Preparation of highly active MCM-41 supported Ni2P catalysts and its dibenzothiophene HDS performance☆
Hua Song*,Qi Yu,Yanguang Chen,Yuanyuan Wang,Ruixia Niu
College of Chemistry and Chemical Engineering,Northeast Petroleum University,Daqing 163318,China
1.Introduction
Sulfur removal from fuels is becoming one ofthe mostsignificantaspects[1].It has been realized that current commercial sulfided hydrodesulfurization(HDS)catalysts are not sufficient to meet the required standards[2,3],and the investigation and development of high-performance HDS catalysts has been stimulated by the challenge of producing cleaner fuels from increasingly low quality petroleum feedstocks[4,5].Recently,a new class of materials,the transition metalphosphides(i.e.MoP,WP,Ni2P)have aroused extensive attention as a new generation of HDS catalysts,due to their high thermal stabilities and high activity for HDS[6,7].Transition metal phosphides used as catalytic materials show excellent activity for certain reactions with activity similar to that of noble metal catalysts,among which,Ni2P shows the most remarkable activities,and numerous works have been reported on their HDS performance[8].
The Ni2P is commonly synthesized using temperature-programmed reduction(TPR)of metal phosphate precursors which were prepared mainly by impregnation of the support with Ni(NO3)2and(NH4)2HPO4(or NH4H2PO4)solutions.
During the preparation of Ni2P,it is necessary to passivate the obtained catalysts prior to exposure to air or moisture in order to form a protective passivation layer.The freshly obtained catalysts are passivated typically by a low concentration of oxygen flow(0.5 vol%O2/N2).In such cases,the catalyst has to be pretreated at elevated temperatures prior to the HDS reaction,and the operation consumes energy.Duan et al.[9]proposed H2S as a passivation agent for Ni2P catalysts and found that a small degree of surface reconstruction occurs in the course of HDS atthe surface of the H2S-passivated catalyst,and it gave a higher HDS activity and required no post re-reduction.This shows that surface modification of Ni2P catalyst would cause a surface reconstruction of catalyst,which could affect performance of the HDS of Ni2P catalyst.
In our previous work[10],we had demonstrated a method for preparing highly active MCM-41 supported Ni2P catalysts by impregnation of a nickelchloride and ammonium hypophosphite solution with MCM-41 zeolite,followed by reduction of the precursors in a flow of H2at 210–390 °C,which is lower than the traditional TPR method by about 200°C.Then the catalysts were passivated with a mixture of O2/N2(0.5 vol%of O2)before they are taken out from the synthesis apparatus.In this study,a simple surface modification method is proposed for preparing highly active Ni2P HDS catalysts.The supported Ni2P catalysts were synthesized from two different phosphorus precursors.One is NH4H2PO2according to the method described previously[10],and the other is(NH4)2HPO4according to the traditional TPR method.Then as-obtained Ni2P catalysts were modified with air instead of the usual passivation with the conventional O2/N2mixture.In this way,preprocessing was not necessary for the catalyst before HDS reaction.For comparison,the as-obtained two Ni2P catalysts were passivated with an O2/N2mixture(0.5 vol%of O2).The effects of surface modification with air on HDS performance of Ni2P catalysts prepared by these two different methods have been investigated.
2.Experimental
2.1.Preparation of support and catalysts
The MCM-41 support was synthesized by the hydrothermal synthesis method in the literature[11].The silicate gel was prepared using tetraethoxysilane(TEOS)as the Si source,and the cationic surfactant cetyltrimethylammoniumbromide(CTAB)as the template.The MCM-41 support obtained was named ‘M41’.
