Pd catalysts supported on rGO-TiO2 composites for direct synthesis of H2O2:Modification of Pd2+/Pd0 ratio and hydrophilic property☆
Shuying Chen,Rui Tu,Jun Li*,Xiaohua Lu*
State Key Laboratory of Materials-Oriented Chemical Engineering,College of Chemical Engineering,Nanjing Tech University,Nanjing 210009,China
1.Introduction
Hydrogen peroxide(H2O2)is a green,versatile chemical commodity with wide applications,such as bleaching ofpulp/paper and synthesis of chemicals[1–4].Currently the industrial production of H2O2is mainly realized by anthraquinone process,which has serious drawbacks including the use of large amount of organic solvent.Compared with the anthraquinone process,the direct synthesis of H2O2from H2and O2is a greener process and thus attracts broad attention[5–17].However,the direct process is still not industrialized,even though it has been studied for decades.The major problem is the low productivity and selectivity of H2O2which resulted from the hydrogenation and decomposition of the generated H2O2.
Many efforts have been made to develop efficient catalysts to address this problem.Among them,Pd supported catalysts have been extensively studied[5,8,10,17].The electronic state of Pd is proved to play a vital role in in fluencing the selectivity of H2O2.It was reported that the selectivity of H2O2on PdO is higher than that on Pd0,due to the lower hydrogenation and decomposition rate of H2O2on PdO[18–20].Pd0is more favorable for H2conversion because H2is more easily dissociated on Pd0sites[21,22].Therefore,the activity of the catalysts is actually determined by the synergy of Pd2+and Pd0.The latest report from Han et al.also confirmed that both Pd2+and Pd0have an important impact on the catalystic performance for H2O2synthesis[17].
The hydrophobicity or hydrophilicity of the catalysts also has significant effects on the performance of the catalysts.Chuang et al.revealed that the repulsive effect between the hydrophobic groups on the catalyst surface and H2O2molecule could reduce the secondary adsorption of H2O2,which could significantly lower the hydrogenation rate of H2O2[23].Wang et al.found that the hydrogenation and decomposition rate of H2O2is much higher on catalyst surface with many hydrophilic groups[24].Therefore,the hydrophobic modification of the catalysts would be an effective mothed to improve the H2O2selectivity.
TiO2is the frequently used support in the direct synthesis of H2O2[13,18,25,26],because it has several advantages including the strong interaction with Pd particles,stability and nontoxicity[27–29].However,the H2O2hydrogenation rate on Pd/TiO2ishigherthan thaton Pd/carbon owing to the hydrophilic property of TiO2[25].According to the above analysis,the hydrophobic nature of the catalyst surface and the electronic state of Pd particles are both controllable factors for the improvement of the H2O2selectivity.If two of them are joined together,the novel catalysts may be more effective for H2O2synthesis.Therefore,we think that the nanostructured composites of TiO2and graphene supported catalystseem to be a practical approach to regulate the above two factors.Graphene is a well-known carbonaceous material with orderly graphite structure which has been proved to be advantageous for high H2O2selectivity[24].Moreover,graphene could modify the electronic structure of Pd due to its unique electronic property[30–33],but the interaction between metal and carbon materials is weak that would lead to the instability of catalysts[23].Thus,the introduction of graphene to composite with TiO2could modify the oxide state of Pd particles and regulate the hydrophilic/hydrophobic nature of catalyst surface simultaneously.In this work,hydrophilic TiO2was combined with reduced graphene oxide(rGO)in hydrothermal method and then Pd nanoparticles were loaded with incipient wetness impregnation.The rGO-TiO2composites supported Pd catalysts showed higher selectivity and productivity ofH2O2compared with Pd/TiO2because ofthe reduced hydrogenation rate of H2O2.
2.Experimental
2.1.Catalyst preparation
Graphene-TiO2(rGO-TiO2)composites with different rGO contents were prepared in a typical hydrothermal method.A certain amount of graphene oxide was well dispersed in deionized water/ethanol mixed solution with ultrasonic treatment,where the graphene oxide solution was acquired by the modified Hummer's method[34].TiO2(P25,20%rutile and 80%anatase,Degussa)was added into the above slurry under vigorous stirring.After 2 h,the suspension was transferred to a 100 ml Te flon-lined autoclave to heat at 180°C for 12 h.The resultant sample was regained by filtration and washed with deionized water,then it was dried at 110°C in vacuum oven overnight to obtain rGOTiO2composite.The composite of rGO and P25 was denoted as rGP-x,x represents the mass percentage of rGO in the composites.
