Oxidative desulfurization of dibenzothiophene over Fe promoted Co–Mo/Al2O3 and Ni–Mo/Al2O3 catalysts using hydrogen peroxide and formic acid as oxidants
Yaseen Muhammad *,Ayesha Shoukat,Ata Ur Rahman ,Haroon Ur Rashid ,Waqas Ahmad
1 Institute of Chemical Sciences,University of Peshawar,Khyber Pakhtunkhwa,Pakistan
2 School of Chemistry and Chemical Engineering,Key Laboratory of Petrochemical Resource Processing and Process Intensification Technology,Guangxi University,Guangxi 530004,China
3 Department of Chemistry,Sarhad University of Science and Information Technology,Peshawar,Pakistan
1.Introduction
Fossil fuels are contributing more than 82%of energy all over the globe,of which,50%comes from fuel oils and hence petroleum is still the primary source to ful fill these needs[1].Fuel oils are generally contaminated with organosulfur compounds comprising of thiols,aromatic sulfides,mercaptanes and benzothiophenes(BTs),where BTs contribute about 85%[2,3].These organosulfur ring compounds exist in non-substituted i.e.BT,dibenzothiophenes(DBT),as well as alkyl derivatives i.e.4-methyldibenzothiophene(4-MDBT)and 4,6-dimethyldibenzothiophene(4,6-DMDBT)[4].These sulfur(S)compounds adversely affect the quality of petroleum products by decreasing the API gravity and calorific value as well as contribute to environmental pollution by getting converted into SOxduring combustion.Moreover,S species also lead to particulates production resulting in black exhaust fumes[5],causing impairment of pipelines,acid rain and poisoning of catalysts in oil re fineries[6].
Worldwide oil re fineries are now producing low S fuel oils(less than 50 ppm)to meet new regulation mandate[7]which is a challenging job.Several reports following different approaches are available in literature to cope with these challenges including hydrodesulfurization(HDS)[8,9],biodesulfurization(BDS)[10],adsorptive desulfurization[11],oxidative desulfurization(ODS)[12,13],and extraction through ionic liquids(ILs)etc.[14,15].HDS,operated at high temperature and pressure in the presence of excess hydrogen over a suitable catalyst,is quite effective for cyclic and aliphatic S containing hydrocarbons but less attractive towards BT and DBT[16].Also harsh operating conditions in HDS cause higher consumption of hydrogen and decrease of catalyst life resulting in highercosts[17].Similarly,the use of otherapproaches like BDS is hindered by low versatility of types of enzymes,slow reaction rates and low miscibility ofthe reactants[18].On the contrary,ODSis an interesting and conventionalprocess characterized by mild temperature and pressure operation in the presence of a suitable catalyst and oxidant i.e.ozone,perchlorate,potassium permanganate,nitric acid,nitrogen oxides,ter-butyl hydroperoxide and hydrogen peroxide where H2O2has been reported with good activity[19].In addition to oxidant selection,various catalytic systems such as polyoxometalates,molybdates supported on alumina,tungsten,vanadium and titanium supported catalysts have been reported elsewhere[20].Apositive pointofthis approach isthatthe mostrefractory S containing compounds i.e.DBT and its derivatives show high reactivity[21]and are easily oxidized to highly polarized sulfones,which can be subsequently removed by distillation and solvent extraction[22].
Mo supported overalumina has been the choice ofresearcher for the ODS of DBT over the years with high activity.For example,Cedeno-Caero,et al.reported enhanced desulfurization activity using Al2O3supported catalysts in the presence of H2O2as oxidant[23].Similarly,Jia,et al.investigated ODS of DBT into sulfones in the presence of H2O2and 14 wt%MoO3/Al2O3catalyst[16].Apart from this,Fe supported over activated carbon using oxygen as the oxidant has been reported with good ODS activity[24].Similarly,good ODS activity has been reported on Fe based ILs for different S compounds earlier[25–27].These reports show that Fe can act as a good promoter to classical desulfurization catalysts.Thus,it can be assumed to test the ODS of DBT in the presence of Fe based catalyst avoiding the use of expensive ILs which can considerably limit process cost.To achieve this task,this study was aimed to develop an efficient and cost effective catalyst for the ODS of DBT under mild operating conditions with special emphasis on the use of extremely low Fe loading(2 wt%)as the promoter to classical Co–Mo/Al2O3and Ni–Mo/Al2O3catalysts in the presence of H2O2and formic acid as oxidant systems.
2.Experimental
2.1.Chemical reagents
Chemical reagents in this study were of analytical grade and used without further purification.DBT was purchased from Acros Organics,New Jersey,USA.n-heptane,acetic acid,HCl and NaOH were purchased from RdH Laborchemikalien,GmbH and Co.Seelze.Alumina support(Al2O3),Cobalt II nitrate hexahydrate Co(NO3)2·6H2O,Nickel II nitrate hexahydrate Ni(NO3)2·6H2O,ammonium heptamolybdate tetrahydrate[(NH4)6Mo7O24·4H2O]and 30 wt%H2O2were purchased from BDHLaboratory Supplies,Poole,England.Iron II sulfate heptahydrate(FeSO4·7H2O)waspurchased from Merck and Co.,Darmstadt,Germany.
2.2.Model fuel oil
The model oil comprised of 1000 ppm DBT(by dissolving 1 g of DBT in 1000 ml of n-heptane)from which working solutions of 200–1000 ppm were prepared to draw calibration curve for DBT.