The supported Ni2P catalyst precursors were prepared using temperature-programmed reduction(TPR)method by impregnating nickel nitrate(Ni(NO3)2·6H2O)and ammonium hypophosphite((NH4)2HPO4)or ammonium hypophosphite(NH4H2PO2)with M41,following procedures previously described by our group[12].After the water was evaporated,the impregnated solid was dried and calcined.The obtained oxidic precursor prepared with ammonium hypophosphite((NH4)2HPO4)as phosphorus sources was named as ‘PNi2P(T)/M41’.The other precursor prepared with ammonium hypophosphite(NH4H2PO2)was named as ‘PNi2P(t)/M41’.Both of the precursors were used for pressing the tablets,then crushed and sieved to obtain a particle diameter of 0.83–1.00 mm.In the fixed-bed reactor,The precursors were heated to 650 °C(PNi2P(T)/M41)or 400 °C(PNi2P(t)/M41),at a rate of 3 °C·min−1with a flow of H2(100 ml·min−1)for 2 h for reduction,then cooled to the subsequent processing temperature naturally in a continuous H2flow.
The obtained catalysts were treated at 100°C under flowing air for 1 h were named as Ni2P(x)/M41-O,where x=T stands for catalysts prepared using(NH4)2HPO4as the phosphorus source,and x=t stands for catalysts prepared using NH4H2PO2as the phosphorus source.
For comparison,the obtained catalysts were also passivated at room temperature in flow of a O2/N2mixture(0.5 vol%of O2)at a rate of 30 ml·min−1for 1 h,and catalysts were named as Ni2P(x)/M41-N.For all the catalysts,Ni loading is 8.8 wt%and an initial Ni/P molar ratio is 1/2.
Prior to reaction,the Ni2P(x)/M41-N catalysts were pretreated with flowing H2(30 ml·min−1)at 500 °C for 2 h and then cool down to the reaction temperature and start the HDS operation.For the Ni2P(x)/M41-O catalysts this pretreatment step was saved.
2.2.Characterization of catalysts
X-ray diffraction(XRD)patterns of the samples were obtained with a D·max−1-2200PC-X-ray diffractometer operated at 40 kV,30 mA,using CuKαradiation,and scan range from 10°to 80°at a rate of 10°·min−1.
Surface areas of catalysts and supports were analyzed according to BET method based on adsorption isotherms at liquid nitrogen−196 °C temperature.All the samples were outgassed at 200 °C until the vacuum pressure was 0.798 Pa,using Micromeritics adsorption equipment of NOVA2000e.
The relationship between surface modification and active sites was investigated using a Micromeritics ASAP 2010 apparatus with a TCD for CO pulsed chemisorption without re-reduction on the catalyst.The catalysts were pre-treated in a continuous He flow to remove moisture;then CO pulses were repeatedly injected into N2until there was no further CO chemisorption after consecutive injections.
The X-ray photoelectron spectroscopy(XPS)spectra were recorded using ESCALAB MKII spectrometer employing a monochromatic Mg Kαradiation(E=1253.6 eV).The XPS measurements equipped with a hemi-spherical analyzer operating at fixed pass energy of 40 eV.All recorded photoelectron binding energies were referenced against the C 1s contamination line at 284.8 eV.
2.3.Catalytic activities
The prepared catalysts were tested in a flowing high-pressure fixed–bed reactor.The HDS catalytic activities were evaluated using a feed consisting of a decalin solution of DBT(1 wt%).The experimental conditions of the HDS reaction were 3.0 MPa,340°C,hydrogen/oil ratio of 500(v/v)and weight hourly space velocity(WHSV)=6 h−1.Sampling of liquid products were collected every hour after a steady reaction conditions has been achieved.Both feed and reaction products were analyzed by flame ionization detector(FID)gas chromatography equipped with a GC-14C-60 column.
3.Results and Discussion
3.1.XRD
Fig.1 shows the XRD patterns of samples synthesized by different methods.The broad feature peak located at2θ=23°is typicalfor amorphous silica of mesoporous M41,which is observed in all spectra[13].The diffraction patterns for all samples showed the peaks at 2θ=40.6°,44.5°,47.1°,54.1°and 54.8°(PDF:03–0953),which are corresponding to the characteristic peaks of Ni2P.This shows that after surface modification with air at 100°C,the crystal structure of Ni2P was retained.The additional phase of related to Ni and P is not observed,indicating the formed phase is mainly Ni2P for all catalysts.For Ni2P(t)/M41-O,the crystallite size(Dc)of the catalyst was calculated according to the Scherrer equation[14,15](column 5 of Table 1),which is 14 nm,smaller than that of corresponding Ni2P(t)/M41-N(18 nm).The same tendency was observed for the samples prepared using(NH4)2HPO4.This demonstrates that the absorbed oxygen promotes highly dispersed smaller Ni2P particles.
