Interaction of antipsychotic drug with novel surfactants:Micellization and binding studies
Naved Azum*,Malik Abdul Rub,Abdullah M.Asiri
Center of Excellence for Advanced Materials Research,King Abdulaziz University,Jeddah 21589,Saudi Arabia Chemistry Department,Faculty of Science,King Abdulaziz University,Jeddah 21589,Saudi Arabia
1.Introduction
Amphiphiles or surface active compounds have both hydrophilic and hydrophobic properties.The amphiphiles have the unique capability of making nano size clusters in solutions,known as micelles.The micelles formed by amphiphiles are capable of solubilizing the sparingly soluble substances.They are able to modify the interfacial properties of the liquid(non-aqueous or aqueous)in which they are present.Amphiphiles are ubiquitous materials,which exhibit a fascinating range of applications in chemical processes,industries,in the formulation of pharmaceuticals,in mineral processing technologies,and in the food processing industries[1–5].When amphiphiles are present in low concentration,they act much as normal electrolytes but deviate at higher concentrations.The solubility of surfactants in water is low because of the presence of the hydrophobic hydrocarbon tail.When these compounds are dissolved in a solvent,the hydrophobic portion segregates from the solvent by self-aggregation leading to the formation of micelles.This phenomenon,known as the hydrophobic effect,spontaneously minimizes the unfavorable hydrocarbon–water contacts and increases the entropy of the system.Two or more types of amphiphiles are mixed together,to make a mixed micelle.The mixed micelle is a result of the self-assembly of different amphiphile monomers present in a solution.Many applications from household to industrial are associated with mixed micelles because of it having great properties of a mixed system in comparison to single micelle[6–17].
In recent times,a new generation of surfactants has been evolved named as gemini or novel surfactants[18–24].In these novel surfactants,the two conventional surfactants are chemically bonded together by a spacer group(Fig.1).The spacer may be a flexible or rigid structure[25].The gemini surfactants(contain ester or amide group)have globally concern to solve the environmental problems.These surfactants are used in carbon steel pipelines in the acidic medium as corrosion inhibitor[26–28].
Noteworthy among these gemini surfactants,the cationic alkanediylα,ω-bis(alkyldimethylammonium bromide)type,designated m-s-m,where the length of the alkyl tails designated by“m”and s refers to the number of methylene units that make up the alkyl spacer,has received more attention because of its low critical micelle concentration(cmc)values,superior surface activity,higher solubilizing capacity,biodegradability,and non-toxic nature.The hydrophobic interactions in surfactants are driven by the micellization,while the repulsion of charged head groups and hydration are the voluntary.In gemini surfactants two amphiphilic moieties are joined by a spacer group,when the spacer is short,these moieties are closed to each other.Due to chemical bond connection,the hydrophobic chain interactions are enhanced;on the other hand,repulsion between the hydrophilic groups is reduced.That's why gemini surfactants are more readily to form aggregates.
Chlorpromazine(CPZ)is an amphiphilic drug[29,30],marketed with the name Thorazine.It is an antipsychotic drug used to treat psychotic disorders such as schizophrenia.It is also used to treat bipolar disorder,nausea,vomiting,and anxiety before surgery.CPZ is a tricyclic amphiphilic compound,which contains a hydrophobic part as an amino group(Fig.2).CPZ has anticholinergic,cardiovascular,and antihistamine side effects[29,31]and induces phospholipidosis(accumulation of excessive intracellular phospholipids)[32].The interaction occurs between the negative phosphate oxygen of the lipids found in the body and protonated amine group of CPZ.
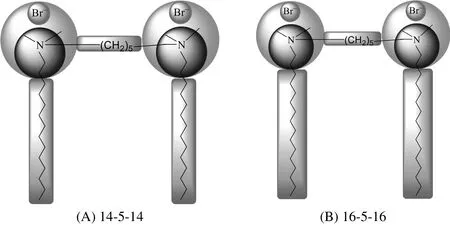
Fig.1.Chemical structure of(A)14-5-14 and(B)16-5-16.
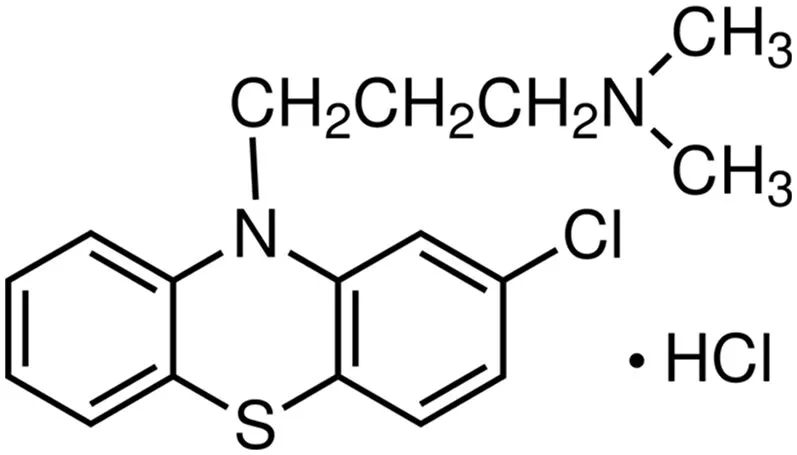
Fig.2.Chemical structure of Chlorpromazine hydrochloride(CPZ).
To lessen these side effects,phenothiazine drugs are used with a carrier(surfactants,polymers,block co-polymers etc.)[33–41].Thus,the aim of our study is to interrogate the adsorption and mixed micellization study of cationic amphiphilic drug(CPZ)with gemini surfactants(alkanediyl-α,ω-bis(alkyldimethylammonium bromide),m-s-m).Such explorations are guided to depict the involvement of the drug monomers in the mixed adsorbed monolayer and mixed aggregates[42,43].The appropriateness of energetic model for binary systems is also perceived.Various theoretical models are being used to analyze the data.
2.Materials and Methods
2.1.Materials
All chemicals were of analytical grade and employed as obtained without further purification.Important information on the resource and purity of the employed chemicals are revealed in Table 1.De-ionized double distilled water(DDW)having specific conductivity(1 to 2)·10−6S·cm−1at 298.15 K was consumed throughout the study to prepare the stock solution.Amphiphilic solutions were prepared by dissolving accurately weighed quantities of amphiphiles(drug and gemini surfactants)in requisite volumes of DDW solution for the experiments.