2.3.Catalyst preparation
Catalysts were prepared by incipient wet impregnation method reported elsewhere[28,29]using powdered alumina as catalytic support.Stoichiometric amount of alumina was successively impregnated with aqueous solution of(NH4)6Mo7O24·4H2O,corresponding to 5 wt%of Mo followed by Co(NO3)2·6H2O or Ni(NO3)2·6H2O impregnation corresponding to 2 wt%of Co or Ni respectively.After each impregnation,the support with metal salt solution was stirred for 2 h in electric shaker.After shaking,solution was filtered and dried in oven at 120°C for 1 h,followed by calcination in muffle furnace at 500°C for 3 h converting each of the metallic species into oxide state.Similar protocol was followed for Fe promoted Co–Mo/Al2O3and Ni–Mo/Al2O3catalysts having 2 wt%Fe(using FeSO4.7H2O as Fe precursor)loadingsin allsamples.Atthe end of calcination,catalysts were stored in inert N2environment.
2.4.Support and catalyst characterization
Textural properties,elemental composition,and surface morphology of the prepared catalyst were characterized by X-ray diffraction(XRD),Atomic Absorption Spectroscopy(AAS),Energy Dispersive X-ray Analysis(EDX)and Scanning electron microscopy(SEM).
2.5.Catalytic activity test
ODS activity of the catalysts was tested using 800 ppm DBT solution(as model oil)in n-heptane in the presence of H2O2and formic acid as oxidants.Effect of experimental parameters was tested including oxidant type,amount of oxidant,catalyst dose,reaction time and pH one by one while keeping others constant.In a classical experiment,10 ml of 800 ppm solution,0.4 g catalyst and 30 wt%oxidant were taken in a flask.The flask was sealed and shaken at fixed temperature for certain reaction time in water-bath shaker.After shaking,the solution was filtered and analyzed using double beam UV–vis spectrophotometer.Catalytic desulfurization activity was recorded in terms of DBT conversion using Eq.(1).

where:

2.6.Analysis of reaction products
ODS reaction products were analyzed using UV–visible spectrophotometer model 160 A,Shimadzu,Japan.The wavelength was swept from 200 to 800 nm where DBT showed a strong peak at wavelength of maximum absorption(λmax)at 320 nm[30].
3.Results and Discussion
3.1.Textural properties and characterization of catalysts
3.1.1.XRD study
XRD technique was used to get insight about the chemical composition,crystallinity and the presence ofCo,Mo,Niand Fe in Al2O3supported catalysts.XRD runs were recorded at 2θ angular range between 10°and 80°and the scans are depicted in Fig.1 which show peaks for Al2O3support,Mo[31,32]and Co attheirrespective positions[33].Furthermore,Fe(having a relatively weaker peak)was confirmed in XRD scan of Fe–Co–Mo–Al2O3indicating the presence of crystalline iron oxide,though in small amount[34].It is further believed that the species in a material present at concentration lower than 5 wt%,cannot be properly detected by XRD technique[35]and hence very weak peaks for Co,Ni,Fe(2 wt%)and Mo 5 wt%were observed for all the oxide phases.The consistency of XRD analysis can be confirmed from the fact that almost all metals appeared at their previously reported peaks position i.e.cobalt oxide(Co3O4),at 2θ =32°,37°,45°,58°and 66°[36],Fe2O3at 2θ:24°,33°,35°,40°,49°,54°and 57°[37]and NiO shows the characteristic peaks at 2θ =37°,43°and 63°[38].The appearance of weak peaks in XRD analysis for metallic species,specially Fe,required further unequivocal characterization tools i.e.EDX and Atomic Absorption Spectroscopy for catalyst characterization which are provided in the proceeding sections.

Fig.1.XRD pattern of fresh catalysts.
3.1.2.SEM and surface area analyses
Attributed to the fact that surface morphology,and distribution of active species play a key role in determining catalytic activity[39],textural characterizations of catalysts were performed using SEM[Model No:Nova NanoSEM-450]and the scans are depicted in Fig.2(a–c).SEM studies showed that there are irregular distributions ofsupportparticles with average particle size less than 10 μm indicating a closely packed particle structure.SEM scans further showed that particles of Co,Mo,Ni and Fe impregnated catalysts were more tightly packed than nascent support.These results were further supported by low surface area of the impregnated catalysts than mere support.
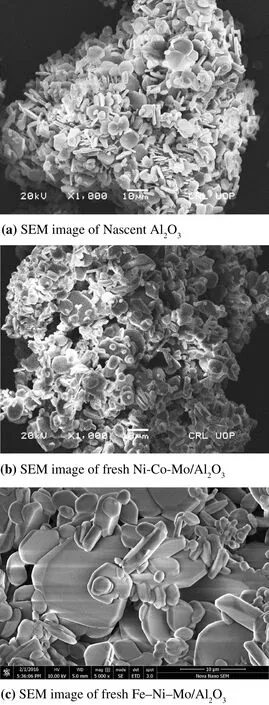
Fig.2.(a)SEM image of Nascent Al2O3.(b)SEM image of fresh Ni–Co–Mo/Al2O3.(c)SEM image of fresh Fe–Ni–Mo/Al2O3.
In order to get further insight of the particles distribution and porosity of the catalysts,surface area of fresh and spent catalysts was determined via N2-adsorption approach using BET surface area and porosity analyzer(Micromeritics TriStar II 3020).The data are shown in Table 1,which indicate that Al2O3support was of low surface area having a structure close to impervious and packed nature.These data are consistent with SEM results showing irregular distribution with less porous nature of the catalyst.It can be seen that surface area of support considerably decreased with the impregnation of metals,which was more dominant in case of tri-metallic catalysts i.e.Fe–Ni–Mo/Al2O3compared to bi-metallic i.e.Co–Mo/Al2O3or Ni–Mo/Al2O3attributed to the presence of extra metal in the earlier one[8].Asupport with low surface area can decrease catalyst cost many times compared to those with high surface area and hence Al2O3with low surface area was chosen in this study without compromising on the over activity of the process.