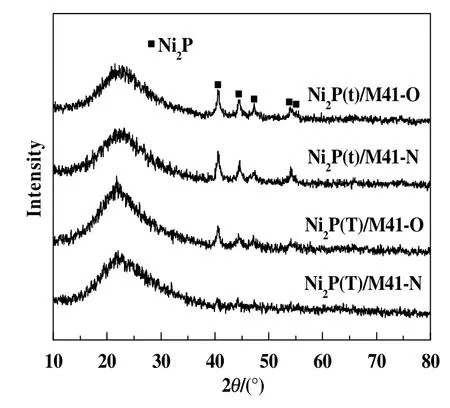
Fig.1.XRD patterns of samples synthesized by different methods.
3.2.BET
The textural characterizations of supports and the catalysts are summarized in Table 1.The surface area and pore volume of the MCM-41 support are 1012 m2·g−1and 0.816 cm3·g−1,respectively.After loading Ni2P precursors,the mass fraction of MCM-41 on the supported catalysts was reduced and some of the pores were blocked,which may explain the decrease of the BET surface area of all the catalysts.The Ni2P(x)/M41-O catalysts obtained by surface modification with air possessed slightly higher surface area than corresponding Ni2P(x)/M41-N catalysts which treated by O2/N2mixture(0.5 vol%of O2).This may have been caused by the surface restructuring after modification withair,which featured lower phosphorus contenton the surface of Ni2P(x)/M41-O as compared to Ni2P(x)/M41-N(Table 2).This will be further discussed in Section 3.4.
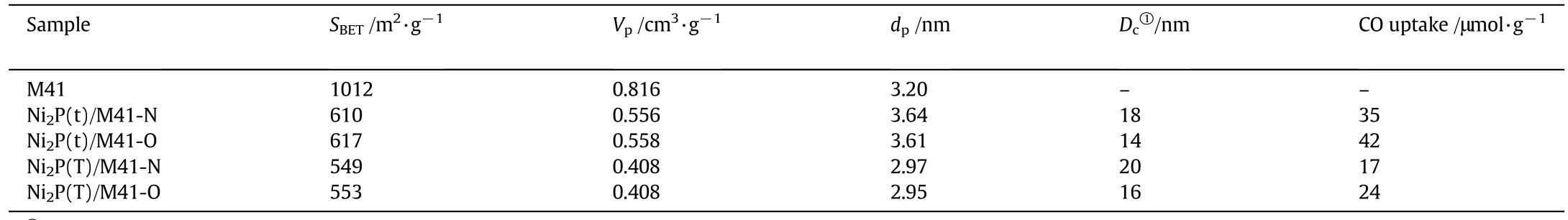
Table 1 Textural characterization of supports and the catalyst samples
3.3.CO chemisorption
The CO chemisorption was obtained for the samples as summarized in column 6 of Table 1.The CO chemisorption measurements were used to titrate the surface Ni atoms and to provide an estimate of the active CO chemisorption sites on the catalysts[16].The CO molecules mainly adsorb on Ni sites and the amount of CO molecules adsorbed on P sites may be very small[17],therefore the enrichment of P atoms on the surface of catalysts makes the amount of exposed nickel atoms decrease.The CO chemisorption of Ni2P(T)/M41-O and Ni2P(t)/M41-O are 24 μmol·g−1and 42 μmol·g−1,much higher than corresponding Ni2P(T)/M41-N(17 μmol·g−1)and Ni2P(t)/M41-N(32 μmol·g−1).Similar results were obtained by Li et al.[18].They reported that the CO uptake value of Ni2P which modified with air at 50°C showed a 1.7 times improvement during surface modification.They concluded that number of active sites increased due to some inactive sites transformed into active sites during the surface modification with air.It can be seen from Table 1,the surface areas of Ni2P(x)/M41-O samples increased slightly as compared to those corresponding Ni2P(x)/M41-N samples,showing increase surface area is not the main reason for the increased CO uptake.Therefore,the increased CO uptake of the samples modified with air at 100°C can be attributed to the smaller Ni2P particles sizes of Ni2P(x)/M41-O samples,which is beneficial to expose more active Ni2P particles on the surface,and the transformation of some inactive sites into active sites during the surface modification.In addition,the lower P atoms on the surface of Ni2P(x)/M41-O(Table 2),which would lead to more exposed Ni atoms is another important reason.This will be further discussed later in Section 3.4.