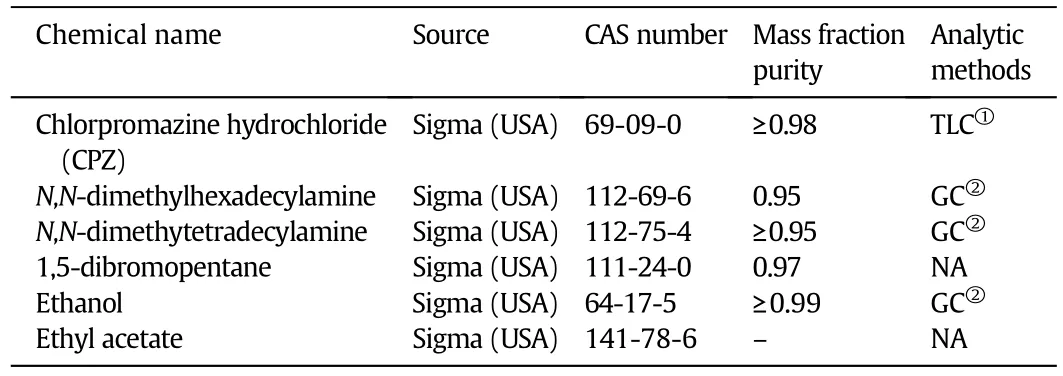
Table 1 Source and purity of the compounds employed in this work
2.2.Synthesis of gemini surfactants(m-s-m)
The synthesis and purification are the two factors,which are important in its preparation.The bis(quaternary ammonium)surfactants were synthesized by adopting the procedure outlined in reference[44,45]and showing in the Fig.3:
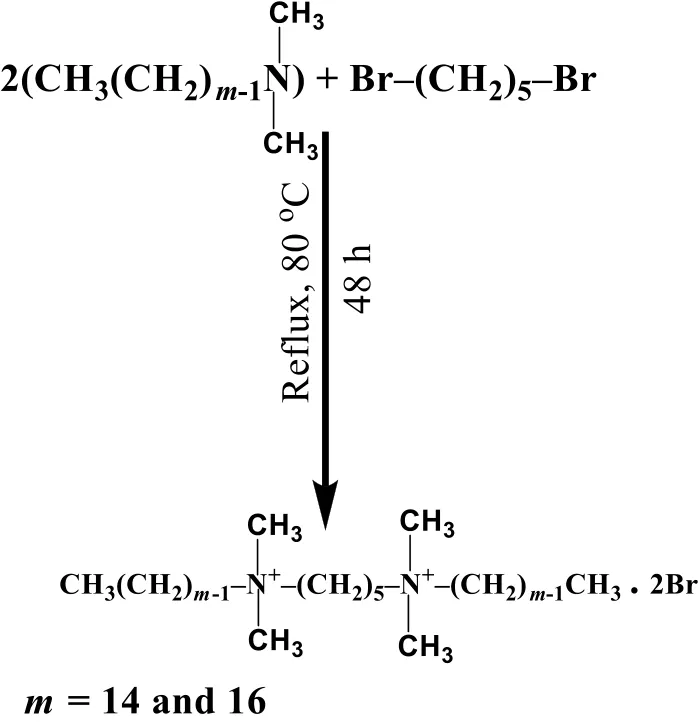
Fig.3.Protocol for the synthesis of gemini(m-5-m)surfactants(m=14 for 14-5-14 and m=16 for 16-5-16).
A 1:2.1 equivalent mixture of corresponding 1,5-dibromo alkane with N,N-dimethyl alkyldecylamines in dry ethanol was re fluxed(at 353.15 K)for 48 h.Thin-layer chromatography method was employed to check the progress of the reaction.The solvent was removed from the reaction mixture under vacuum to obtain the product.The hexane/ethyl acetate mixture was used to re-crystallized the solid and get the product in pure form.The overall yield of the gemini surfactants ranged from 70%–90%.The synthesized gemini surfactants(m-s-m)was checked by1HNMR(Table 2)spectroscopy using CDCl3asa solvent.
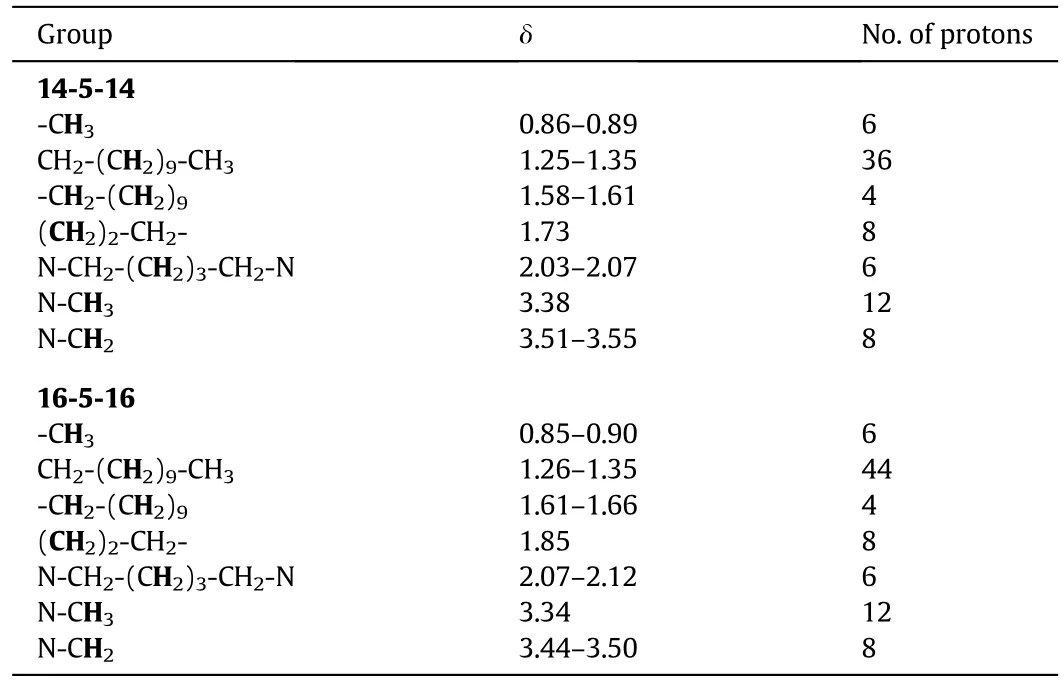
Table 2 1HNMR data for gemini surfactants
2.3.Surface tension measurements
The platinum ring detachment technique is used to get the surface tension of CPZ,gemini as well as their binary mixed systems by using Attension tensiometer(Sigma 701,Germany)at 298.15 K.The temperature of the studied solution was kept constant having error±0.1 K by frequently circulating thermo-stated H2Othrough a jacketed vessel.The Attension tensiometer works on Du Nouy principle.We have added the different aliquot volume of stock solution to a fixed amount of solvent(water)in the vessel.The ethanol flame was used to clean the ring after each set of experiments.The precision of the measurements was around±1.0 m·Nm−1.The Fig.4 represents a relation between measured surface tension and logarithm of surfactant concentration.
2.4.Fluorescence measurements
All fluorescence measurements were performed on Hitachi F-7000 spectrometer.This apparatus is equipped with 150 W Xenon lamp as the excited source.Fluorescence data were collected by using excitation wavelength of 315 nm.We choose the size of both emission and excitation slits,5 nm,by using a 10-mm quartz cell for all experiments.
2.5.UV–visible measurements
The absorbance ofCPZ solution with increasing concentration ofsurfactants was measured by using double beam UV–visible spectrometer(Thermo Scientific,Evolution 300).The double distilled and de-ionized water was used for baseline correction.The temperature was kept constant(298.15 K).