The Pd/rGP-x catalysts used in this work were synthesized in the incipient-wetness impregnation method.PdCl2was chosen as metal precursors(Sigma–Aldrich,99.9%),which was dissolved in hydrochloric acid solution.An aqueous solution of PdCl42−was added into support dropwise with stirring until the paste was formed.After aging for several hours,the sample was dried at 110°C in vacuum oven overnight.Before the reaction,the catalyst was reduced at 300°C in H2flow.The theoretical loading of Pd for all the catalysts was 3 wt%.
2.2.Catalyst characterization
X-ray diffraction(XRD)spectrum was recorded on a Bruker D/8 Advance X-ray diffractometer working at a voltage of 40 kV and a current of 100 mA with CuKαradiation in the 2θ range of 10°–80°.Laser Raman spectroscopic measurements were performed on Horiba–Jobin Yvon Labram HR800 Raman spectrometer with a 514.5 nm Ar+laser.Fourier transform infrared(FTIR)spectra were carried out using Perkin-Elmer spectrometer in the frequency range of 4000–450 cm−1with a resolution of 4 cm−1.The morphologies and dispersion of particle size of the catalysts were examined by transmission electron microscopy(TEM:JEOL JEM-2100,Japan)at120 kV.Axis AXIS UltraDLD using AlKαX-rays source was employed to measure X-ray photoelectron spectroscopy(XPS)of catalysts.
2.3.Catalyst performance
The directsynthesis ofH2O2from H2and O2wasperformed in a glass reactor at atmospheric pressure and 10°C,which is similar to the one reported in the literature[17].In the process,the reagent gases with a total flow rate of 60 ml·min−1(H2:O2:N2=9:36:15)were blended in a pre-mixer and then imported into the reactor.50 mg catalyst was dispersed in reaction medium contained 60 ml ethanol and 0.38 ml concentrated H2SO4.The rate of agitation in the reactor was kept at 950 r·min−1to minimize masstransferresistance.Gaschromatography with thermal conductivity detector was used to analyze the conversion of H2on-line.The concentration of H2O2was detected by colorimetry after the complexation of the reaction slurry with a TiOSO4/H2SO4reagent.The selectivity of H2O2was calculated with the following formula:

Moreover,the hydrogenation and decomposition of H2O2over catalysts were also investigated in the similar reaction conditions described above.The initial H2O2added to reaction solvent had a concentration of 0.5 wt%.And the reactant gas was fixed with a flow of H2/N2(9:51 ml·min−1)for hydrogenation and a flow of N2(60 ml·min−1)for decomposition reaction.
3.Results and Discussion
3.1.Characterization
Raman spectra of P25,rGO,and rGP-x composites were displayed in Fig.1.It could be seen that both rGO and rGP-x composites showed two Raman bands at 1330 cm−1(D band)and 1590 cm−1(G band),which are attributed to the graphite substrate[35].And these two bands do not appear atthe spectrum ofP25.As expected,the intensity ofD peaks and G peaks of rGP-x composites reduced along with the decreasing rGO content in rGP-x composites.Even the content of rGO is only 0.025 wt%,the signal of D band and G band are still detectable in Raman spectra,which verifies the successful introduction of rGO in the composites.