3.4.XPS
The XPS spectra in the Ni 2p,P 2p and O 1s regions for samples are shown in Fig.2,and the binding energy values are presented in Table 2.As shown in Fig.2(a),all spectra were decomposed,taking into account the spin-orbital splitting of the Ni 2p3/2and Ni 2p1/2lines(about 17 eV)and the presence of satellite peaks at about 5 eV higher than the binding energy of the parent signal[19].The bands centered at 852.1–852.6 eV and 856.5–856.7 eV are assigned to Niδ+in Ni2P phase and Ni2+species[20].The binding energies for Ni species over Ni2P(x)/M41-O catalysts are remained unchanged after surface modification with air.
As shown in Fig.2(b),the P 2p binding energy was observed,and the peaks at128.8–129.6 eVfor Pδ−ofNi2P[19]and 134.3–134.8 eV for surface nickel phosphate(PO43−,P5+)species due to the superficial oxidation of Ni2P.For Ni2P(x)/M41-N,the peaks at 133.2 and 133.5 eV,which are attributed to the H2PO3−species,can be seen.On the contrary,for Ni2P(x)/M41-O,the peaks attributed to H2PO3−species are diminished.This means that the H2PO3−species transformed to PO43−species by absorbed oxygen at higher temperature.This is possibly due to the interaction between oxygen and phosphorus,which revealed that phosphorus in the catalyst tended to further oxidize[18].
As shown in Fig.2(c),the peak at around 532.6 eV of Ni2P(x)/M41-N catalysts could be attributed to OH−.However,the binding energy of OH−shifts to a slightly higher value for the Ni2P(x)/M41-O catalyst.This indicates that OH−ion could possibly transfer some of its electrons to Ni[21]and oxygen was strongly adsorbed on the surface of Ni2P(x)/M41-O.
XPS analyses were used to calculate the surface Ni/P atomic ratios(Table 2).Allthe samples showed lower Ni/P values than the theoretical Ni/P ratio which corresponding to the precursor materials was 0.5.This may be the consequence of the aggregation of phosphorous on the surface of the catalysts.For Ni2P(x)/M41-O catalysts,the increase of Ni/P was observed as compared to corresponding Ni2P(x)/M41-N samples which was prepared by the same phosphorus.This indicates that the P atoms on the surface of the catalysts decreased,which was in conformity with the analysis of CO uptake(Section 3.3).As compared to Ni2P(x)/M41-N samples,the oxygen contents on the surface of the corresponding Ni2P(x)/M41-O samples increased.This confirmed that the addition of the highly electronegative element of oxygen on the surface of Ni2P(x)/M41-O samples happened during surface modification with air and the existence of the interaction between Ni and O atoms on the surface of the catalysts.