3.Results and Discussion
3.1.Critical micelle concentration
The representative plots of surface tension vs.lg[amphiphile]for binary mixtures are shown in Fig.4.The cmc values for pure 14-5-14,16-5-16 and CPZ amphiphiles tally with the earlier literature values[44–48].The drug molecules hardly adjust in the curve area of the micelle because the hydrophobic portion of the drug is rigid.It is not easy for the drug molecule to amend in the curve area of the micelle because of its rigid hydrophobic part,hence make micelles at higher concentration.The hydrophobic interaction is the major driving force of micelle formation.At the time of micellization activity,water molecules from the hydration shells in the region of the hydrophobic portions of a monomer are liberated,and entropy increases.The chain of the surfactant breaks the water structure around it as a result increases in entropy;therefore,on increasing the chain length ofthe surfactantmolecule the micellization takes place at a lower concentration.Hence,the cmc of 16-5-16 is less than that of 14-5-14.Further,it is also clear that the cmc values for all CPZ-14-5-14/16-5-16 mixtures fall in between the cmc values of pure components.In mixed geminis-CPZ systems,the cmc values increase with increasing the mole fractions of CPZ.It means that the gemini formed micelles first,the CPZ molecules penetrated into the gemini micelle,and the hydrophobic interactions between the rigid hydrophobic part of CPZ and alkyl chains of the gemini increased.
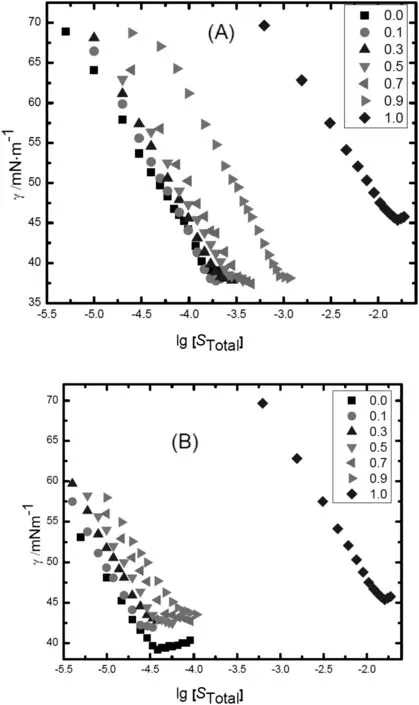
Fig.4.Plots of surface tension(γ)vs.the logarithm oftotalamphiphile concentration(S Total)ofbinary mixtures atvarious mole fractions ofCPZ(A)CPZ+14-5-14(B)CPZ+16-5-16 at 298.15 K.
The Clint model[49]can be used to predict the cmc of an ideal mixture(the individual components are non-interacting),where the ideal cmc of mixed amphiphiles is shown by the Eq.(1)

where αiand cmciare the stoichiometric mole fraction and cmc of i th component in the mixture.The comparison between ideal and nonideal mixtures can be easily predicted by this model.It is confirmed from Fig.5 that experimental values(cmcexp)are lower than that of ideal values(cmcideal),which signifies synergistic interaction among components in the mixture.There isa hydrophobic interaction between the hydrophobic part of CPZ and the two hydrophobic chains of gemini surfactant;hence,aggregation takes place at inferior concentrations in comparison to an ideal state.

Fig.5.Plots of experimental cmc(cmc exp)and ideal cmc(cmc ideal)versus mole fraction of CPZ(αCPZ)for(A)CPZ+14-5-14 and(B)CPZ+16-5-16.
3.2.Interaction and interaction parameter(β)
In the light of regular solution theory(RST),the mutual interaction between amphiphiles in the mixed systems leading to non-ideality has been evaluated theoretically by Rubingh[50].The micelle mole fraction of CPZ(X1),as well as interaction parameter(β),can be calculated using the following equations:
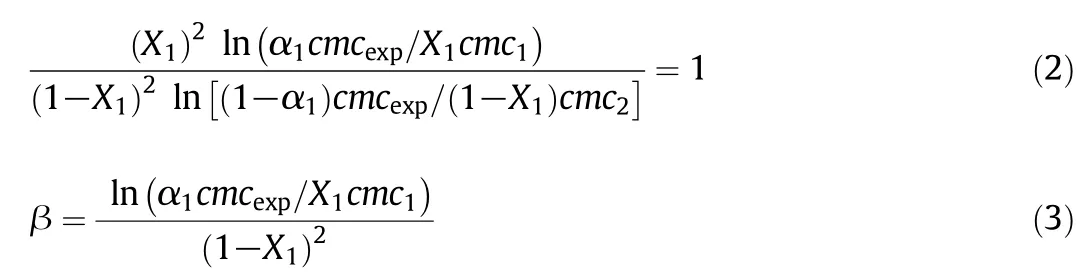
The magnitude of interaction operating between components of mixed systems,compared to their self-interaction before mixing in akin situations,can be judged by using a parameter of interaction(β).A negative β value shows that the attractive interaction between two different amphiphiles(Table 3)because the value of the β is comparative to the free energy of mixing.
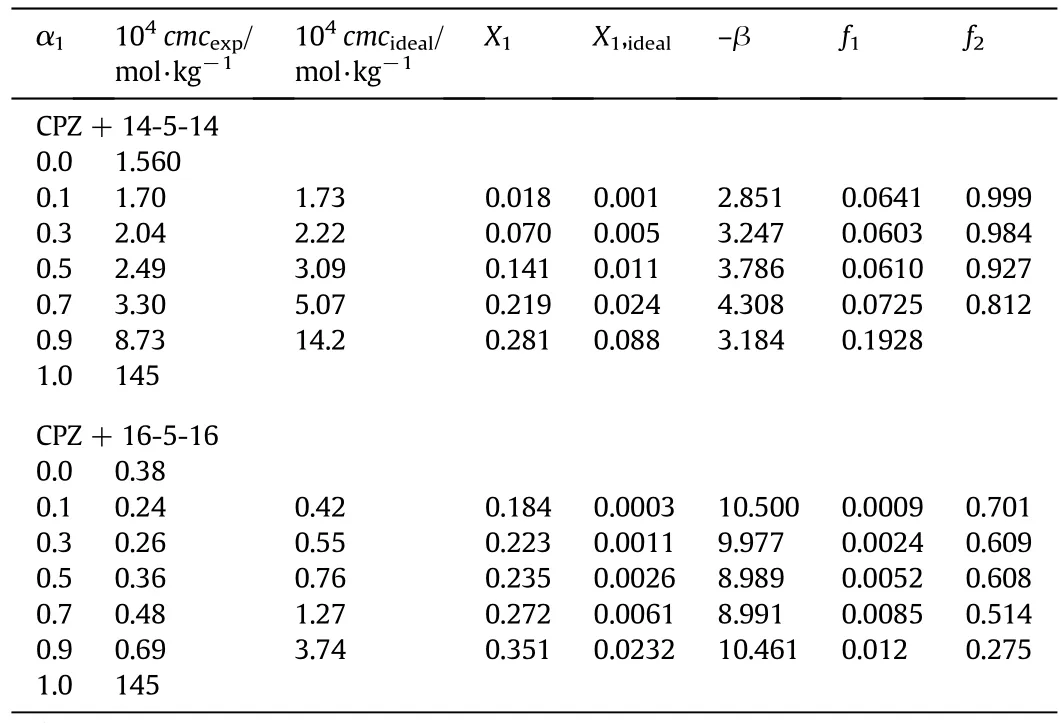
Table 3 Different physicochemical parameters at bulk for CPZ+14-5-14/16-5-16 mixtures at 298.15 K and pressure p=0.1 MPa①
The strong attraction among components in the mixed systems comes out by the higher negative β values while approximately ideal mixing by zero.A positive value indicates that the attractive interaction between amphiphiles is weaker.The strength of interaction between the different molecules is notalone responsible for the existence of synergism in the mixtures of amphiphiles,but synergism also depends on the association properties of all amphiphiles in the solution.Due to the more hydrophobic nature of16-5-16 compared to 14-5-14,itgenerates strong synergistic interactions with CPZ as the clear from the more negative value of βavof the 16-5-16+CPZ system(βav= −9.784).It may be due to the longer hydrophobic twin tails of the gemini surfactant which interact more favorably with CPZ to form the core of mixed micelles.Similar synergistic effects in m-2-m+CTAB mixtures have been reported by Sood et al.[51].
