X-ray diffraction(XRD,Bruker,Model D8 with Cu Kαexcitation)and Raman spectra.The elemental composition and chemical state of the samples were analyzed by X-ray photoelectron spectrometer(XPS,Physical Electronics 5600).The UV–vis diffuse reflectance spectra(UV–vis DRS)was obtained by a UV/vis spectrometer(Perkin-Elmer Lambda 950).Surface morphologies of the samples were studied by using fieldemission scanning electron microscope(FESEM,Hitachi S-4800).Photoluminescence(PL)spectrum was obtained on an Edinburgh FLS-920 spectrometer.
Raman measurement:The Raman spectrum was collected using a Horiba–Jobin Yvon system(Horiba Labram HR800)with a 514 nm air-cooled Ar+laser line.The laser power is controlled at~5 mW.The size of the laser beam on the sample is about 1 μm.The typical spectral collection condition is a 20 s exposure time and two accumulation.The obtained Raman signals are fitted by a Lorenzian function using the LabSpec5 software,and then the Raman shifts,intensity,full width at the half-maximum intensity can be obtained.
3.Results and Discussion
3.1.Characteristics of nitrogen-doped bicrystalline TiO2 nano fibres
Fig.1 shows the XRDpatterns of commercial P25 and various N-TiO2samples.The characteristic peaks of sintered K2Ti2O5disappear in the hydrolytic reaction,assigned to the phase transformation to noncrystalline H2Ti2O5(Fig.S1).As they were deduced from the appearance of phase TiO2(B)in NT500,the amorphous precursor may contain H2Ti5O11,which is in agreement with the findings in our previous work[31,32].All N doping samples show characteristic diffraction peaks of the anatase crystal phase of TiO2,locating at 2θ=25.4°(101)and 48°(200).It is noteworthy that the amount of TiO2(B)decreased with increasing the sintering temperature,and completely disappeared in NT700.

Fig.1.XRD patterns of P25 and N-TiO2 nano fibers(N represents nitrogen doped samples,T500,T600,T700 denotes sintering temperature).

Fig.2.FESEM of P25 and N-TiO2 nano fibers:(a)P25,(b)NT500,(c)NT600,(d)NT700.
The microstructures of P25 and prepared N-TiO2samples were characterized by FESEM as shown in Fig.2.As indicated in Fig.2a,large aggregates with particles of 25 nm were observed in P25.The average particle size of protonic titanate precursor-H2Ti2O5is about 5–10 nm(Fig.S3)with nano-voids existed between the particles.Such stacked void space would facilitate the diffusion of ammonia and maximize nitrogen doping.As shown in Fig.2b,significant microstructure change could be observed after thermal treatment at 500°C.The particle size increased to nearly 15–20 nm,and it is also noticed that those nanovoids were gradually changed into numerous nano-pores with average pore size of about 10 nm.With increasing temperature to 600°C,slitshaped nanopores were observed in Fig.2c after the reorganization of nanoparticles.Further increasing temperature to 700°C leads to a nonporous structure of NT700,Fig.2d.The high treatment temperature is the major reason for the collapsed pore structure and thus solid structure is obtained.Such nonporous structure blocks the diffusion of ammonia and thus negatively affects the nitrogen-uptake during the doping process.

Fig.3.UV–vis spectra of P25 and N-TiO2 nano fibers(the dotted line represents the absorption edge of TiO2.Inset:Tauc plots showing the bandgap energies calculated by the transformed Kubelka–Munk function).

Fig.4.Deconvoluted XPS spectra of NT600.
Ion doping has a significant influence on light absorption properties of TiO2[33,34].The UV–vis absorption spectra ofP25 and various N-TiO2samples were shown in Fig.3.It was found that there was a clear red shift of the absorption edge and enhanced absorption in the visible light region as compared to P25.The red-shift of the absorption edge demonstrated a decrease in the band gap energy.This expanded optical absorption should arise from the formation of doping energy levels by the integration of nitrogen atoms in TiO2,which allows the sub-bandgap excitation[35].In addition,visible light absorption for N-TiO2increased with increasing sintering temperature(500–600 °C)of the doping process.However,as doing temperature increased to 700°C,the absorbance decreased in the visible light range that could be attributed to the release of nitrogen element from the TiO2lattice at high temperatures.