3.5.HDS activity and selectivity
The activities of catalysts evaluated by the HDS of DBT are shown in Fig.3.With increasing the time on stream,the activities of all the samples were increased rapidly at first and then increased gradually,tended to stabilize finally.As mentioned above,prior to reaction,the Ni2P(x)/M41-N catalysts were pretreated in situ with flowing H2(30 ml·min−1)at 500 °C for 2 h and then cool down to the reaction temperature and start the HDS operation.However,the initial activitiesofNi2P(T)/M41-N(48.3%at1 h on stream)and Ni2P(t)/M41-Ncatalysts(56.0%at 1 h on stream)are much lower when compared to those of Ni2P(T)/M41-O(63.5%at 1 h on stream)and Ni2P(t)/M41-O catalysts(80.8%at 1 h on stream),showing that more active Ni2P sites were formed on Ni2P(x)/M41-O catalysts which was modified with air.In addition,after 8 h the DBT conversions reached 83.6%and 98.7%for Ni2P(T)/M41-Oand Ni2P(t)/M41-O,respectively,which was an increase of 4.6%and 7.1%when compared with that found for corresponding samples which were prepared with same method and passivated by O2/N2mixture.The higher activities of Ni2P(x)/M41-O catalysts can be attributed to the smaller Ni2P particles sizes(Table 1)and the increased hydrogen dissociation activity due to the surface modification.The improvement of HDS performance was related to the activation of atomic hydrogen.It was reported that when P inserted into Ni,a small charge transferred from Ni to P,which allowed a high activity for the dissociation of molecular hydrogen[22,23].This suggested that the additional highly electronegative elements could potentially accelerate hydrogenation performance.The oxygen possessed relatively high electronegativity which would increase the hydrogenation activity of Ni2P since number of active sites increased due to some inactive sites transformed into active sites during the surface modification of Ni2P with air.Our results exhibited that the surface modification with air at 100°C realized the addition ofthe highly electronegative element of oxygen on the surface of Ni2P(x)/M41-O catalysts(See Table 2,oxygen contents).During HDS,the highly electronegative element oxygen could activate the hydrogen and trans fer some of itselectrons to Ni,and oxygen was strongly adsorbed on the surface of Ni2P(x)/M41-O.Therefore,the hydrogen dissociation activities of Ni2P(x)/M41-O were enhanced since the transformation of some inactive sites into active sites.The CO uptake results also confirmed that more active Nisites were exposed on surface of the samples modified with air(See Table 1,CO uptakes).Thereby,the HDS performances of Ni2P(x)/M41-O catalysts were higher than those of corresponding Ni2P(x)/M41-N catalysts.
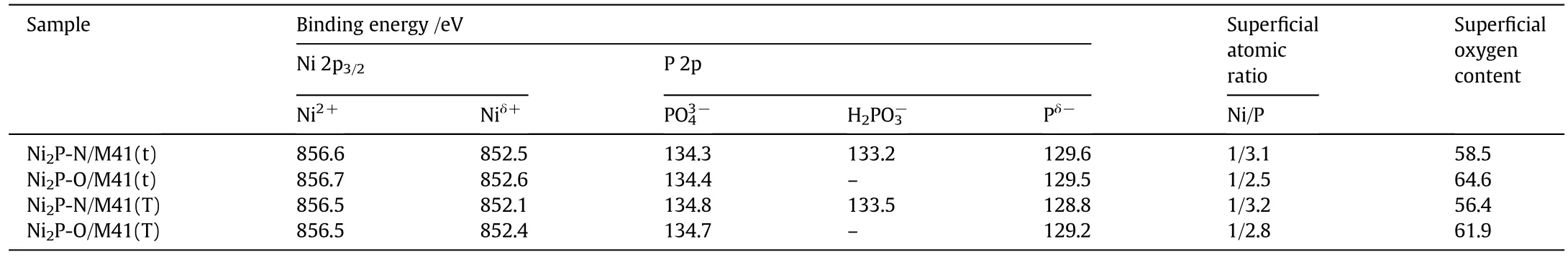
Table 2 Spectral parameters obtained by XPS analysis
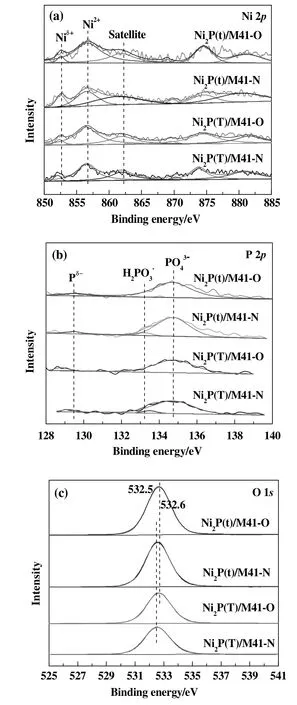
Fig.2.XPS spectra in the Ni 2p and P 2p regions for Ni2P/M41 catalyst samples.(a)Ni 2p core level spectra;(b)P 2p core level spectra;(c)O 1s core level spectra.