The mole fraction of component 1(X1,ideal)in the mixed micelle in an ideal condition can be calculated as:

The values of X1and X1,idealare shown in Table 3.The evaluated X1values are higher than X1,idealfor all mole fractions.It is concluded that the mixed micelle of geminis+CPZ has the large involvement of CPZ than in its ideal state.The activity coefficients(f1,f2)for present binary systems inside the micelle are connected to the interaction parameter(β)that can be calculated from the following equations:

The activity coefficients are thermodynamic quantities without unit and for an ideal solution have a unity value.The values greater or lower than 1 indicates the increase of attractive or repulsive interactions.The micellar mole fraction of the CPZ is higher in the mixed systems even in the high concentration of gemini in the mixtures;as a result,signifying their higher tendency to take part in mixed micellization and well supported by their activity coefficients.The activity coefficients are found to be below 1,indicating non-ideal behavior as well as attractive interaction.
3.3.Mixed monolayer formation
In the binary amphiphilic systems,the interfacial composition and interaction parameter between two amphiphiles at the Langmuir monolayer formation was evaluated by using Rosen model[52].In the case of an ideal monolayer,a negative value of interaction parameter(βS)indicates synergistic while a positive value indicates antagonistic amphiphile–amphiphile interaction.However,for an ideal monolayer,interaction parameter is zero.Theand βSvalue in case of the mixed monolayer at the interfacial surface are evaluated by means of using Eqs.(7)and(8)
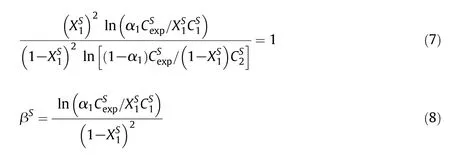
whereis mole fraction of CPZ in the totalmixed monolayer andandare the molalconcentrations in the solution phase ofCPZ,14-5-14/16-5-16 and their mixture,respectively,at the mole fraction α1of CPZ.Eq.(7)solved numerically for,which is then substituted into Eq.(8)to calculate βS.The βSandvalues of mixtures are given in Table 4.
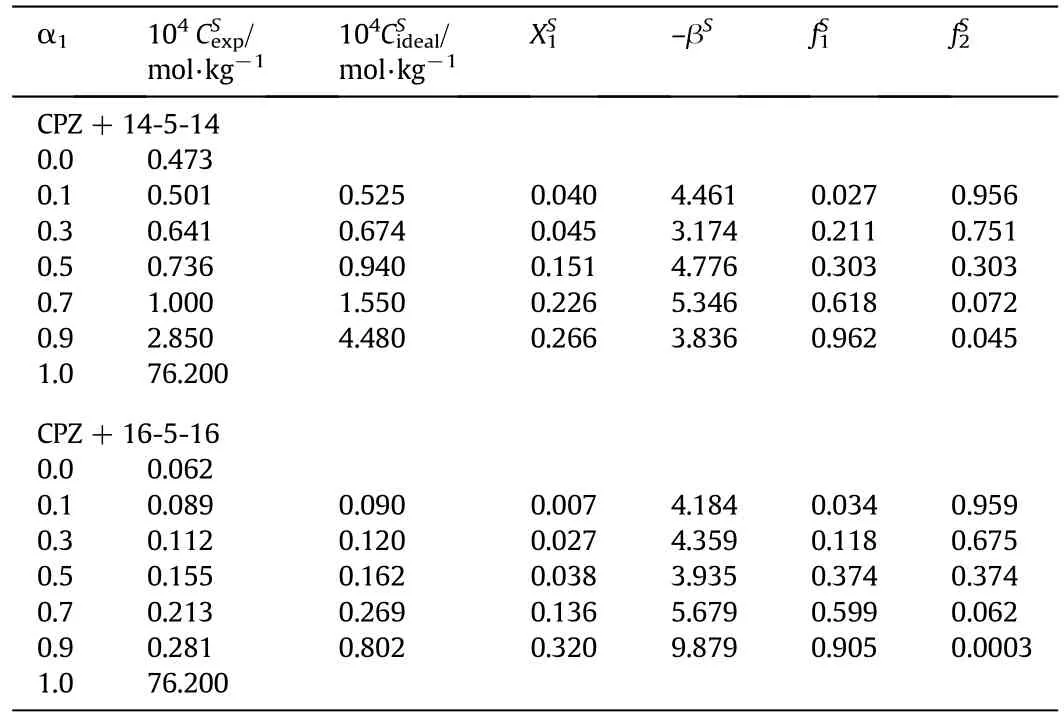
Table 4 Different physicochemical parameters atinterface for CPZ+14-5-14/16-5-16 mixtures at 298.15 K and pressure p=0.1 MPa①
The negative value of βSindicates synergistic interaction in the mixed monolayer.Higher the values of α1compared toindicates more propensities of gemini surfactants to preferentially populate the interface as compared to CPZ.
The activity coefficientsandof the amphiphiles in mixed monolayer are related to βS.

From Table 4,it was observed that all the systems exhibit values of activity coefficients less than one,which shows a synergistic effect between components of mixed surfactant systems at the interface.
3.4.Adsorption behavior
The surface tension of pure or mixed solution decreases when amphiphiles adsorb atthe air–water interface.The breakdown of hydrogen bonds at the surface is responsible for the reduction in surface tension.On increasing the concentration of amphiphiles after the saturation at the interface,the amphiphilic molecules start to aggregate into nanostructured assemblies called as the micelle.After this association,the surface tension remains constant(Fig.4).The Gibbs adsorption isotherm can be used to calculate the amount of adsorbed amphiphiles per unit area at various concentrations by the fundamental Gibbs adsorption equation[53].
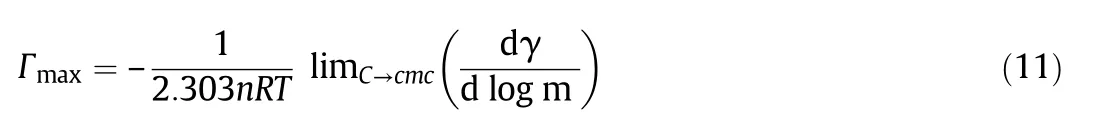
where R,T,and m is the gas constant(8.314 J·mol−1·K−1),the temperature in Kelvin and concentration(STotal)in mol·kg−1respectively;n is the number of ionic species whose concentration at interface varies with the change in the[amphiphile]in the solution.For CPZ,n is taken as 2,and for geminis n is taken as 3.For mixtures,n is calculated by using the relation n=X1n1+X2n2.The surface excess is a measure of the effectiveness of the surfactant adsorption at the interface.The packing and tightness of molecules at the interface depends on the value of Γmax,higher value of corresponds to maximum packing and strong tighten surfactant molecules at the interface.The physico-chemical properties like foaming,wetting,and emulsification can be determined by the adsorption effectiveness.The surfactant concentration required to reduce the surface tension by 20 mN·m−1is a useful measure of efficiency a surfactant.It is a negative logarithm of C20values,pC20=−lg C20.The pC20for pure CPZ is lower than the mixtures,indicates that the concentration required to decrease the surface tension of water in 20 mN·m−1is lower in the presence of CPZ(Table 5).