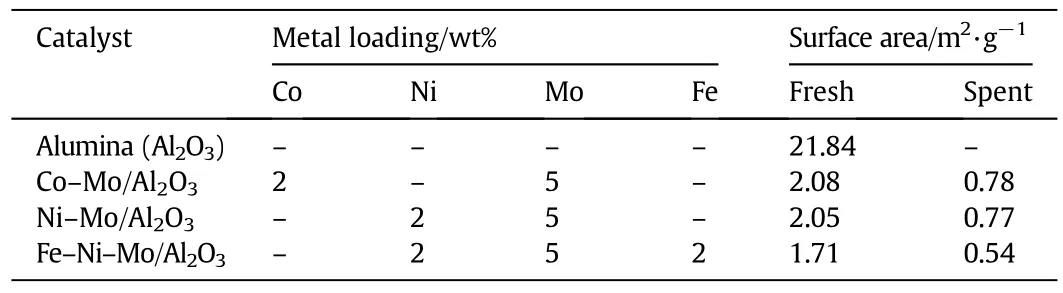
Table 1 BET surface area and metal loadings of different catalysts
3.1.3.EDX and AAS analyses
In order to confirm the presence and incorporation of metallic species onto Al2O3support in the prepared catalysts,EDX spectra were recorded for Fe–Ni–Mo/Al2O3using SEM[Model No:Nova NanoSEM-450].The data are shown in Table 1 and Fig.3,which shows corresponding peaks for Al,Ni,Mo and Fe,confirming results of XRD analysis.
Similarly,success fulincorp oration ofFe in catalytic samples was also tested via quantitative analyses for Fe impregnated catalysts(Fe–Ni–Mo/Al2O3)using AAS technique.In a classical AAS analysis,the catalyst sample(0.125 g)was digested in 10 ml mixture comprised of HNO3,H2SO4,HCl and HF in a ratio of 3:3:2.5:1.5 ml respectively.2.5 ml portion of the solution was then diluted to about 1.2 L and analyzed by AAS(GBC Avanta Ver.1.32 Atomic Absorption Spectrophotometer)at a wavelength of 248.3 nm operated in flame mode using air–acetylene mixture as fuel gas.The amount of Fe contents in the catalyst sample as calculated from AAS analysis,was 1.8 wt%,which is close to the theoretical value of 2 wt%.The minor loss in Fe contents could be attributed to experimental handling errors or leaching during the impregnation process.AAS results are in good agreement with EDX and XRD results,suggesting the presence of Fe as the promoter in catalysts under study.
3.2.Catalytic activity tests
3.2.1.Effect of initial DBT concentration
The effect of initial DBT concentration over Ni–Co–Mo/Al2O3and Fe–Ni–Mo/Al2O3was investigated in a range of 200–1000 ppm as shown in Fig.4.A direct relation between conversion and initial DBT concentration exists which reaches to maximum at 800 ppm and becomes constant onward.As catalysis being strongly affected by surface interaction,thus higher concentration of DBT provides more chances of interaction between DBT molecules and catalytic active sites leading to higher conversion[40,41].It is believed that at lower concentration,there are phase transfer limitations among molecules of S and polar H2O2resulting in lower DBT conversion,while higher DBT concentration allows greater such interactions leading to higher DBT conversion as visible from Fig.4.Based on these results,800 ppm is selected as optimum concentration for onward activity tests.
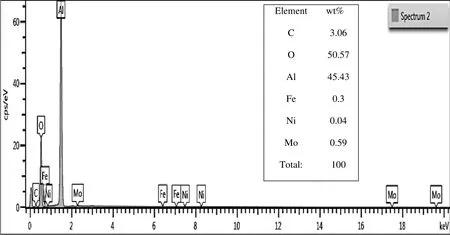
Fig.3.EDX spectrum of Fe–Ni–Mo/Al2O3.
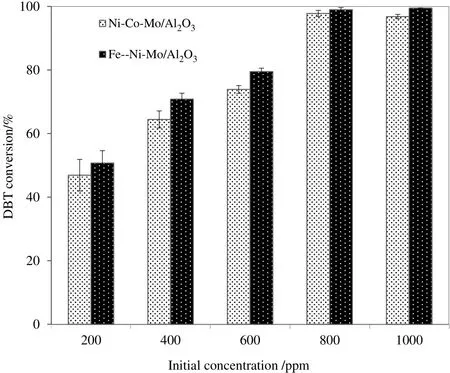
Fig.4.Conversion of DBT as a function of initial concentration over Ni–Co–Mo/Al2O3 and Fe–Ni–Mo/Al2O3 catalyst.