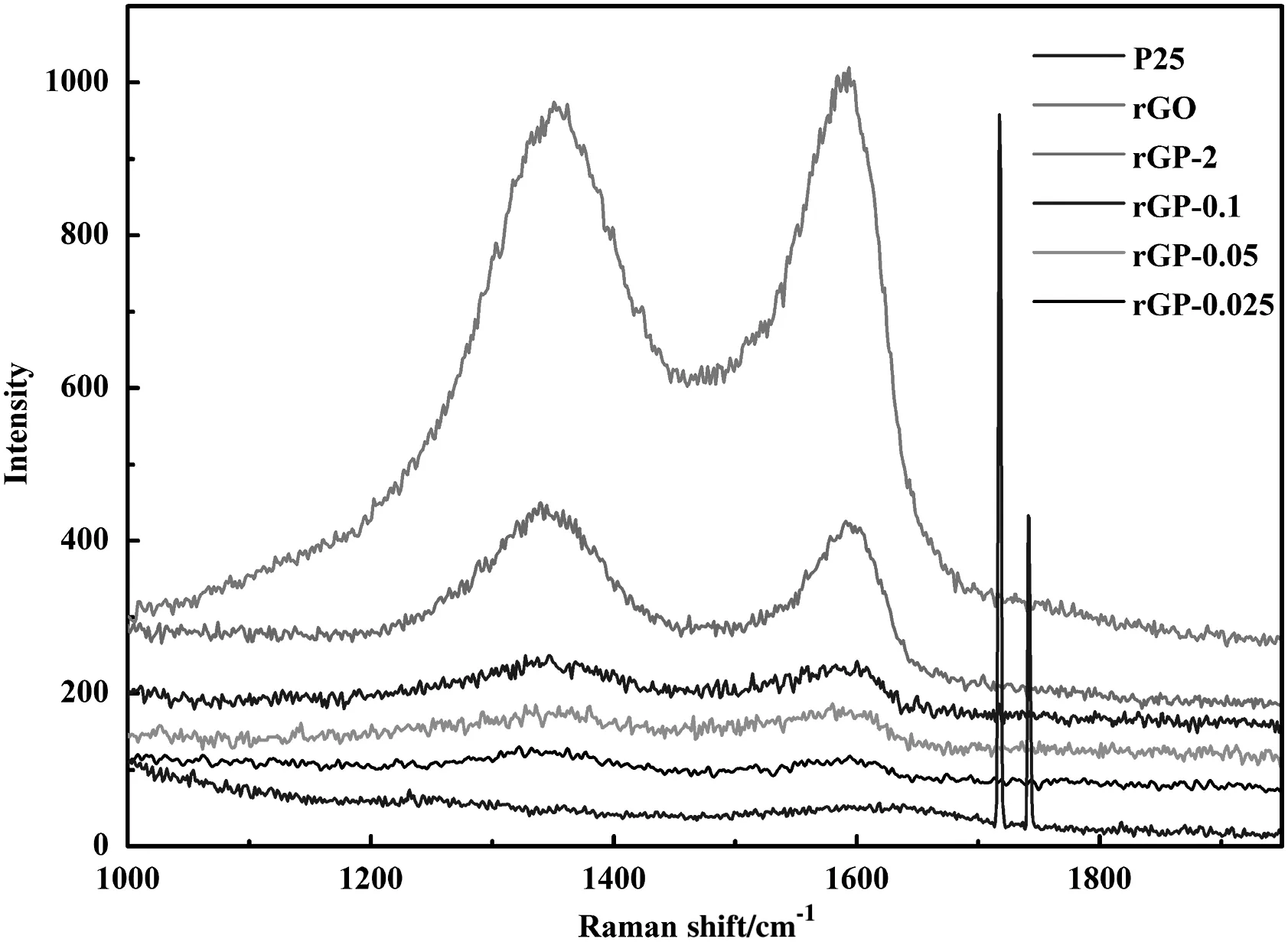
Fig.1.Raman spectra of series of rGP-x composites.
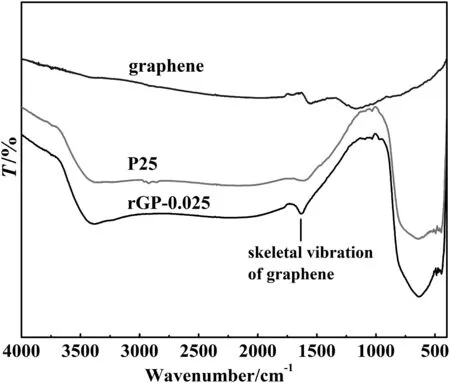
Fig.2.FTIR spectra of graphene,P25 and rGP-0.025 composite.
The FTIR spectra of P25,graphene,and rGP-0.025 were exhibited in Fig.2.The absorption bands located at around 1600 cm−1are both found in the curve of graphene and rGP-0.025,which is attributed to the skeletal vibration of the graphene sheets[36].It also indicates that the graphene oxide is reduced to graphene after the hydrothermal treatment.For pure P25 particles,the spectrum showed a peak around at 641 cm−1,which results from the vibration of the Ti--O--Ti bond.However,the absorption peak below 1000 cm−1of rGP-0.025 was much deeper than the corresponding peak of P25,which can be identified as the combination ofthe vibration ofTi--O--Tibond and Ti--O--C bond(798 cm−1)[36].In otherwords,thatmeans the rGOand P25 have been successfully chemically combined by the interaction between the residualcarboxylic groups on the surface ofgraphene oxide and the surface hydroxyl groups of P25 during the hydrothermal process.