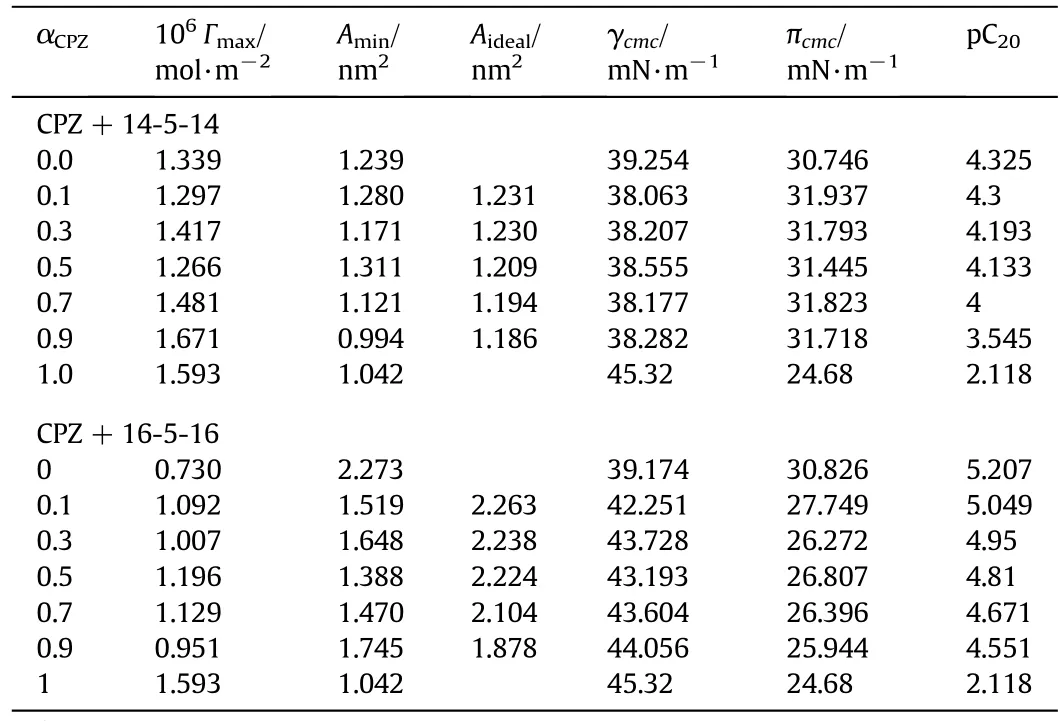
Table 5 Interfacial parameters CPZ+14-5-14/16-5-16 mixtures at 298.15 K and pressure p=0.1 MPa①

The efficiency increase with increasing length of the gemini surfactant.The values ofminimum area per molecule(Amin)for all the studied mixed systems were calculated using the equation:where NAis the Avogadro's number.Γmaxand Aminare expressed in mol·m−2and nm2units,respectively.Table 5 lists the Γmaxand Aminfor these systems.The data show that the Γmaxof CPZ is more than that of the gemini surfactants.The lower Γmaxvalues in the case of gemini are due to the fact that these molecules,having two hydrophobic head groups covalently bonded with the help of a spacer and thus occupy more area.Moreover,with the increase in chain length of these surfactants,the surface area occupied by each molecule increase further and hence 16-5-16 has less Γmaxvalue in comparison to 14-5-14.The data recorded in Table 5 specify that the CPZ has higher surface adsorption(less Amin),while the adsorption tendency of CPZ molecule in the binary mixtures decreases.The balance between hydrophilicity and hydrophobicity ofa particular amphiphile is responsible for the surface activity of that surfactant.The adsorbed molecules at solution–air interface occupy more at lower values of the Γmax(higher Amin),due to the lower compactness.The Table 5 data show that the area occupied per molecule in the mixtures is more than those taken by CPZ,which confirmed that the interacting components are loosely packed atthe interface,and their orientation is almost perpendicular to the interface.The obtained outcomes also advocate that the geminisurfactants somewhat engaged in the adsorption on the surface.Table 5 also lists the ideal mixing values,Aideal,calculated from the equation:

whereandare the micellarmolarfraction of component1 and 2 at the interface,respectively.We found that the ideal values(Aideal)in maximum cases are larger than the corresponding experimental values(Amin).It means that there is some van der Waals self-attraction among the hydrophobic parts of gemini molecules before mixing,which decreased upon mixing with CPZ.The areas occupied by the amphiphiles heads are greater than the idealstate,as a result of loose monolayer formation by binary mixtures.
The difference between the interfacial tension of water(γo)and interfacial tension at cmc(γcmc)is known as surface pressure(πcmc).It is a measure of the effectiveness of interfacial tension reduction.It can be expressed as:

The πcmcvalues of mixtures are larger than CPZ(Table 5),which indicates a significant reduction in the effectiveness of the amphiphiles in lowering the surface tension.
3.5.Thermodynamics of micellization
The standard Gibbs free energies of micellization and adsorption have been calculated as per the following equations[54,55]:

where Xcmcis the cmc in mole fraction unit obtained from surface tension studies.The calculated values ofare given in Table 6.
Theare found to be negative for pure amphiphiles as well as mixed amphiphiles,indicating that the process of micellization is spontaneous.The adsorption process can be measured as a distribution between the bulk and surface phases.The values ofof the mixtures are more negative than those of pure CPZ,which signify that in the attendance of surfactants molecules are dragged up to the interface more easily.More negative values of thethanconfirm that the micellization is secondary in nature as compared to surface adsorption.Sugihara etal.[56]have projected a thermodynamic quantity,Gmin,for evaluating synergism in mixed systems and given as:

Gminis considered as the work required for making an interface or the free energy change accompanied by the transition from bulk phase to the interface of the components in the solution.In other words,the lower the value of Gmin,the more thermodynamically stable surface is formed.The value of Gminalso measures the evaluation of synergism in the mixed systems.It is clear from the Table 6 that the values Gminare lower in magnitude.It means thermodynamically stable surfaces are formed with synergistic interaction.
The excess free energy of mixing(ΔGex)can be calculated by using the activity coefficients by the relation[57]:
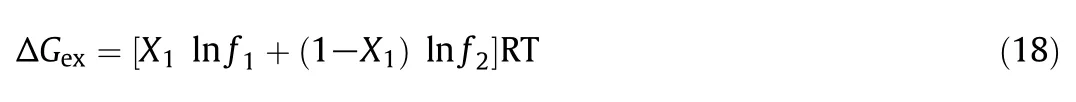
The values are given in Table 6.The ΔGexvalues found to be negative confirms that the formed mixed micelles are additionally thermodynamically stable in comparison to micelles formed from pure single amphiphiles.