3.2.2.Effect of contact time
Catalytic activity is strongly affected by the time given to the interaction of solute molecules and catalyst active sites.Thus,effect of contact time ODS of DBT at different intervals was tested and results are summarized in Fig.5,which shows that maximum DBT conversion was achieved at 90 min.These and other similar reports are supported by the fact that longer exposure facilitates better DBT–catalyst interaction leading to higher S removal[42,43].Comparatively longer reaction time in this study to previous reports can be attributed to low Fe loading keeping economic aspects in mind.120 min with 99%DBT conversion on Fe loaded catalysts is quite comparable with earlier reports[44]and Fenton's like reagent for the ODS of DBT[27].From Fig.5,it can be seen that Fe–Ni–Mo/Al2O3exhibited maximum activity i.e.99%DBT conversion with overall ODS activity order as:Fe–Ni–Mo/Al2O3> Fe–Co–Mo/Al2O3> Ni–Co–Mo/Al2O3> Ni–Mo/Al2O3> Co–Mo/Al2O3> Mo/Al2O3.Furthermore,this work can be considered superior as higher DBT conversion(90%)with Mo loading much lower(5 wt%)was reported compared to previous reports(16 wt%Mo loading)with only 83%DBT conversion[45].From the comparison of reported literature shown in Table 2 for ODS of DBT as model oil,it can be concluded that low operating temperature and cost effective Fe based catalysts with 99%conversion in the current work can be considered as a promising forward step in the field of fuel oil desulfurization.
3.2.3.Effect of reaction temperature
It is believed that both DBT oxidation and H2O2decomposition are favored athig her temperature[50–52].Catalytic activity of the catalysts as a function of reaction temperature was tested in a range of 20–60 °C at 150 min reaction time and 0.4 g of catalyst dose.Results compiled in Fig.6 show a direct relation of DBT conversion with temperature reaching to maximum i.e.97%at60°C over Fe–Ni–Mo/Al2O3.An optimal temperature is required in ODS system ashighertemperature can cause side reaction leading to thermal decomposition of H2O2which can adversely affect quality of the fuel[53].Furthermore,5 wt%Mo loading achieving a DBT conversion of 90%at 50°C demonstrates superior efficiency of the current catalyst–oxidant system to previous reports having 16 wt%Mo loading and 100°C reaction temperature[54].At any temperature,Fe–Ni–Mo/Al2O3exhibited maximum activity among different catalysts tested supporting the data mentioned in Section 3.2.2 above,indicating the consistency and accurateness of the current experimental set-up.
3.2.4.Influence of catalyst dose on ODS process
Experiments were performed over a catalyst dose ranging from 0.2–1.4 g per 10 ml of 800 ppm DBT to check its effect on ODS activity.Higher catalytic dose provides more interactions between catalytic intermediates and DBT molecules and hence leads to greaterconversion[50,51].It is clear from Fig.7 that%DBT conversion over all catalysts increases with increase in catalyst dosage and stays above 95%at 0.4 g dose.Conversion above 98%means that extra catalyst dose would remain un-interacted with DBT molecules and hence no further change in the trend can be seen in Fig.7 at a catalyst dose of 0.4 g onward.
3.2.5.Effect of amount of oxidant on ODS process
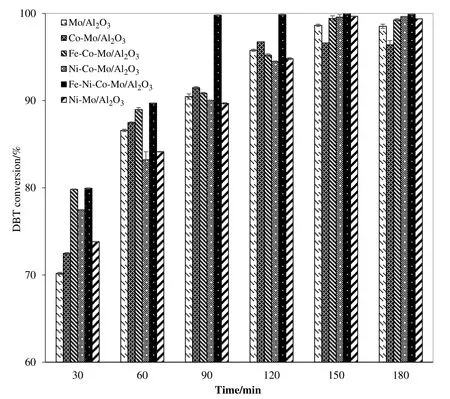
Fig.5.Catalytic conversion of DBT as a function of reaction time.
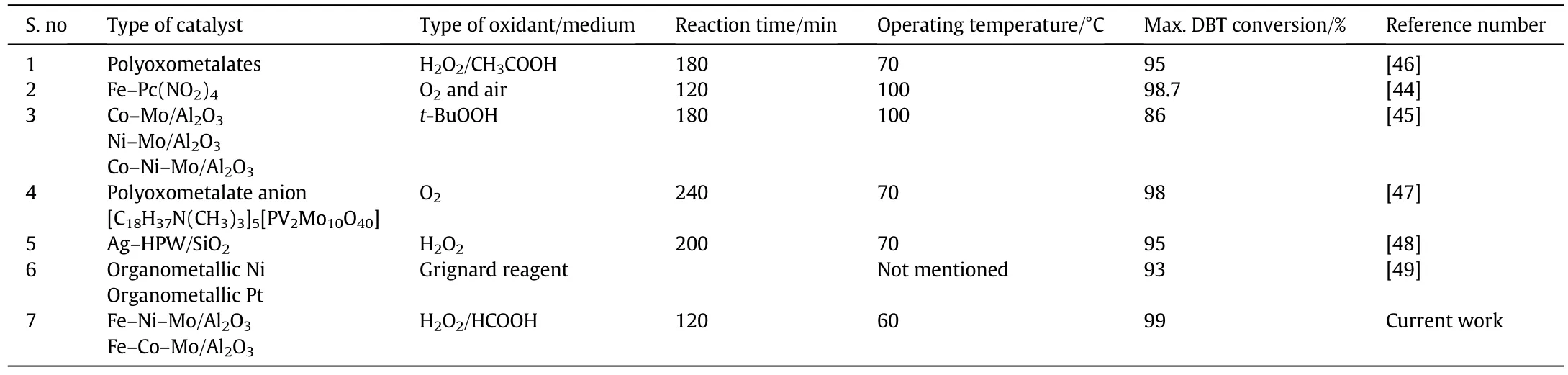
Table 2 Comparative analysis of DBT conversion via ODS from reported literature and current study
In ODS,H2O2and formic acid have been tested in the presence of various Mo/Al2O3supported catalysts[55]using Liquid–Liquid extraction approach.Amount of oxidant consumed in oxidation process can directly affect the efficiency as well as cost of the process.Unlike previous reports which did notfocus on the amount of oxidant in ODS of DBT[56],effect of the amount of H2O2and formic acid were tested in the presence of Fe promoted Mo–Al2O3catalysts and data are shown in Figs.8 and 9 respectively.It can be seen that ODS activity in case of H2O2is higher compared to formic acid while 0.5 ml was the optimum amount of each oxidant for maximum DBT conversion(86%in case of H2O2and 83%for formic acid).Moreover,Ni based catalysts ranked higher in activity than Co based ones which can be attributed to the fact that Ni2+with a 3d84S0external orbital possesses higher reduction potential(Eo)(−25 V)than Co(3d74S0)(−28 V),hence readily oxidizing the electron rich S center of DBT,exposing it for oxidation by H2O2or formic acid leading to sulfoxide and finally sulfones formation[57].Apart from reduction potential,S of the DBT acts as a soft acid on Pearson theory,which would prefer to interact with a soft base.The crystal field splitting of“d”orbitals of Co2+and Ni2+result in low spin species with ionic radii of 79 and 83 picometer(pm)respectively.Ni2+with larger ionic radii acts as a softer base compared to Co2+and thus preferentially interacts with S of DBT(a soft acidic center)in the reaction mixture,leading to higher DBT conversion by Ni based catalysts.Figs.8 and 9 further show that Fe,in the presence of both,H2O2and formic acid,enhances the catalytic activity of Co or Ni based catalysts based on Fenton's reaction.