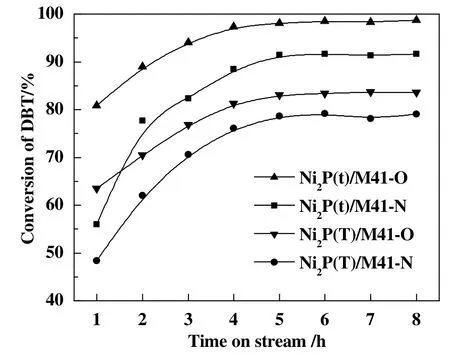
Fig.3.HDS activity of the Ni2P(x)/M41catalysts.Temperature,340°C;Pressure,3.0 MPa;H2/oil ratio,500(v/v);WHSV,6 h−1.
It is worth noting that the modification temperature and oxygen content may both play the important role in surface modification.Li et al.[18]reported that for sample modified with air at 50°C the oxidation layer was about 6.18 nm,while the sample modified with air at room temperature maintained a thinner oxidation layer of 1.06 nm.This suggested that modification temperature is one of the key factors during the surface modification.The oxygen content would also affect the oxidation degree of the surface.Therefore,the Ni2P(x)/M41-N catalysts,which obtained with low oxygen content(O2/N2mixture with 0.5 vol%of O2)at low modification temperature,are much different from Ni2P(x)/M41-O catalysts.The XPS analysis(Fig.2)exhibited that oxygen was more strongly adsorbed on the surface of Ni2P(x)/M41-O catalysts than that on surface of the Ni2P(x)/M41-N catalysts.In addition,the changes in the surface Ni/P molar ratios and oxygen content of Ni2P(x)/M41-O(See Table 2)confirmed that surface modification with air at 100°C caused a surface reconstruction of catalyst.
The HDS catalytic selectivity of the catalysts is given in Fig.4.For all the samples,the yield of BP is much higher than that of CHB,indicating that DBT primarily removed by the DDS pathway over all the catalysts[24].Moreover,as compared to the Ni2P(x)/M41-N catalysts,the selectivity of CHB over Ni2P(x)/M41-O catalysts is increased,indicating the HYD pathway is enhanced after surface modification with air.
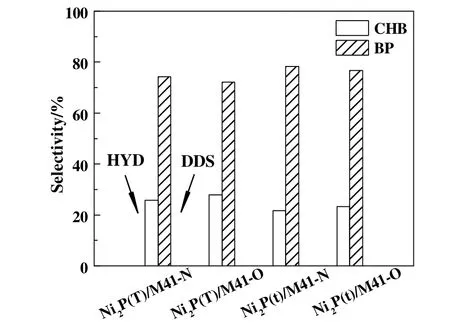
Fig.4.HDS selectivity of the Ni2P(x)/M41catalysts.Temperature,340°C;Pressure,3.0 MPa;H2/oil ratio,500(v/v);WHSV,6 h−1.