In order to understand the chemical state of nitrogen dope TiO2,XPS technique was employed to analyze the binding state of nitrogen,specifically for NT600.As seen in Fig.4,the peak located at a binding energy value of 399.8 eV is attributed to the O–Ti–N structure[33].The presence of a peak at 396.2 eV corresponds to the formation of Ti–N–Ti[36].Another peak observed at402.2 eVis assigned to the interstitial Ti–O–N[37].The nitrogen content in NT600 is about 3.34%in atom ratio.However,owing to the resolution limit of the XPS instrument in our experiment,the nitrogen percentage in NT500 and NT700 is difficult to quantified because the content is very low(Fig.S2).But the nitrogen difference among the samples may be differentiated from the absorption intensity in the UV–vis spectra discussed above.P25 presented an onset of absorption at 405 nm as commonly observed,while doping with nitrogen greatly enhanced the absorption at a wavelength of 400–550 nm.Therefore,it can be concluded that nitrogen content varies in the order NT600>NT500>NT700,indicated from the XPS and UV–vis date.
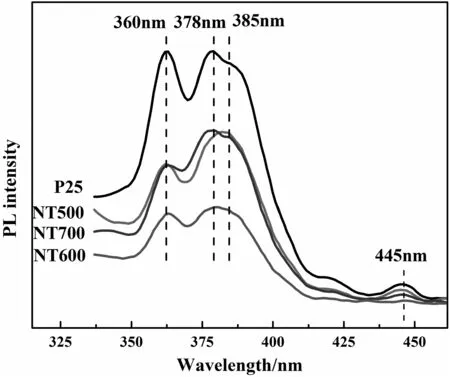
Fig.6.Photoluminescence(PL)spectra of P25 and N-doped bicrystalline TiO2 nano fibers.
3.2.SERS activity of bicrystalline TiO2 nano fibres
Fig.5 shows the SERS spectra of 4-MBA molecules adsorbed on bicrystalline TiO2nano fibres at room temperature.All peaks are consistent with those previously reported for4-MBAadsorbed on TiO2nanoparticles[22].Two strong bonds observed at 1593 and 1074 cm−1correspond to ν8a(a1)and ν12(a1)aromatic ring characteristic vibrations,respectively.Other weak bonds located at 1149(ν15,b2)and 1182 cm−1(ν9,a1)are assigned to the C--H deformation modes.The S--H group of 4-MBA is known to be dissociated on the metal surface.Fig.S4 verifies this fact since the characteristic S--H band is not observed in the SERS spectra.The NT600 is dispersed in the 4-MBA ethanol solution,the 4-MBA molecule should be bonded to the NT600 via the S atom.It is clear that4-MBA adsorbed on N-doped TiO2substrates exhibitstronger signals than that observed on pure P25 and undoped TiO2nano fibers.For undoped TiO2nano fibers,bicrystalline T500,T600 nano fibres exhibitbetter SERS activity in comparison with T700 single crystal.Remarkably,NT600 shows the best activity toward SERS signals.These results indicate thatnitrogen doping can enhance the SERS effectofTiO2corresponding to surface-adsorbed analytes,and the SERS signals increase evidently with increasing nitrogen content.Foreach sample,Raman spectra from 10 randomly selected spots were collected,and the signal intensity was averaged for final analysis.The relative standard deviation(RSD)of NT600 was calculated to be~0.12(Figs.5 and S4),confirming the excellent SERS signal uniformity of the N-TiO2substrate.