3.6.UV–visible spectroscopy
The two-adsorption band belongs to a neutral phenothiazine compound; firstat the 250–265-nm region corresponding to theπ→π∗transition,and second in the longer wavelength range(300–320 nm)region attributed to the n→π∗transitions due to the presence of the nitrogen lone pair(Fig.6)[58].
In our case,the firstpeak appears at259 nm and the other at306 nm.The adsorption spectrum of CPZ has been studied in the presence of increasing equivalents of the surfactants to study the interactions between CPZ and gemini surfactants.It is important to state here that pure geminisurfactants do notshow any peak in the UV–visible spectra.The characteristic peaksat306 nm,which isdue to the tricyclic region of CPZ,on the addition of gemini surfactants,the absorption intensity of CPZ decreases,but no shifting occurs.These results indicate the binding of the surfactants with the CPZ molecule.In the mixed micellar systems,the geminisurfactants taken by the micelle ofCPZ is often insufficientto produce high absorbance.
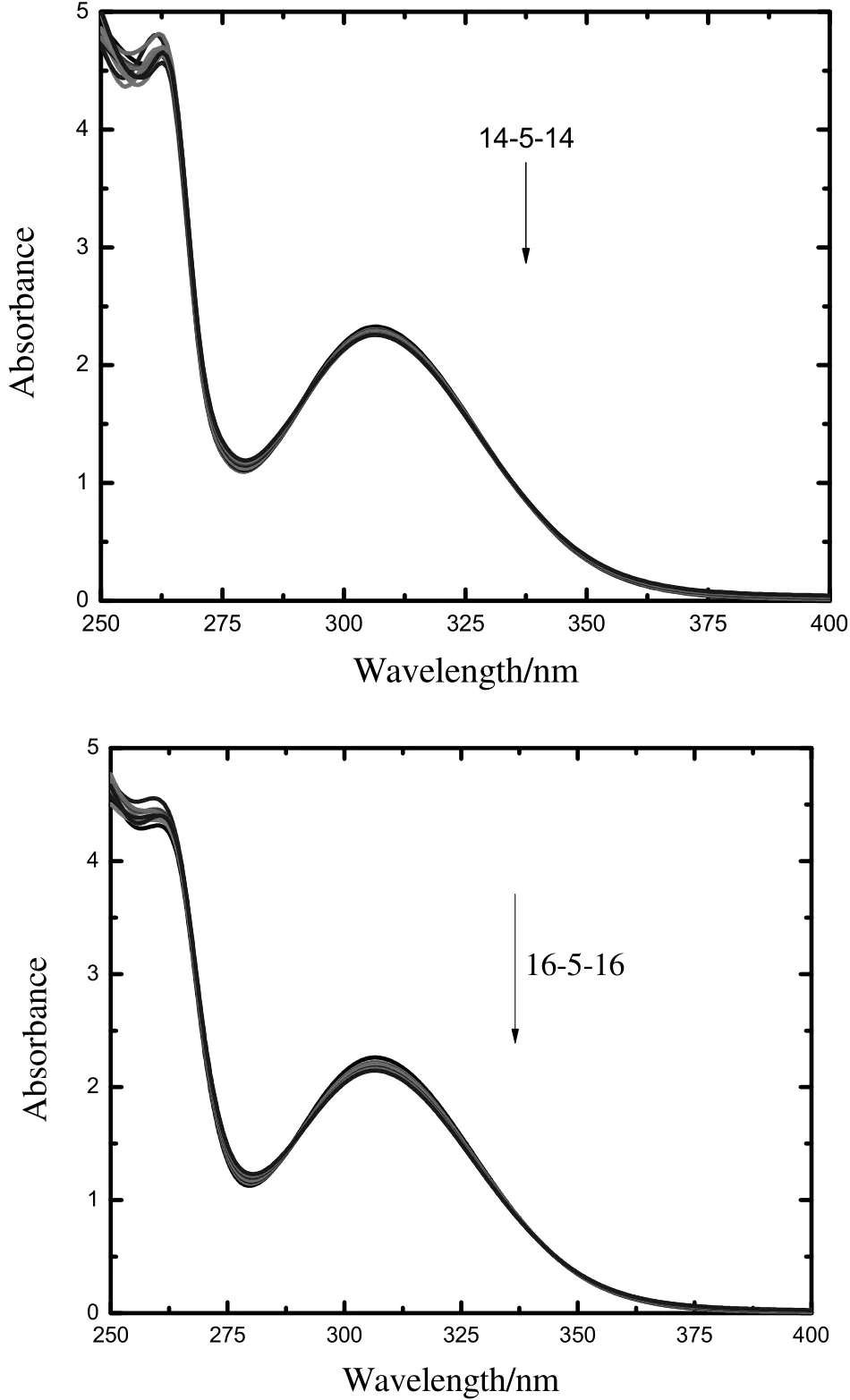
Fig.6.Electronic absorption spectra of pure CPZ in the presence of increasing the concentration of 14-5-14/16-5-16.
3.7.Fluorescence quenching
To get firm conclusions about the interactions between CPZ and cationic gemini surfactants,more experimentation is needed.Therefore,we carried out fluorescence measurements by using the same solution prepared for the UV–visible measurements.Fig.7 shows that the fluorescence intensity increases with the increase of the concentration of gemini surfactants with a slightly red shift of spectra from 458 to 464 nm(14-5-14)and 458–460 nm(16-5-16).The red shift of spectra denotes the binding.The red shift in 14-5-14 is more than that in 16-5-16,which implies that the binding of 16-5-16 with CPZ is not as strong compared to that of 14-5-14 with CPZ.
4.Conclusions
The mixed micellization,spectroscopic,and thermodynamic properties of the amphiphilic drug(CPZ)and cationic novel surfactants(14-5-14 and 16-5-16)have been studied.The cmc s of the mixed amphiphiles were measured by surface tension measurement.The results suggested that the two components formed mixed micelles and mixed monolayer.The values of experimental cmc(cmcexp)are lower than that of the ideal cmc(cmcideal)which suggested that the mixed micelles formed due to attractive interactions with greater contribution from CPZ.The spontaneity of the current systems is confirmed by the negative values of the standard free energy of micellization(ΔGmo).Values of free energies indicate that the process of adsorption at the interface is primary;whereas,micellization is secondary.The rapid increase of fluorescence intensity of CPZ with surfactants is due to the strong binding of surfactants with CPZ by a hydrophobic interaction.
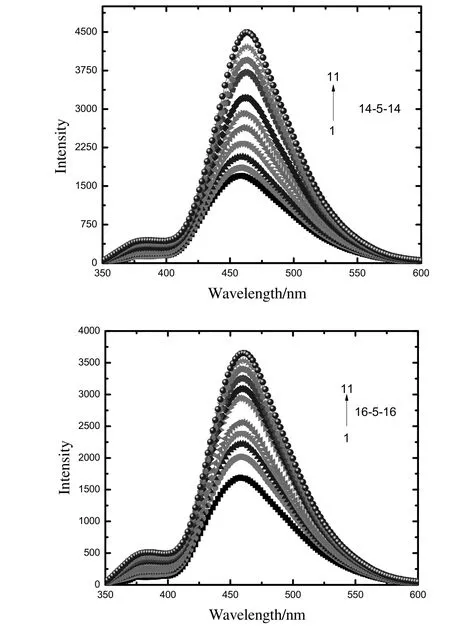
Fig.7.Fluorescence spectra of pure CPZ at λex=315 nm in the presence of increasing concentration of 14-5-14/16-5-16.