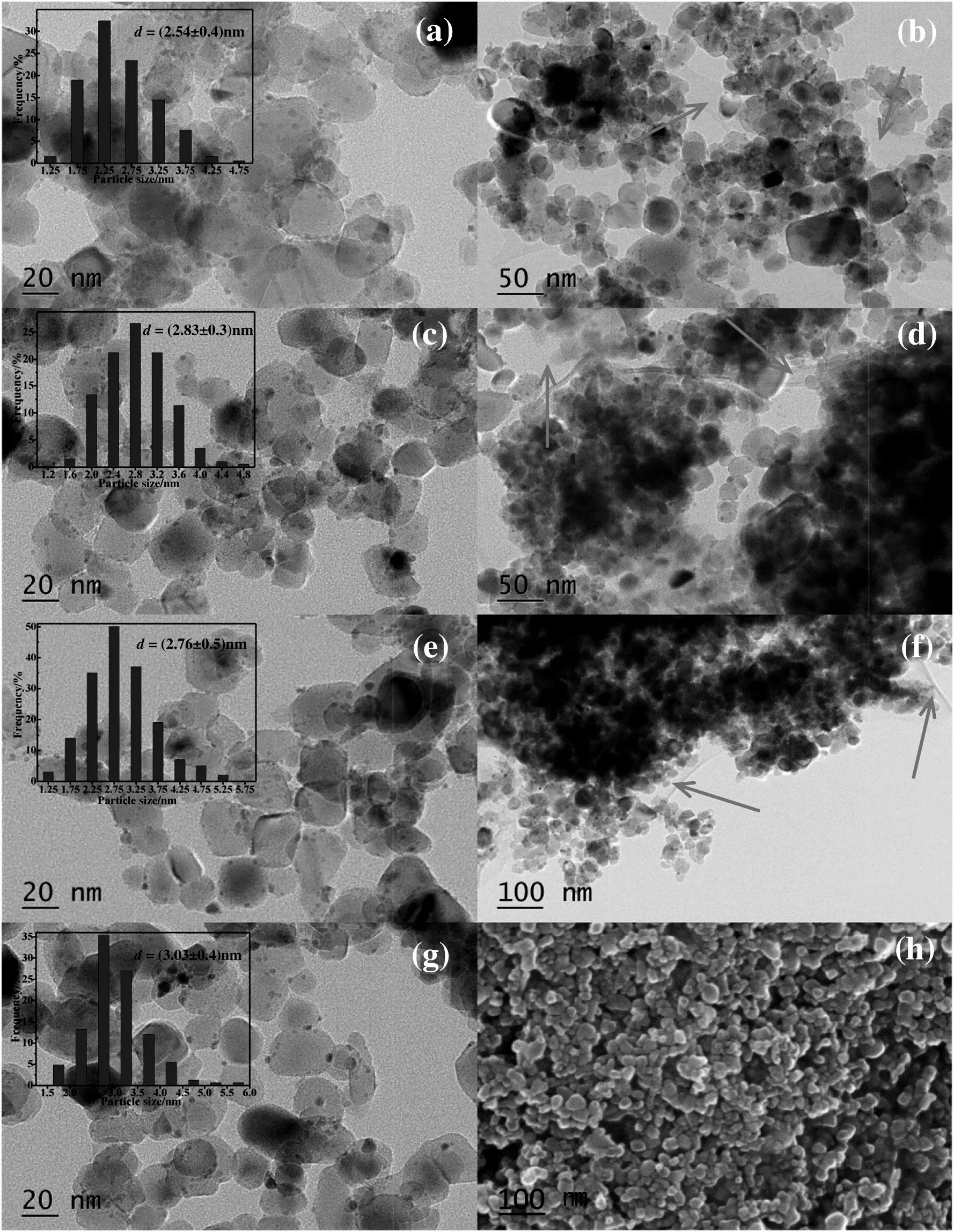
Fig.3.TEM images of series of Pd/rGP-0.5(a),(b),Pd/rGP-0.05(c),(d),Pd/rGP-0.025(e),(f)and Pd/P25(g),(h).