4.Conclusions
Highly active MCM-41 supported nickel phosphide catalysts for hydrodesulfurization(HDS)were synthesized by two different phosphorus sources,in which the surface of Ni2P catalysts were modified with air at 100°C for 1 h instead of being passivated by O2/N2mixture.The preparation method need not pre-treat catalyst prior to HDS reaction as traditional method.The XRD analysis demonstrated that the surface modification with air did not destruct the crystal structure of Ni2P.Moreover,the CO uptakes of Ni2P(T)/M41-O and Ni2P(t)/M41-O are 24 μmol·g−1and 42 μmol·g−1,much higher than corresponding Ni2P(T)/M41-N(17 μmol·g−1)and Ni2P(t)/M41-N(32 μmol·g−1),showing that the dispersion ofNi2P particles was improved after surface modification and more nickel atoms were exposed on Ni2P(x)/M41-O samples.Both of the catalysts modified by air showed a higher HDS activity than corresponding catalysts prepared by traditional method.The higher activities of Ni2P(x)/M41-O catalysts can be attributed to the smaller Ni2P particles sizes(Table 1)and the increased hydrogen dissociation activity due to the surface modification.At 3.0 MPa and 613 K,the DBT conversion of the Ni2P(t)/M41-O catalyst modified with air was 98.7%,which was an increase of 7.1%when compared with that found for Ni2P(t)/M41-N catalyst passivated by O2/N2mixture.As compared to the Ni2P(x)/M41-N catalysts,the selectivity of CHB over Ni2P(x)/M41-Ocatalysts is increased,indicating the HYD pathway is enhanced after surface modification with air.
[1]H.Y.Zhao,S.T.Oyama,H.J.Freund,R.Włodarczyk,M.Sierka,Nature of active sites in Ni2P hydrotreating catalysts as probed by iron substitution,Appl.Catal.,B.164(2015)204–216.
[2]A.Stanislaus,A.Mara fi,M.S.Rana,Recent advances in the science and technology of ultra low sulfur diesel(ULSD)production,Catal.Today 153(2010)1–68.
[3]H.Ziaei-Azad,N.Semagina,Iridium addition enhances hydrodesulfurization selectivity in 4,6-dimethyldibenzothiophene conversion on palladium,Appl.Catal.,B.191(2016)138–146.
[4]Z.Vít,H.Kmentová,L.Kaluzˇa,D.Gulková,M.Boaro,Effect of preparation of Pd and Pd–Pt catalysts from acid leached silica–alumina on their activity in HDS of thiophene and benzothiophene,Appl.Catal.,B 108–109(2011)152–160.
[5]G.N.Yun,Y.K.Lee,Dispersion effects of Ni2P catalysts on hydrotreating of light cycle oil,Appl.Catal.,B 150–151(2014)647–655.
[6]V.Teixeira da Silva,L.A.Sousa,R.M.Amorimb,L.Andrini,S.J.A.Figueroa,F.G.Requejo,F.C.Vicentini,Lowering the synthesis temperature of Ni2P/SiO2by palladium addition,J.Catal.279(2011)88–102.
[7]A.Infantes-Molina,J.A.Cecilia,B.Pawelec,J.L.G.Fierro,E.Rodríguez-Castellón,A.Jiménez-López,Ni2P and CoP catalysts prepared from phosphite-type precursors for HDS–HDN competitive reactions,Appl.Catal.,A.390(2010)253–263.
[8]L.Yang,X.Li,A.J.Wang,R.Prins,Y.Wang,Y.Y.Chen,X.P.Duan,Hydrodesulfurization of 4,6-dimethyldibenzothiophene and its hydrogenated intermediates over bulk Ni2P,J.Catal.317(2014)144–152.
[9]X.P.Duan,Y.Teng,A.J.Wang,V.M.Koganb,X.Lia,Y.Wang,Role of sulfur in hydrotreating catalysis over nickel phosphide,J.Catal.261(2009)232–240.
[10]H.Song,M.Dai,H.L.Song,X.Wan,X.W.Xu,A novel synthesis of Ni2P/MCM-41 catalysts by reducing a precursor of ammonium hypophosphite and nickel chloride at low temperature,Appl.Catal.,A 462–463(2013)247–255.
[11]H.I.Meléndez-Ortiz,L.A.García-Cerda,Y.Olivares-Maldonado,G.Castruita,J.A.Mercado-Silva,Y.A.Perera-Mercado,Preparation of spherical MCM-41 molecular sieve at room temperature:in fluence of the synthesis conditions in the structural properties,Ceram.Int.38(2012)6353–6358.