Fig.5.(A)Raman spectra of 4-MBA adsorbed on P25 and bicrystalline TiO2 nano fibers.(B)The relative standard deviation of the 1593 cm−1 Raman vibration mode of 4-MBA on NT600.

Fig.7.The schematic diagram of the proposed charge transfer mechanism.A:undoped TiO2;B:N-TiO2.
The PL technique has been widely used to study the photophysical and photochemical properties of semiconductors due to its high sensitivity and non-destructive character[38].Itcan be usefulto reveal in formation such as surface oxygen vacancies and defects as well as the separation and recombination of photo-induced charge carriers[39].Fig.6 shows the PL spectra of P25 and N-doped TiO2samples at 325 nm excitation wavelength.It is quite apparent that all samples showobvious PL spectra with similarshaped curves,having distinguishable peaks atabout360,378,385 and 445 nm,respectively.According to the theory reported previously,the peak at 360 nm is attributed to the band-band PL process[40]in which the electron transition takes place from the bottom of conduction band(CB)to the top of valence band(VB).And the PL peaks at 378 and 445 nm correspond to the excitonic signals[40,41]relating to the sub-band(surface defects).A broad emission band centered at385 nmwas observed in allsamples,which corresponds to the band edge transition.Nitrogen doping in TiO2enriches its oxygen vacancies,which can act as the traps to bind photo-generated electrons,suppress electron–hole recombination and thus greatly reduce the PL intensity.It can be seen from Fig.6 that the PL intensity decreases in the order of NT600<NT500<NT700<P25,depending on the amount of dopant.Thus,it can be concluded that nitrogen doping in TiO2can effectively separate the photo-generated electron–hole pairs by introducing doping energy levels.This SERS enhancement phenomenon is in good agreement with the evidences supported by PL measurement.It is well known that for most semiconductor materials,the chemical enhancement mechanism[22]should dominate SERS effects.In this case,the observed enhancementis due to charge transfer interactions between TiO2and the surface-adsorbed molecule,4-MBA.
3.3.SERS Mechanism for 4-MBA Adsorbed on Bicrystalline TiO2 Nano fibres
A mechanism in Fig.7 is proposed to explain the charge transfer observed in the bicrystalline TiO2nano fibres and in the N-TiO2nano fibres.The bicrystalline structure of TiO2provides additional charge transfer from anatase to TiO2(B)besides the anatase to probing molecule transition.This process lowers the energy for charge transfer,which is responsible for those additional SERS enhancement in the present bicrystalline TiO2system.As shown in Fig.7(A),the photoinduced electrons in the CB of TiO2(B)migrate to the anatase phase first which has a lower CB potential.Then,electrons move the CB electrons of anatase to the available sub band gap and finally inject into the adsorbed molecules.At the same time,the holes stimulated in anatase would migrate toward the TiO2(B)phase because of the higher VB edge potentials.Nitrogen doping in TiO2can further improve the separation of photo-generated electron–hole pairs by introducing doping energy levels,as shown in Fig.7(B),which is favorable for the TiO2-to-molecule charge transfer and SERS for the adsorbed molecules.Thus,the SERS signals of 4-MBA adsorbed on the N-TiO2exhibit a larger enhancement as compared to the one on pure TiO2.
4.Conclusions
In summary,we have successfully fabricated nitrogen-doped bicrystalline TiO2nano fibres as SERS active substrates.The difference between the conduction band edges of the two phases may produce charge transfer from one phase to the other,which results in effective separation of photo-generated charge and thus facilitates SERS enhancement.In addition,we have compared the SERS enhancement of the 4-MBA molecules adsorbed on N-TiO2of different nitrogen content.The SERS of 4-MBA adsorbed on NT600,containing the highest percentage of nitrogen,facilitates the charge transfer process and thus SERS intensity.The introduction of nitrogen dopant in TiO2induces facile charge transfer between the molecules and substrate by creating doping energy levels and surface defects.These results demonstrate that nitrogen-doped bicrystalline TiO2substrates are good candidates to improve the SERS performance.This work is expected to attract broad research interest in semiconductor based SERS-active substrates.