The morphology and distribution of the nanoparticles for Pd/rGP-x catalysts were detected by TEM as shown in Fig.3.The rGO sheets with some wrinkles can be found in Pd/rGP-x catalysts in Fig.3(b),(d),(f),as shown by the red arrows,which is consistent with the results of the Raman spectra.The microstructure of Pd/P25 particles was showed in Fig.3(h).It is revealed that the morphology of TiO2didn't change after hydrothermal treatment.After the statistics,the final mean particle size of Pd in Pd/rGP-0.5,Pd/rGP-0.05,Pd/rGP-0.025 and Pd/P25 catalysts were 2.54 nm,2.83 nm,2.76 nm,and 3.03 nm,respectively.It is illustrated that the Pd particles with small size disperse uniformly on the supports.There is also little difference among them,which reveals that the introduction of rGO has negligible influences on the particle size of Pd.
The XRDpatterns for series ofPd/rGP-x catalysts were given in Fig.4.The diffraction peaks of Pd/P25 were indexed to anatase(JCPDF 21–1272)and rutile(JCPDF 21–1276)[37].While almost nothing had changed in the XRD patterns of Pd/rGP-x after the hydrothermal treatment and introduction of rGO,compared with Pd/P25.It might be ascribed to the low content or obscured diffraction peak of rGO at 24.5°shielded by the peak of anatase TiO2at 25.4°[37].In addition,no diffraction peaks for Pd had been found in Fig.4,which indicated the good dispersion of Pd on supports.

Fig.4.XRD patterns of Pd/P25 and series of Pd/rGP-x catalysts.

Fig.5.XPS spectroscopy of catalysts:(1)Pd/rGP-0.5;(2)Pd/rGP-0.05;(3)Pd/rGP-0.025;(4)Pd/P25.
The XPS spectra of Pd3d for Pd/rGP-x catalysts were given in Fig.5.There are two pairs of peaks that appeared for Pd3d.One pairs center at the binding energy of 335.1 eV and 340.4 eV are assigned to metallic Pd03d5/2and Pd03d3/2respectively,the other one locate at 336.4 eV and 341.7 eV,corresponding to Pd2+[38].The atomic ratios of Pd0/Pd2+are believed to be a vital influence on the performance of catalysts,which can be quantified according to the peak area of fitting curves.The detailed results are shown in Table 1.Both Pd0and Pd2+are identified for all the catalysts,the ratio of Pd2+increased from 46.3%(Pd/P25)to 56.4%(Pd/rGP-0.5).It can be found that the introduction of rGO obviously modified the surface atomic ratios of Pd2+and Pd0of the catalysts.Due to the strong ability for electronic transmissions of rGO,it is facile to transfer electron from Pd which is located on the surface of rGP-x to the support[28,29].Therefore,it is easier to form the Pd2+species than Pd/P25[39].As a result,the ratio of Pd2+increases along with the rising content of rGO in catalysts.

Table 1 The quantified XPS data of the surface Pd atoms
The disperse state of the samples in the biphasic solvent of water and carbon tetrachloride were displayed in the Fig.6.The bottom layer is carbon tetrachloride phase and the upper layer is water phase.P25 powder is dispersed homogeneously in the phase of water while rGO is located at the bottom,indicating the different hydrophilicity and hydrophobicity.In addition,all the rGO-P25 composites disperse in the carbon tetrachloride phase;it means that the composites are all hydrophobic.And they tend to focus on the interface of biphasic solvent with the decreasing concentration of rGO in composites,indicating that the corresponding hydrophobicity becomes weaker.The difference in hydrophobicity brings about different interaction forces between H2O2and support,which has an important impact on the selectivity of H2O2according to the previous works in the literatures[24,40].The weaker interaction can decrease the hydrogenation rate of H2O2,resulting in the higher H2O2selectivity.Moreover,the hydrophobicity of catalyst surface could reduce the transfer resistance for the diffusion of H2to the catalysts surface[23].

Fig.6.The hydrophobic and hydrophilic performance ofrGP-x composites:1–rGO,2–rGP-2,3–rGP-0.5,4–rGO-0.1,5–rGP-0.05,6–rGP-0.025 and 7–P25.