3.3.Proposed mechanism of ODS

Fig.6.Catalytic conversion of DBT as a function of temperature.
ODS process usually leads to the formation of sulfones in the presence of H2O2and/or formic acid[50,58]through a two-step mechanism with each step involving attack of oxygen atom on the S atom of DBT leading to final DBTO2molecule over Mo/Al2O3type catalysts[59].Higher oxidation ability by Fe promoted catalysts in the present study can be explained on the facts that Fe3+promotes the formation of performic acid.Performic acid with higher oxidizing power than formic acid converts MO2(M=Co,Mo,Ni or Fe)into MO3type species,which readily react with DBT,gradually oxidizing it into sulfones[60].It is also assumed that enhanced activity of Fe based catalysts can be attributed to the fact that in the presence of H2O2,Fe2+readily oxidizes into Fe3+,dissociating H2O2into OH−and OH•species,resulting in superoxide formation i.e.•OOH.This superoxide readily reacts with DBT molecule converting it into sulfoxide and ultimately into sulfone.These factors are summarized in a proposed mechanism depicted in Fig.10.
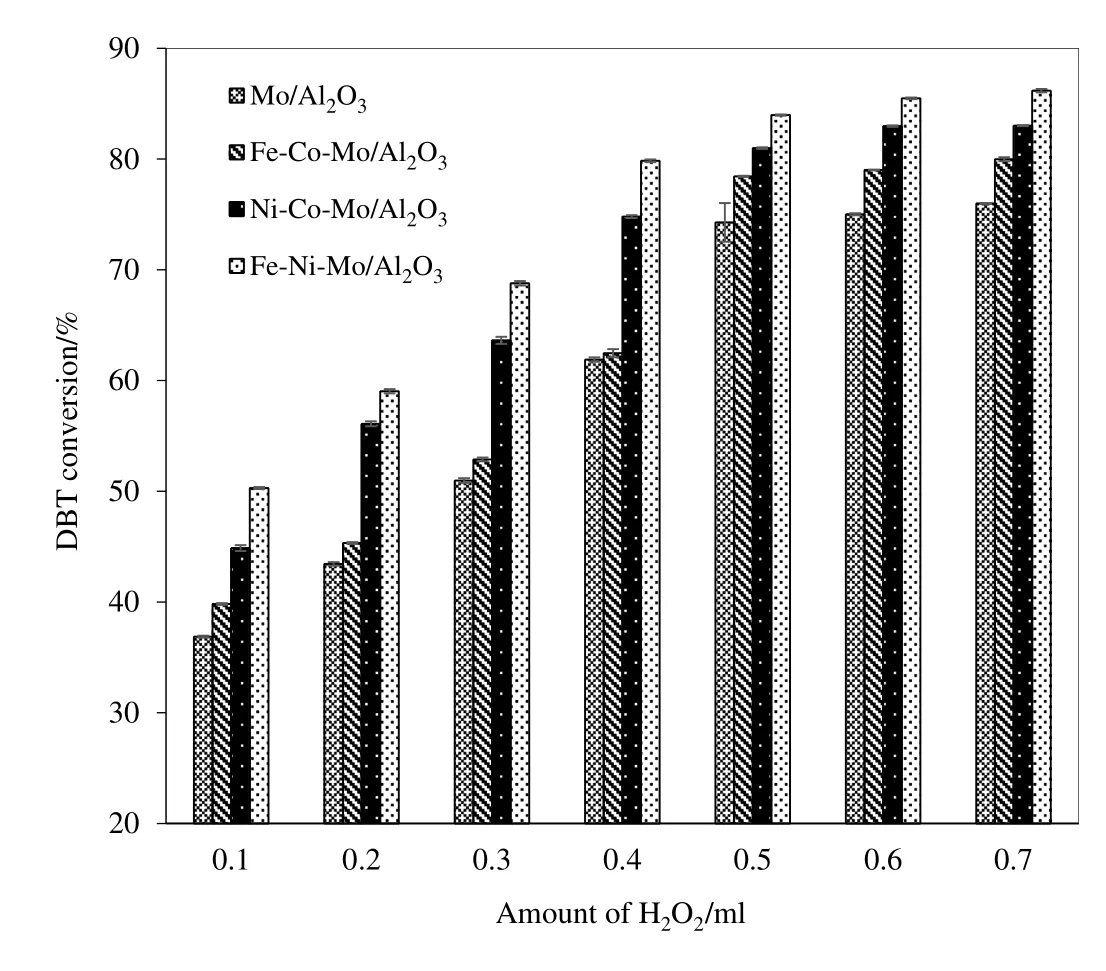
Fig.8.Conversion of DBT as a function of amount of H2O2.