Acknowledgments
Chemistry Department and Centre of Excellence for Advanced Materials Research,King Abdulaziz University,Jeddah are highly acknowledged.
[1]M.Malmsten,Surfactants and Polymers in Drug Delivery,Taylor&Francis,New York,2002.
[2]K.Holmberg,D.O.Shah,M.J.Schwuger,Handbook of Applied Surface and Colloid Chemistry,Wiley,New York,2002.
[3]T.F.Tadros,Phase behaviour of surfactant systems,Applied Surfactants,Principles and Applications,Wiley-VCH Verlag GmbH&Co.,KGA,2005.
[4]Y.Liu,X.Dong,Y.Sun,New development of reverse micelles and applications in protein separation and refolding,Chin.J.Chem.Eng.16(2008)949–955.
[5]A.Z.Naqvi,M.A.Rub,Kabir-ud-Din,Study of phospholipid induced phase separation in amphiphilic drugs,Colloid J.77(2015)525–531.
[6]Y.Konodo,H.Uchiyama,N.Yoshino,K.Nishiyama,M.Abe,Spontaneous vesicle formation from aqueous solutions of didodecyldimethylammonium bromide and sodium dodecyl sulfate mixtures,Langmuir 11(1995)2380–2384.
[7]A.Khan,E.F.Marques,Synergism and polymorphism in mixed surfactant systems,Curr.Opin.Colloid Interf.Sci.4(1999)402–410.
[8]C.Tondre,C.Caillet,Properties of the amphiphilic films in mixed cationic/anionic vesicles:a comprehensive view from a literature analysis,Adv.Colloid Interf.Sci.93(2001)115–134.
[9]N.Azum,M.A.Rub,A.M.Asiri,Analysis of surface and bulk properties of amphiphilic drug ibuprofen and surfactant mixture in the absence and presence of electrolyte,Colloids Surf.B 121(2014)158–164.
[10]N.Azum,M.A.Rub,A.M.Asiri,Experimental and theoretical approach to mixed surfactant system of cationic gemini surfactant with nonionic surfactant in aqueous medium,J.Mol.Liq.196(2014)14–20.
[11]I.A.Khan,R.Mohammad,M.S.Alam,Surface properties and mixed micellization of cationic gemini surfactants with ethyleneamines,J.Chem.Eng.Data 55(2009)370–380.
[12]N.Azum,M.A.Rub,A.M.Asiri,Micellization and interfacial behavior of binary and ternary mixtures in aqueous medium,J.Mol.Liq.216(2016)94–98.
[13]M.A.Rub,N.Azum,A.M.Asiri,Interaction of cationic amphiphilic drug nortriptyline hydrochloride with TX-100 in aqueous and urea solutions and the studies of physicochemical parameters of the mixed micelles,J.Mol.Liq.218(2016)595–603.
[14]M.A.Rub,N.Azum,F.Khan,A.M.Asiri,Surface,micellar,and thermodynamic properties of antidepressant drug nortriptyline hydrochloride with TX-114 in aqueous/urea solutions,J.Phys.Org.Chem.30(2017),e3676.
[15]I.A.Khan,R.Mohammad,M.S.Alam,Kabir-ud-Din,Mixed micellization of cationic gemini surfactants with primary linear alkylamines,J.Surfactant Deterg.13(2010)179–188.
[16]N.Azum,M.A.Rub,A.M.Asiri,W.A.Bawazeer,Micellar and interfacial properties of amphiphilic drug–non-ionic surfactants mixed systems:surface tension, fluorescence and UV–visible studies,Colloids Surf.A 522(2017)183–192.
[17]N.Azum,M.A.Rub,A.M.Asiri,Interaction of triblock copolymer with cationic gemini and conventional surfactants:a physicochemical study,J.Dispers.Sci.Technol.(2017)https://doi.org/10.1080/01932691.2017.1283510.
[18]F.M.Menger,C.A.Littau,Gemini surfactants:a new class of self-assembling molecules,J.Am.Chem.Soc.115(1993)10083–10090.
[19]D.Danino,Y.Talmon,R.Zana,Alkanediyl-a,x-bis(dimethylalkylammonium bromide)surfactants(dimeric surfactants).5.Aggregation and microstructure in aqueous solutions,Langmuir 11(1995)1448–1456.
[20]J.Zhao,D.C.Sherril,B.M.Fung,Mixtures of monomeric and dimeric cationic surfactants,J.Phys.Chem.B 102(1998)7613–7618.
[21]N.Azum,A.Z.Naqvi,M.A.Rub,A.M.Asiri,Multi-technique approach towards amphiphilic drug-surfactant interaction:A physicochemical study,J.Mol.Liq.240(2017)189–195.
[22]H.Siddiqui,M.Kamil,M.Panda,Kabir-ud-Din,Solubilization of phenanthrene and fluorine in equimolar binary mixtures of gemini/conventional surfactants,Chin.J.Chem.Eng.22(2014)1009–1015.
[23]M.A.Rub,D.Kumar,N.Azum,F.Khan,A.M.Asiri,Study of the interaction between promazine hydrochloride and surfactant(conventional/gemini)mixtures at different temperatures,J.Solut.Chem.43(2014)930–949.
[24]N.Azum,A.M.Asiri,M.A.Rub,A.O.Al-Youbi,Thermodynamic aspects of polymersurfactant interactions:gemini(16-5-16)-PVP-water system,Arab.J.Chem.9(2016)S1660–S1664.
[25]A.R.Tehrani-Bagha,K.Holmberg,Cationic ester-containing gemini surfactants:physical–chemical properties,Langmuir 26(2010)9276–9282.
[26]X.Wang,H.Yang,F.Wang,A cationic gemini-surfactant as effective inhibitor for mild steel in HCl solutions,Corros.Sci.52(2010)1268–1276.
[27]M.Mobin,S.Masroor,Cationic gemini surfactants as novel corrosion inhibitor for mild steel in 1.0 M HCl,Int.J.Electrochem.Sci.7(2012)6920–6940.
[28]M.A.Hegazy,Novel cationic surfactant based on triazole as a corrosion inhibitor for carbon steel in phosphoric acid produced by dihydrate wet process,J.Mol.Liq.208(2015)227–236.
[29]W.C.Bowman,M.J.Rand,Textbook of pharmacology,Blackwell,Cambridge,UK,1990.
[30]L.R.S.Barbosa,I.Rosangela,W.Caetano,D.S.Neto,M.Tabak,Self-aggregation of phenothiazine compounds investigated by small angle scattering and electron paramagnetic resonance spectroscopy,J.Phys.Chem.B 112(2008)4261–4269.
[31]E.M.Glaser,S.B.Newling,Side effects of chlorpromazine hydrochloride,Br.J.Pharmacol.10(1955)429–433.
[32]W.H.Halliwell,Cationic amphiphilic drug-induced phospholipidosis,Toxicol.Pathol.25(1997)53–60.