3.2.Catalytic performance
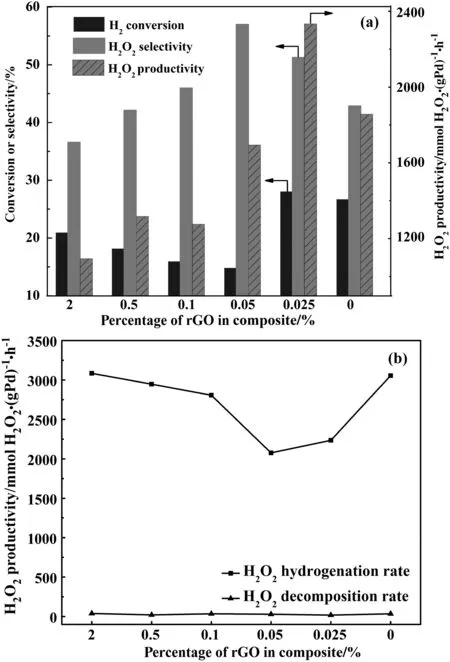
Fig.7.The H2 conversion and H2O2 selectivity,H2O2 productivity(a)and H2O2 hydrogenation and decomposition rate(b)of catalysts.
The catalytic performance and H2O2hydrogenation/decomposition of Pd/rGP-x catalysts were shown in Fig.7.As shown in Fig.7,when the rGO was introduced,the activities of the catalysts were significantly changed compared with Pd/P25.Firstly,the H2O2selectivity improved from 51%to 57%and H2conversion decreased evidently along with the amount of rGO in catalysts increasing from 0.025%to 0.05%.However,as the content of rGO continued to increase and were more than 0.05%,the H2O2selectivity presented a downward trend,while a small increase in the H2conversion was observed.To address this,the side reactions including the hydrogenation and decomposition of H2O2were investigated.The detailed data are given in Table 2.And the experimental data of Pd/P25 what we achieved as reference were similar with the data in the literature[17].The concentration of H2O2had a little change in N2flow,which reduced evidently when H2/N2mixture was bubbled into the reactor.The fact indicates that H2O2hydrogenation is the main cause for the decrease of the H2O2selectivity.According to the characterization results mentioned above,the surface ratio of Pd2+and hydrophobicity of catalysts increased with the rising content of rGO.It was reported that the Pd2+and hydrophobicity of catalysts surface is beneficial for depressing the hydrogenation of H2O2[18,23].On the other hand,the Pd0sites are favorable for the H2conversion.Thus,the H2O2selectivity of Pd/rGP-x(x=0.025,0.05)turned out an upward drift and was higher than Pd/P25,the H2conversion of Pd/rGP-0.05 decreased obviously and it led to a low H2O2productivity.However,H2O2is an active intermediate,and it is inclined to leave from the catalyst sites through liquid phase rather than gas phase[23].If the surface of catalysts is too hydrophobic,H2O2generated on the catalysts site will tend to hydrogenate successively to form H2O.Yet the higher hydrophobicity could reduce the transfer resistance for H2to reach the active sites[23].Therefore,as the content of rGO was more than 0.05%,the H2O2hydrogenation rate of catalysts was much higher than Pd/rGP-x(x=0.025,0.05)and the conversion of H2increased gradually.Then H2O2selectivity of Pd/rGP-x(x=0.1,0.5,2)decreased and showed a descending tendency.The change of H2conversion and H2O2selectivity indicates that only introducing appropriate amount of graphene is advantageous for H2O2synthesis.
For Pd/rGP-0.025,the hydrophobicity of catalyst surface caused by the introduction of rGO could reduce the transfer resistance of reactant gas and promote the desorption of H2O2.While compared with Pd/P25,a small decrease was observed in the ratio of Pd0of Pd/rGP-0.025.The combined effects of increased hydrophobicity and decreased ratio of Pd0resulted in that the H2conversion of Pd/rGP-0.025(28.1%)only increased a little than Pd/P25(26.2%).The H2O2hydrogenation rate of the Pd/rGP-0.025 was 2235.46 mmol H2O2·(g Pd)−1·h−1,which reduced a lot compared with Pd/P25.Ultimately,a H2conversion of 28%and a relative high H2O2selectivity of 51%towards Pd/rGP-0.025 brought about a H2O2productivity of 2333.2 mmol H2O2·(g Pd)−1·h−1,which increased by 26%than Pd/P25.