4.Conclusions

Fig.10.Proposed mechanism for the catalytic ODS of DBT using H2O2 as oxidant.
The main focus of this study revolves around the promotion effect of cost effective Fe to classical Co or Ni based Mo/Al2O3catalysts for the ODS of DBT in the presence of H2O2and formic acid as oxidants at mild operating conditions.Experimental data showed that Fe greatly enhanced the oxidative desulfurization process following catalytic activity order as:Fe–Ni–Mo/Al2O3> Fe–Co–Mo/Al2O3> Ni–Co–Mo/Al2O3>Ni–Mo/Al2O3>Co–Mo/Al2O3>Mo/Al2O3.H2O2exhibited higher efficiency towards the oxidation process compared to formic acid.BET surface area,EDX,XRD,AAS and SEM characterization techniques were used to get insight about the textural structure and morphology of the catalysts.Current approach based on the application of mild operating conditions,low catalyst cost,high DBT conversion(99%)and simple mechanization can be considered as forward steps in the industrial desulfurization processing for fuel oil.

Fig.9.Conversion of DBT as a function of amount of formic acid.
Acknowledgments
The authors highly acknowledge Centralized Resource Laboratory(CRL),University of Peshawar for their technical and instrumental facilities.
[1]A.Al-Abduly,V.K.Sharma,Oxidation of benzothiophene,dibenzothiophene,and methyl-dibenzothiophene by ferrate(VI),J.Hazard.Mater.279(2014)296–301.
[2]J.L.García-Gutiérrez,G.C.Laredo,P.García-Gutiérrez,F.Jiménez-Cruz,Oxidative desulfurization of diesel using promising heterogeneous tungsten catalysts and hydrogen peroxide,Fuel 138(2014)118–125.
[3]F.Al-Shahrani,T.Xiao,S.A.Llewellyn,S.Barri,Z.Jiang,H.Shi,G.Martinie,M.L.Green,Desulfurization of diesel via the H2O2oxidation of aromatic sul fides to sulfones using a tungstate catalyst,Appl.Catal.B 73(2007)311–316.
[4]V.C.Srivastava,An evaluation of desulfurization technologies for sulfur removal from liquid fuels,RSC Adv.2(2012)759–783.
[5]A.Stanislaus,A.Mara fi,S.M.Rana,Recent advances in the science and technology of ultra low sulfur diesel(ULSD)production,Catal.Today 153(2010)1–68.
[6]A.Trehoux,Y.Roux,R.Guillot,J.Mahy,F.Avenier,Catalytic oxidation of dibenzothiophene and thioanisole by a diiron(III)complex and hydrogen peroxide,J.Mol.Catal.A Chem.396(2015)40–46.
[7]A.Ates,G.Azimi,K.Choi,H.W.Green,T.M.Timko,The role of catalyst in supercritical water desulfurization,Appl.Catal.B 147(2014)144–155.
[8]Y.Muhammad,Y.Lu,C.Shen,C.Li,Dibenzothiophene hydrodesulfurization over Ru promoted alumina based catalysts using in situ generated hydrogen,Energy Convers.Manag.52(2011)1364–1370.
[9]F.Dai,Y.Muhammad,X.Gong,C.Li,Z.Li,S.Zhang,Low-temperature and lowpressure fuel hydrodesulfurization by solid catalyst coupling with ionic liquids,Fuel 134(2014)74–80.
[10]H.Tang,Q.Li,Z.Wang,D.Yan,J.Xing,Simultaneous removal of thiophene and dibenzothiophene by immobilized Pseudomonas dela fieldii R-8 cells,Chin.J.Chem.Eng.20(2012)47–51.
[11]A.Srivastav,V.C.Srivastava,Adsorptive desulfurization by activated alumina,J.Hazard.Mater.170(2009)1133–1140.
[12]J.Wang,Q.Guo,C.Zhang,K.Li,One-pot extractive and oxidative desulfurization of liquid fuels with molecular oxygen in ionic liquids,RSC Adv.4(2014)59885–59889.
[13]J.Zhang,A.Wang,Y.Wang,H.Wang,J.Gui,Heterogeneous oxidative desulfurization of diesel oil by hydrogen peroxide:Catalysis of an amphipathic hybrid material supported on SiO2,Chem.Eng.J.245(2014)65–70.
[14]B.K.Saikia,K.Khound,B.P.Baruah,Extractive de-sulfurization and de-ashing of high sulfur coals by oxidation with ionic liquids,Energy Convers.Manag.81(2014)298–305.
[15]Z.Song,D.Yu,Q.Zeng,J.Zhang,H.Cheng,L.Chen,Z.Qi,Effect of water on extractive desulfurization of fuel oils using ionic liquids:A COSMO-RS and experimental study,Chin.J.Chem.Eng.25(2017)159–165.
[16]Y.Jia,G.Li,G.Ning,Ef ficient oxidative desulfurization(ODS)of model fuel with H2O2catalyzed by MoO3/γ-Al2O3under mild and solvent free conditions,Fuel Process.Technol.92(2011)106–111.