[12]H.Song,J.Wang,Z.D.Wang,H.L.Song,F.Li,Z.S.Jin,Effect of titanium content on dibenzothiophene HDS performance over Ni2P/Ti-MCM-41 catalyst,J.Catal.311(2014)257–265.
[13]J.A.Cecilia,A.Infantes-Molina,E.Rodríguez-Castellón,A.Jiménez-López,A novel method for preparing an active nickel phosphide catalyst for HDS of dibenzothiophene,J.Catal.263(2009)4–15.
[14]X.Wang,P.Clark,S.T.Oyama,Synthesis,characterization,and hydrotreating activity of several iron group transition metal phosphides,J.Catal.208(2002)321.
[15]S.T.Oyama,X.Wang,Y.K.Lee,W.J.Chun,Active phase of Ni2P/SiO2in hydroprocessing reactions,J.Catal.221(2004)263–273.
[16]S.T.Oyama,X.Wang,Y.K.Lee,K.Bando,F.G.Requejo,Effect of phosphorus content in nickel phosphide catalysts studied by XAFS and other techniques,J.Catal.210(2002)207–217.
[17]K.A.Layman,M.E.Bussell,Infrared spectroscopic investigation of CO adsorption on silica-supported nickel phosphide catalysts,J.Phys.Chem.B 108(2004)10930–10941.
[18]R.G.Li,Q.X.Guan,R.C.Wei,S.Q.Yang,Z.Shu,Y.Dong,J.Chen,W.Li,A potential regularity for enhancing the hydrogenation properties of Ni2P,J.Phys.Chem.C 119(2015)2557–2565.
[19]I.K.Tamás,V.Zdeněk,G.P.Dilip,R.Ryong,S.K.Hei,J.M.H.Emiel,SBA-15-supported nickel phosphide hydrotreating catalysts,J.Catal.253(2008)119–131.
[20]Y.N.Guo,P.H.Zeng,S.F.Ji,N.Wei,H.Liu,C.Y.Li,Effect of Mo promoter content on performance of Mo-Ni2P/SBA-15/cordierite monolithic catalyst for hydrodesulfurization,Chin.J.Catal.31(2010)329–334.
[21]K.Sutthiumporn,S.Kawi,Promotional effectof alkaline earth over Ni–La2O3catalyst for CO2reforming of CH4:role of surface oxygen species on H2production and carbon suppression,Int.J.Hydrog.Energy 36(2011)14435–14446.
[22]J.A.Rodriguez,J.Y.Kim,J.C.Hanson,S.J.Sawhill,M.E.Bussell,Physical and chemical properties of MoP,Ni2P,and MoNiP hydrodesulfurization catalysts:time-resolved X-ray diffraction,density functional,and Hydrodesulfurization activity studies,J.Phys.Chem.B 107(2003)6276–6285.
[23]P.Liu,J.A.Rodriguez,T.Asakura,J.Gomes,K.Nakamura,Desulfurization reactions on Ni2P(001)and α-Mo2C(001)surfaces:complex role of P and C sites,J.Phys.Chem.B 109(2005)4575–4583.
[24]H.Song,X.W.Xu,M.Dai,H.L.Song,Effect of Pt on hydrodesulfurization performance of the Ni2P/MCM-41 catalyst,Chem.J.Chin.Univ.34(2013)2609–2616.
 Chinese Journal of Chemical Engineering2018年3期
Chinese Journal of Chemical Engineering2018年3期
- Chinese Journal of Chemical Engineering的其它文章
- Numerical investigation on flow and heat transfer characteristics of corrugated tubes with non-uniform corrugation in turbulent flow
- Investigations on pool boiling critical heat flux,transient characteristics and bonding strength of heater wire with aqua based reduced graphene oxide nano fluids
- Heavy metals adsorption by banana peels micro-powder:Equilibrium modeling by non-linear models
- Potential aspect of rice husk biomass in Australia for nanocrystalline cellulose production
- Fouling evaluation on membrane distillation used for reducing solvent in polyphenol rich propolis extract
- Investigation on a vertical radial flow adsorber designed by a novel parallel connection method☆