Supplementary Material
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.cjche.2017.05.020.
[1]A.Wang,X.Kong,Review of recent progress of plasmonic materials and nanostructures for surface-enhanced Raman scattering,Materials 8(6)(2015)3024.
[2]X.M.Qian,S.M.Nie,Single-molecule and single-nanoparticle SERS:From fundamental mechanisms to biomedical applications,Chem.Soc.Rev.37(5)(2008)912–920.
[3]J.Chao,W.Cao,S.Su,L.Weng,S.Song,C.Fan,L.Wang,Nanostructure-based surfaceenhanced Raman scattering biosensors for nucleic acids and proteins,J.Mater.Chem.B 4(10)(2016)1757–1769.
[4]W.Ji,B.Zhao,Y.Ozaki,Semiconductor materials in analytical applications of surface-enhanced Raman scattering,J.Raman Spectrosc.47(1)(2016)51–58.
[5]X.X.Han,W.Ji,B.Zhao,Y.Ozaki,Semiconductor-enhanced Raman scattering:active nanomaterials and applications,Nano 9(15)(2017)4847–4861.
[6]J.R.Lombardi,R.L.Birke,A unified view of surface-enhanced Raman scattering,Acc.Chem.Res.42(6)(2009)734–742.
[7]S.L.Kleinman,E.Ringe,K.L.Wustholz,E.Phillips,K.A.Scheidt,G.C.Schatz,R.P.V.Duyne,Single-molecule surface-enhanced Raman spectroscopy of crystal violet isotopologues:Theory and experiment,J.Am.Chem.Soc.133(11)(2011)4115–4122.
[8]W.Ji,W.Song,I.Tanabe,Y.Wang,B.Zhao,Y.Ozaki,Semiconductor-enhanced Raman scattering for highly robust SERS sensing:The case of phosphate analysis,Chem.Commun.51(36)(2015)7641–7644.
[9]I.Alessandri,L.E.Depero,All-oxide Raman-active traps for light and matter:probing redox homeostasis model reactions in aqueous environment,Small 10(7)(2014)1294–1298.
[10]W.Song,W.Ji,S.Vantasin,I.Tanabe,B.Zhao,Y.Ozaki,Fabrication of a highly sensitive surface-enhanced Raman scattering substrate for monitoring the catalytic degradation of organic pollutants,J.Mater.Chem.A 3(25)(2015)13556–13562.
[11]L.Li,T.Hutter,A.S.Finnemore,F.M.Huang,J.J.Baumberg,S.R.Elliott,U.Steiner,S.Mahajan,Metal oxide nanoparticle mediated enhanced Raman scattering and its use in direct monitoring of interfacial chemical reactions,Nano Lett.12(8)(2012)4242–4246.
[12]L.C.Perez,L.Kador,B.Peng,M.Thelakkat,Characterization of the adsorption of Ru-bpy dyes on mesoporous TiO2films with UV–Vis,Raman,and FTIR spectroscopies,J.Mater.Chem.B 110(17)(2006)8723–8730.
[13]A.Musumeci,D.Gosztola,T.Schiller,N.M.Dimitrijevic,V.Mujica,D.Martin,T.Rajh,SERS of semiconducting nanoparticles(TiO2hybrid composites),J.Am.Chem.Soc.131(17)(2009)6040–6041.
[14]W.Xu,J.Xiao,Y.Chen,Y.Chen,X.Ling,J.Zhang,Graphene-veiled gold substrate for surface-enhanced Raman spectroscopy,Adv.Mater.25(6)(2013)928–933.
[15]R.Livingstone,X.Zhou,M.C.Tamargo,J.R.Lombardi,L.G.Quagliano,F.Jeanmary,Surface enhanced Raman spectroscopy of pyridine on CdSe/ZnBeSe quantum dots grown by molecular beam epitaxy,J.Phys.Chem.C 114(1)(2010)17460–17464.