[17]A.F.Shojaei,M.A.Rezvani,M.H.Loghmani,Comparative study on oxidation desulphurization of actual gas oil and model sulfur compounds with hydrogen peroxide promoted by formic acid:Synthesis and characterization of vanadium containing polyoxometalate supported on anatase crushed nanoleaf,Fuel Process.Technol.118(2014)1–6.
[18]L.Kong,G.Li,X.Wang,Mild oxidation of thiophene over TS-1/H2O2,Catal.Today 93-95(2004)341–345.
[19]C.Lanju,G.Shaohui,Z.Dishun,Oxidation of thiophenes over silica gel in hydrogen peroxide/formic acid system,Chin.J.Chem.Eng.14(2006)835–838.
[20]M.Te,C.Fairbridge,Z.R,Oxidation reactivities of dibenzothiophenes in polyoxometalate/H2O2and formic acid/H2O2systems,Appl.Catal.A Gen.219(2001)267–280.
[21]S.Liu,B.Wang,B.Cui,L.Sun,Deep desulfurization of diesel oil oxidized by Fe(VI)systems,Fuel 87(2008)422–428.
[22]W.Wang,S.Wang,H.Liu,Z.Wang,Desulfurization of gasoline by a new method of electrochemical catalytic oxidation,Fuel 86(2007)2747–2753.
[23]C.L.Caero,E.Herna'ndez,F.Pedraza,F.Murrieta,Oxidative desulfurization of synthetic diesel using supported catalysts Part I.Study of the operation conditions with a vanadium oxide based catalyst,Catal.Today 107–108(2005)564–569.
[24]X.Ma,A.Zhou,C.Song,A novel method for oxidative desulfurization of liquid hydrocarbon fuels based on catalytic oxidation using molecular oxygen coupled with selective adsorption,Catal.Today 123(2007)276–284.
[25]W.Zhu,P.Wu,L.Yang,Y.Chang,Y.Chao,H.Li,Y.Jiang,W.Jiang,S.Xun,Pyridiniumbased temperature-responsive magnetic ionic liquid for oxidative desulfurization of fuels,Chem.Eng.J.229(2013)250–256.
[26]H.Li,W.Zhu,Y.Wang,J.Zhang,J.Lu,Y.Yan,Deep oxidative desulfurization of fuels in redox ionic liquids based on iron chloride,Green Chem.11(2009)810–815.
[27]J.Zhang,W.Zhu,H.Li,W.Jiang,Y.Jiang,W.Huang,Y.Yan,Deep oxidative desulfurization of fuels by Fenton-like reagent in ionic liquids,Green Chem.11(2009)1801–1807.
[28]Y.Muhammad,C.Li,Dibenzothiophene hydrodesulfurization using in situ generated hydrogen over Pd promoted alumina-based catalysts,Fuel Process.Technol.92(2011)624–630.
[29]Y.Yoshimura,N.Matsubayashi,T.Sato,H.Shimada,A.Nishijima,Molybdate catalysts prepared by a novel impregnation method:Effect of citric acid as a ligand on the catalytic activities,Appl.Catal.A Gen.79(1991)145–159.
[30]N.Farzin Nejad,E.Shams,M.K.Amini,J.C.Bennett,Synthesis of magnetic mesoporous carbon and its application for adsorption of dibenzothiophene,Fuel Process.Technol.106(2013)376–384.
[31]V.Piriyawong,V.Thongpool,P.Asanithi,P.Limsuwan,Preparation and characterization of alumina nanoparticles in deionized water using laser ablation technique,J.Nanomater.2012(2012)2.
[32]H.M.AbdelDayem,M.A.Al-Omair,Phase composition and catalytic activity of α-NiMoO4reduced with hydride anion,Ind.Eng.Chem.Res.47(2008)1011–1016.
[33]R.Manigandan,K.Giribabu,R.Suresh,L.Vijayalakshmi,A.Stephen,V.Narayanan,Cobalt oxide nanoparticles:Characterization and its electrocatalytic activity towards nitrobenzene,Chem.Sci.Trans.2(2013)S47–S50.
[34]S.A.Kulkarni,P.Sawadh,P.K.Palei,K.K.Kokate,Effect of synthesis route on the structural,optical and magnetic properties of Fe3O4nanoparticles,Ceram.Int.40(2014)1945–1949.
[35]E.Warren,B.Ransom,The in fluence of analytical error upon the interpretation of chemical variations in clay minerals,Clay Miner.27(1992)193–209.
[36]S.K.Chang,Z.Zainal,K.B.Tan,N.A.Yusof,W.Yusoff,S.Prabaharan,Surface morphology and crystallinity of metal oxides in nickel–cobalt binary system,Sains Malaysiana 41(2012)465–470.
[37]M.Batin,V.Popescu,Synthesis and characterization of iron oxide powders,Powder Metall.Prog.11(2011)201–205.
[38]W.Xing,F.Li,Z.-f.Yan,G.Lu,Synthesis and electrochemical properties of mesoporous nickel oxide,J.Power Sources 134(2004)324–330.
[39]M.Ishaq,S.Sultan,I.Ahmad,H.Ullah,M.Yaseen,A.Amir,Adsorptive desulfurization of model oil using untreated,acid activated and magnetite nanoparticle loaded bentonite as adsorbent,J.Saudi Chem.Soc.21(2017)143–151.
[40]A.T.Nawaf,S.A.Gheni,A.T.Jarullah,I.M.Mujtaba,Improvement of fuel quality by oxidative desulfurization:Design of synthetic catalyst for the process,Fuel Process.Technol.138(2015)337–343.