4.Conclusions
rGP-x(x=0,0.025,0.05,0.1,0.5,2,)supports with different rGO contents(x,wt%)were prepared in hydrothermal method.Then Pd/rGP-x catalysts prepared in an incipient wetness method were applied for the direct synthesis of H2O2from H2and O2.The ratio of Pd2+and the hydrophobicity of the catalysts increased along with the rising amount of rGO.Both the modified electronic structure of Pd and the hydrophobicity of the catalyst surface play an important role in enhancing the productivity of H2O2.The selectivity of H2O2appeared to increase firstly and then decrease at the content of 0.1 wt%,as the amount of rGO varied in the range of 0.025 wt%–2 wt%.It indicates that only the modified ratio of Pd2+/Pd0and hydrophobicity of catalysts caused byintroducing appropriate amount of rGO can achieve a high selectivity of H2O2.Among all the catalysts,the Pd/rGP-0.025 catalyst with relatively high selectivity of H2O2showed the largest H2O2productivity of 2333.2 mmol H2O2·(g Pd)−1·h−1,that increased by 26%compared with Pd/P25.Thus,the composites of reduced graphene oxide and TiO2could serve as a promising route forthe design of efficient catalysts.

Table 2 Catalytic performance of Pd catalysts supported on rGP-x composites
[1]J.K.Edwards,G.J.Hutchings,Palladium and gold–palladium catalysts for the direct synthesis of hydrogen peroxide,Angew.Chem.Int.Ed.47(2008)9192–9198.
[2]C.Samanta,Direct synthesis of hydrogen peroxide from hydrogen and oxygen:An overview of recent developments in the process,Appl.Catal.A Gen.350(2008)133–149.
[3]R.Hage,A.Lienke,Applications of transition-metal catalysts to textile and woodpulp bleaching,Angew.Chem.Int.Ed.45(2005)206–222.
[4]Y.Y.Lu,Y.Liu,B.W.Xia,W.Q.Zuo,Phenol oxidation by combined cavitation water jet and hydrogen peroxide,Chin.J.Chem.Eng.20(4)(2012)760–767.
[5]V.R.Choudhary,C.Samanta,Role of chloride or bromide anions and protons for promoting the selective oxidation of H2by O2to H2O2over supported Pd catalysts in an aqueous medium,J.Catal.238(2006)28–38.
[6]C.Samanta,V.R.Choudhary,Direct formation of H2O2from H2and O2and decomposition/hydrogenation of H2O2in aqueous acidic reaction medium over halide-containing Pd/SiO2catalytic system,Catal.Commun.8(2007)2222–2228.
[7]V.R.Choudhary,P.Jana,Direct oxidation of H2to H2O2over different supported PdO catalysts in aqueous acidic medium:In fluence of the reduction,calcination temperature and support of the catalyst on its net H2O2formation activity,Catal.Commun.9(2008)1624–1629.
[8]D.P.Dissanayake,J.H.Lunsford,The direct formation of H2O2from H2and O2over colloidal palladium,J.Catal.214(2003)113–120.
[9]Q.Liu,J.Lunsford,The roles of chloride ions in the direct formation of H2O2from H2and O2over a Pd/SiO2catalyst in a H2SO4/ethanol system,J.Catal.239(2006)237–243.
[10]Q.Liu,J.C.Bauer,R.E.Schaak,J.H.Lunsford,Supported palladium nanoparticles:An efficient catalyst for the direct formation of H2O2from H2and O2,Angew.Chem.Int.Ed.47(2008)6221–6224.
[11]P.Landon,P.J.Collier,A.J.Papworth,C.J.Kiely,G.J.Hutchings,Direct formation of hydrogen peroxide from H2/O2using a gold catalyst,Chem.Commun.(2002)2058–2059.
[12]G.Li,J.K.Edwards,A.F.Carley,G.J.Hutchings,Direct synthesis of hydrogen peroxide from H2and O2and in situ oxidation using zeolite-supported catalysts,Catal.Commun.8(2007)247–250.
[13]J.K.Edwards,E.Ntainjua,A.F.Carley,A.Herzing,C.J.Kiely,G.J.Hutchings,Direct synthesis of H2O2from H2and O2over gold,palladium,and gold–palladium catalysts supported on acid-pretreated TiO2,Angew.Chem.Int.Ed.48(2009)8512–8515.
[14]B.S.Jennifer,J.K.Edwards,N.N.Edwin,A.F.Carley,J.A.Herzing,G.J.Hutchings,Switching off hydrogen peroxide hydrogenation in the direct synthesis process,Science 23(2009)1037–1041.