[16]D.Y.Qi,L.J.Lu,L.Z.Wang,J.L.Zhang,Improved SERS sensitivity on plasmon-free TiO2photonic microarray by enhancing light-matter coupling,J.Am.Chem.Soc.136(28)(2014)9886–9889.
[17]Z.L.Zhang,D.N.Li,Y.L.Mao,Effects of trap density on the surface-enhanced Raman scattering of molecules adsorbed on TiO2(Degussa P25),J.Raman Spectrosc.43(12)(2012)1920–1923.
[18]S.In,A.Orlov,R.Berg,F.Garcia,S.Pedrosa-Jimenez,M.S.Tikhov,D.S.Wright,R.M.Lambert,Effective visible light-activated B-doped and B,N-Codoped TiO2photocatalysts,J.Am.Chem.Soc.129(45)(2007)13790–13791.
[19]M.Liu,L.Piao,L.Zhao,S.Ju,Z.Yan,T.He,C.Zhou,W.Wang,Anatase TiO2single crystals with exposed{001}and{110}facets:Facile synthesis and enhanced photocatalysis,Chem.Commun.46(10)(2010)1664–1666.
[20]Y.Hou,C.Zheng,Z.Zhu,X.Wang,Microwave-assisted fabrication of porous hematite photoanodes for efficient solar water splitting,Chem.Commun.52(42)(2016)6888–6891.
[21]L.B.Yang,M.D.Gong,X.Jiang,D.Yin,X.Y.Qin,B.Zhao,W.D.Ruanb,Investigation on SERS of different phase structure TiO2nanoparticles,J.Raman Spectrosc.46(3)(2015)287–292.
[22]L.Yang,X.Jiang,W.Ruan,B.Zhao,W.Xu,J.R.Lombardi,Observation of enhanced Raman scattering for molecules adsorbed on TiO2nanoparticles:Charge-transfer contribution,J.Phys.Chem.C 112(50)(2008)20095–20098.
[23]X.Xue,W.Ji,Z.Mao,Z.Li,W.Ruan,B.Zhao,J.R.Lombardi,Effects of Mn doping on surface enhanced Raman scattering properties of TiO2nanoparticles,Spectrochim.Acta A 95(2012)213–217.
[24]R.Asahi,T.Morikawa,T.Ohwaki,K.Aoki,Y.Taga,Visible-light photocatalysis in nitrogen-doped titanium oxides,Science 293(5528)(2001)269–271.
[25]C.D.Valentin,G.Pacchioni,A.Selloni,Theory of carbon doping of titanium dioxide,Chem.Mater.17(26)(2005)6656–6665.
[26]V.Kiran,S.Sampath,Enhanced Raman spectroscopy of molecules adsorbed on carbon-doped TiO2obtained from titanium carbide:A visible-light-assisted renewable substrate,ACS Appl.Mater.Interfaces 4(8)(2012)3818–3828.
[27]S.Livraghi,M.C.Paganini,E.Giamello,A.Selloni,V.C.Di,G.Pacchioni,Origin of photoactivity of nitrogen-doped titanium dioxide under visible light,J.Am.Chem.Soc.128(49)(2006)15666–15671.
[28]K.Nishijima,Y.Fujisawa,N.Murakami,T.Tsubota,T.Ohno,Development of an S-doped titania nanotube(TNT)site-selectively loaded with iron(III)oxide and its photocatalytic activities,Appl.Catal.B 84(3–4)(2008)584–590.
[29]X.Cheng,X.Yu,Z.Xing,Enhanced photoelectric property and visible activity of nitrogen doped TiO2synthesized from different nitrogen dopants,Appl.Surf.Sci.268(2013)204–208.
[30]Y.Bai,W.Li,C.Liu,Z.H.Yang,X.Feng,X.H.Lu,K.Y.Chan,Stability of Pt nanoparticles and enhanced photocatalytic performance in mesoporous Pt-(anatase/TiO2(B))nanoarchitecture,J.Mater.Chem.19(38)(2009)7055–7061.