[41]S.Mikhail,T.Zaki,L.Khalil,Desulfurization by an economically adsorption technique,Appl.Catal.A Gen.227(2002)265–278.
[42]O.Gonzalez-Garcia,L.Cedeno-Caero,V–Mo based catalysts for oxidative desulfurization of diesel fuel,Catal.Today 148(2009)42–48.
[43]Y.Tian,Y.Yao,Y.Zhi,L.Yan,S.Lu,Combined extraction–oxidation system for oxidative desulfurization(ODS)of a model fuel,Energy Fuel 29(2015)618–625.
[44]X.Zhou,J.Li,X.Wang,K.Jin,W.Ma,Oxidative desulfurization of dibenzothiophene based on molecular oxygen and iron phthalocyanine,Fuel Process.Technol.90(2009)317–323.
[45]D.Wang,E.W.Qian,H.Amano,K.Okata,A.Ishihara,T.Kabe,Oxidative desulfurization of fuel oil:Part I.Oxidation of dibenzothiophenes using tert-butyl hydroperoxide,Appl.Catal.A Gen.253(2003)91–99.
[46]W.Trakarnpruk,K.Rujiraworawut,Oxidative desulfurization of gas oil by polyoxometalates catalysts,Fuel Process.Technol.90(2009)411–414.
[47]H.Lu,J.Gao,Z.Jiang,Y.Yang,B.Song,C.Li,Oxidative desulfurization of dibenzothiophene with molecular oxygen using emulsion catalysis,Chem.Commun.(2007)150–152.
[48]X.M.Yan,G.S.Su,L.Xiong,Oxidative desulfurization of diesel oil over Ag-modified mesoporous HPW/SiO2catalyst,J.Fuel Chem.Technol.37(2009)318–323.
[49]J.Torres-Nieto,A.Arévalo,J.J.García,Catalytic desulfurization of dibenzothiophene and its hindered analogues with nickel and platinum compounds,Organometallics 26(2007)2228–2233.
[50]F.Yu,R.Wang,Deep oxidative desulfurization of dibenzothiophene in simulated oil and real diesel using heteropolyanion-substituted hydrotalcite-like compounds as catalysts,Molecules 18(2013)13691–13704.
[51]K.P.Cheng,H.Yang,J.H.Wang,H.P.Liu,C.Z.Qiao,Immobilization of acidic ionic liquid on silica gel for catalytic oxidative desulfurization of fuel oils(II):Desulfurization,Advanced Materials Research,Trans Tech Publ 2015,pp.183–187.
[52]J.Zhang,A.Wang,X.Li,X.Ma,Oxidative desulfurization of dibenzothiophene and diesel over[Bmim]3PMo12O40,J.Catal.279(2011)269–275.
[53]A.Haghighat Mamaghani,S.Fatemi,M.Asgari,Investigation of in fluential parameters in deep oxidative desulfurization of dibenzothiophene with hydrogen peroxide and formic acid,Int.J.Chem.Eng.2013(2013).
[54]D.Wang,W.E.Qian,H.Amano,K.Okata,A.Ishihara,T.Kabe,Oxidative desulfurization offueloilPartI.Oxidation ofdibenzothiophenes using tert-butylhydroperoxide,Appl.Catal.A Gen.253(2003)91–99.
[55]L.Hao,S.Benxian,X.Zhou,An improved desulfurization process based on H2O2/formic acid oxidation system followed by liquid–liquid extraction.Part 1.Coker gas oil feedstocks,Pet.Sci.Technol.23(2005)991–999.
[56]M.Te,C.Fairbridge,Z.Ring,Oxidation reactivities of dibenzothiophenes in polyoxometalate/H2O2and formic acid/H2O2systems,Appl.Catal.A Gen.219(2001)267–280.
[57]K.K.Sarda,A.Bhandari,K.K.Pant,S.Jain,Deep desulfurization of diesel fuel by selective adsorption over Ni/Al2O3and Ni/ZSM-5 extrudates,Fuel 93(2012)86–91.
[58]J.Wang,D.Zhao,K.Li,Oxidative desulfurization of dibenzothiophene using ozone and hydrogen peroxide in ionic liquid,Energy Fuel 24(2010)2527–2529.
[59]J.L.García-Gutiérrez,G.A.Fuentes,M.E.Hernández-Terán,F.Murrieta,J.Navarrete,F.Jiménez-Cruz,Ultra-deep oxidative desulfurization of diesel fuel with H2O2catalyzed under mild conditions by polymolybdates supported on Al2O3,Appl.Catal.A Gen.305(2006)15–20.
[60]W.Ahmad,I.Ahmad,M.Yaseen,Desulfurization of liquid fuels by air assisted peracid oxidation system in the presence of Fe-ZSM-5 catalyst,Korean J.Chem.Eng.33(2016)2530–2537.
 Chinese Journal of Chemical Engineering2018年3期
Chinese Journal of Chemical Engineering2018年3期
- Chinese Journal of Chemical Engineering的其它文章
- Numerical investigation on flow and heat transfer characteristics of corrugated tubes with non-uniform corrugation in turbulent flow
- Investigations on pool boiling critical heat flux,transient characteristics and bonding strength of heater wire with aqua based reduced graphene oxide nano fluids
- Heavy metals adsorption by banana peels micro-powder:Equilibrium modeling by non-linear models
- Potential aspect of rice husk biomass in Australia for nanocrystalline cellulose production
- Fouling evaluation on membrane distillation used for reducing solvent in polyphenol rich propolis extract
- Investigation on a vertical radial flow adsorber designed by a novel parallel connection method☆