[33]M.A.Rub,N.Azum,A.M.Asiri,M.E.M.Zayed,A.O.Al-Youbi,Solution properties of phenothiazine drug promazine hydrochloride with cationic hydrotropes in aqueous/electrolyte solution at different temperature,J.Phys.Org.Chem.29(2016)476–489.
[34]N.Azum,M.A.Rub,A.M.Asiri,Energetics of clouding phenomenon in amphiphilic drug imipramine hydrochloride with pharmaceuticals excipients,Pharm.Chem.J.48(2014)201–208.
[35]F.Khan,M.A.Rub,N.Azum,D.Kumar,A.M.Asiri,Interaction of an amphiphilic drug and sodium bis(2-ethylhexyl)sulfosuccinate at low concentrations in the absence and presence of sodium chloride,J.Solut.Chem.44(2015)1937–1961.
[36]M.A.Rub,N.Azum,A.M.Asiri,Self-association behavior of an amphiphilic drug nortriptyline hydrochloride under the in fluence of inorganic salts,Russ.J.Phys.Chem.B 10(2016)1007–1013.
[37]M.S.Alam,A.Z.Naqvi,Kabir-ud-Din,Surface and micellar properties of some amphiphilic drugs in the presence of additives,J.Chem.Eng.Data 52(2007)1326–1331.
[38]N.Azum,M.A.Rub,A.M.Asiri,Micellization and interfacial behavior of the sodium salt of ibuprofen-BRIJ-58 in aqueous/brine solutions,J.Solut.Chem.45(2016)791–803.
[39]M.A.Rub,N.Azum,A.M.Asiri,Binary mixtures of sodium salt of ibuprofen and selected bile salts:interface,micellar,thermodynamic,and spectroscopic study,J.Chem.Eng.Data(2017).https://doi.org/10.1021/acs.jced.7b00298.
[40]N.Azum,M.A.Rub,A.M.Asiri,Self-assocaition and microenvironmental properties of sodium salt of ibuprofen with brij-56 under the in fluence of aqueous/urea solution,J.Dispers.Sci.Technol.38(2017)96–104.
[41]N.Azum,A.M.Asiri,M.A.Rub,A.O.Al-Youbi,Thermodynamic properties of ibuprofen sodium salt in aqueous/urea micellar solutions at 298.15 K,Russ.J.Phys.Chem.A 91(2017)685–691.
[42]M.A.Rub,F.Khan,M.S.Sheikh,N.Azum,A.M.Asiri,Tensiometric, fluorescence and1H NMR study of mixed micellization of non-steroidal anti-in flammatory drug sodium salt of ibuprofen in the presence of non-ionic surfactant in aqueous/urea solutions,J.Chem.Thermodyn.96(2016)196–207.
[43]M.K.Al-Muhanna,M.A.Rub,N.Azum,S.B.Khan,A.M.Asiri,Effect of gelatin on micellization and microstructural behavior of amphiphilic amitriptyline hydrochloride drug solution:a detailed study,J.Chem.Thermodyn.89(2015)112–122.
[44]S.De,V.K.Aswal,P.S.Goyal,S.Bhattacharya,Role of spacer length in dimeric micellar organization.Small angle neutron scattering and fluorescence studies,J.Phys.Chem.100(1996)11664–11671.
[45]I.A.Khan,R.Mohammad,M.S.Alam,Kabir-ud-Din,The interaction of cationic gemini surfactant 1,4-butanediyl-α,ω-bis(dimethyl cetyl ammonium bromide)with primary linear alkanols,J.Dispers.Sci.Technol.31(2009)129–137.
[46]S.Schreier,S.V.P.Malheiros,E.de Paula,Surface active drugs:self association and interaction with membranes and surfactants.Physico-chemical and biological aspects,Biochim.Biophys.Acta 1508(2000)210–234.
[47]N.Azum,A.Z.Naqvi,M.Akram,Kabir-ud-Din,Properties of mixed aqueous micellar solutions formed by cationic alkanediyl-α,ω-bis(tetradecyl dimethyl ammonium bromide)and alkyltrimethylammonium bromides:Fluorescence and conductivity studies,J.Chem.Eng.Data 54(2009)1518–1523.
[48]N.Azum,A.M.Asiri,M.A.Rub,A.A.P.Khan,A.Khan,M.M.Rahman,D.Kumar,A.O.Al-Youbi,Mixed micellization of gemini surfactant with nonionic surfactant in aqueous media:a fluorometric study,Colloid J.75(2013)263–268.
[49]J.H.Clint,Surfactant Aggregation,Chapman and Hall,New York,1992.
[50]D.N.Rubingh,Solution Chemistry of Surfactants,Plenum,New York,1979.
[51]A.K.Sood,H.Kaur,T.S.Banipal,Interactions in the mixed micelles of monomeric and gemini surfactants:in fluence of some co-solvents as a function of temperature,Arab.J.Chem.(2016)https://doi.org/10.1016/j.arabjc.2015.12.009.
[52]M.J.Rosen,X.Y.Hua,Surface concentrations and molecular interactions in binary mixtures of surfactants,J.Colloid Interface Sci.86(1982)164–172.
[53]D.K.Chattoraj,K.S.Birdi,Adsorption and Gibbs surface excess,Plenum,New York,1984.
[54]K.Tsubone,Y.Arakawa,M.J.Rosen,Structural effects on surface and micellar properties of alkanediyl-α,ω-bis(sodium N-acyl-β-alaninate)gemini surfactants,J Colloid Interface Sci.262(2003)516–524.
[55]R.Miller,V.Dutschk,V.B.Fainerman,In fluence of molecular processes at liquid interfaces on dynamic surface tensions and wetting kinetics,J.Adhes.80(2004)549–561.
[56]G.Sugihara,A.M.Miyazono,S.Nagadome,T.Oida,Y.Hayashi,J.S.Ko,Adsorption and micelle formation of mixed surfactant systems in water ii:a combination of cationic gemini-type surfactant with mega-10,J.Oleo Sci.52(2003)449–461.
[57]M.A.Rub,N.Azum,A.M.Asiri,S.Y.M.Alfai fi,S.S.Alharthi,Interaction between antidepressant drug and anionic surfactant in low concentration range in aqueous/salt/urea solution:a conductometric and fluorometric study,J.Mol.Liq.227(2017)1–14.
[58]C.Párkányi,C.Boniface,J.J.Aaron,M.Maa fi,A quantitative study of the effect of solvent on the electronic absorption and fluorescence spectra of substituted phenothiazines:evaluation of their ground and excited singlet-state dipole moments,Spectrochim.Acta 49(1993)1715–1725.
 Chinese Journal of Chemical Engineering2018年3期
Chinese Journal of Chemical Engineering2018年3期
- Chinese Journal of Chemical Engineering的其它文章
- Numerical investigation on flow and heat transfer characteristics of corrugated tubes with non-uniform corrugation in turbulent flow
- Investigations on pool boiling critical heat flux,transient characteristics and bonding strength of heater wire with aqua based reduced graphene oxide nano fluids
- Heavy metals adsorption by banana peels micro-powder:Equilibrium modeling by non-linear models
- Potential aspect of rice husk biomass in Australia for nanocrystalline cellulose production
- Fouling evaluation on membrane distillation used for reducing solvent in polyphenol rich propolis extract
- Investigation on a vertical radial flow adsorber designed by a novel parallel connection method☆