[31]M.He,X.H.Lu,X.Feng,L.Yu,Z.H.Yang,A simple approach to mesoporous fibrous titania from potassium dititanate,Chem.Commun.19(2004)2202–2203.
[32]W.Li,C.Liu,Y.X.Zhou,Y.Bai,X.Feng,Z.H.Yang,L.H.Lu,X.H.Lu,K.Y.Chan,Enhanced photocatalytic activity in anatase/TiO2(B)core-shell nano fiber,J.Phys.Chem.C 112(51)(2008)20539–20545.
[33]Y.Cong,Z.Jinlong,F.Chen,A.Masakazu,D.He,Preparation,photocatalytic activity,and mechanism of nano-TiO2co-doped with nitrogen and iron(III),J.Phys.Chem.C 111(28)(2007)10618–10623.
[34]E.Wang,P.Zhang,Y.Chen,Z.Liu,T.He,Y.Cao,Improved visible-light photocatalytic activity of titania activated by nitrogen and indium modification,J.Mater.Chem.22(29)(2012)14443–14449.
[35]G.Xin,H.Pan,D.Chen,Z.Zhang,B.Wen,Synthesis and photocatalytic activity of N-doped TiO2produced in a solid phase reaction,J.Phys.Chem.Solids 74(2)(2013)286–290.
[36]Z.G.Li,S.Miyake,Characteristics of N-doped TiO2thin films grown on unheated glass substrate by inductively coupled plasma assisted dc reactive magnetron sputtering,Appl.Surf.Sci.255(22)(2009)9149–9153.
[37]G.Zhang,X.Ding,F.He,X.Yu,J.Zhou,Y.Hu,J.Xie,Preparation and photocatalytic properties of TiO2-montmorillonite doped with nitrogen and sulfur,J.Phys.Chem.Solids 69(69)(2008)1102–1106.
[38]L.Jing,Y.Qu,B.Wang,S.Li,B.Jiang,L.Yang,W.Fu,H.Fu,J.Sun,Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity,Sol.Energy Mater.Sol.Cells 90(12)(2006)1773–1787.
[39]S.Cong,Y.Yuan,Z.Chen,J.Hou,M.Yang,Y.Su,Y.Zhang,L.Li,Q.Li,F.Geng,Z.Zhao,Noble metal-comparable SERS enhancement from semiconducting metal oxides by making oxygen vacancies,Nat.Commun.6(2015)7800.
[40]J.Yu,H.Yu,B.Cheng,X.Zhao,J.C.Yu,W.-K.Ho,The effectof calcination temperature on the surface microstructure and photocatalytic activity of TiO2thin films prepared by liquid phase deposition,J.Phys.Chem.B 107(50)(2003)13871–13879.
[41]L.Jing,H.Fu,B.Wang,D.Wang,B.Xin,S.Li,J.Sun,Effects of Sn dopant on the photoinduced charge property and photocatalytic activity of TiO2nanoparticles,Appl.Catal.B Environ.62(3)(2006)282–291.
 Chinese Journal of Chemical Engineering2018年3期
Chinese Journal of Chemical Engineering2018年3期
- Chinese Journal of Chemical Engineering的其它文章
- Numerical investigation on flow and heat transfer characteristics of corrugated tubes with non-uniform corrugation in turbulent flow
- Investigations on pool boiling critical heat flux,transient characteristics and bonding strength of heater wire with aqua based reduced graphene oxide nano fluids
- Heavy metals adsorption by banana peels micro-powder:Equilibrium modeling by non-linear models
- Potential aspect of rice husk biomass in Australia for nanocrystalline cellulose production
- Fouling evaluation on membrane distillation used for reducing solvent in polyphenol rich propolis extract
- Investigation on a vertical radial flow adsorber designed by a novel parallel connection method☆