[15]J.K.Edwards,J.Pritchard,L.Lu,M.Piccinini,G.Shaw,A.F.Carley,D.J.Morgan,C.J.Kiely,G.J.Hutchings,The direct synthesis of hydrogen peroxide using platinumpromoted gold-palladium catalysts,Angew.Chem.Int.Ed.53(2014)2381–2384.
[16]S.J.Freakley,J.H.Harrhy,L.Lu,D.A.Crole,D.J.Morgan,J.K.Edwards,A.F.Carley,A.Y.Borisevich,G.J.Hutchings,Palladium-tin catalysts for the direct synthesis of H2O2with high selectivity,Science 351(2016)965–968.
[17]L.K.Ouyang,P.F.Tian,G.J.Da,X.C.Xu,C.Ao,T.Y.Chen,Y.F.Han,The origin of active sites for direct synthesis of H2O2on Pd/TiO2catalysts:Interfaces of Pd and PdO domains,J.Catal.321(2015)70–80.
[18]V.R.Choudhary,C.Samanta,T.V.Choudhary,Direct oxidation of H2to H2O2over Pdbased catalysts:In fluence of oxidation state,support and metal additives,Appl.Catal.A Gen.308(2006)128–133.
[19]V.R.Choudhary,P.Jana,Direct oxidation of H2to H2O2over PdO/Al2O3catalysts in aqueous acidic medium:In fluence on H2O2formation of Pd loading,calcination temperature and reduction of catalyst and presence of halide anions,Catal.Commun.9(2008)2371–2375.
[20]V.R.Choudhary,C.Samanta,T.V.Choudhary,Influence of nature/concentration of halide promoters and oxidation state on the direct oxidation of H2to H2O2over Pd/ZrO2catalysts in aqueous acidic medium,Catal.Commun.8(2007)1310–1316.
[21]J.Lunsford,The direct formation of H2O2from H2and O2over palladium catalysts,J.Catal.216(2003)455–460.
[22]S.Chinta,A mechanistic study of H2O2and H2O formation from H2and BO2catalyzed by palladium in an aqueous medium,J.Catal.225(2004)249–255.
[23]L.Fu,K.T.Chuang,R.Fiedorow,Selective oxidation ofhydrogen to hydrogen peroxide,Stud.Surf.Sci.Catal.72(1992)33–41.
[24]B.Z.Hu,W.P.Deng,R.S.Li,Q.H.Zhang,Y.Wang,F.Delplanque-Janssens,D.Paul,F.Desmedt,P.Miquel,Carbon-supported palladium catalysts for the direct synthesis of hydrogen peroxide from hydrogen and oxygen,J.Catal.319(2014)15–26.
[25]J.K.Edwards,A.Thomas,B.E.Solsona,P.Landon,A.F.Carley,G.J.Hutchings,Comparison of supports for the direct synthesis of hydrogen peroxide from H2and O2using au–Pd catalysts,Catal.Today 122(2007)397–402.
[26]L.K.Ouyang,G.J.Da,P.F.Tian,T.Y.Chen,G.D.Liang,J.Xu,Y.F.Han,Insight into active sites of Pd–Au/TiO2catalysts in hydrogen peroxide synthesis directly from H2and O2,J.Catal.311(2014)129–136.
[27]S.J.Tauster,S.C.Fung,R.L.Garten,Strong metal-support interactions:Group 8 noble metals supported on titanium dioxide,J.Am.Chem.Soc.100(1978)170–175.
[28]Q.S.Yang,Y.J.Liao,L.L.Mao,Kinetics of photocatalytic degradation of gaseous organic compounds on modified TiO2/AC composite photocatalyst,Chin.J.Chem.Eng.20(3)(2012)572–576.
[29]M.J.Sampaio,L.M.Pastrana-Martinez,A.M.Silva,J.G.Buijnsters,C.Han,C.G.Silva,A.C.Carabineiro,D.D.Dionysiou,J.L.Faria,Nanodiamond–TiO2composites for photocatalytic degradation of microcystin-LA in aqueous solutions under simulated solar light,RSC Adv.5(2015)58363–58370.
[30]A.H.Castro,F.Guinea,M.R.Peres,K.S.Novoselov,A.K.Geim,The electronic properties of graphene,Rev.Mod.Phys.81(2009)109–162.
[31]Y.Nishina,J.Miyata,R.Kawai,K.Gotoh,Recyclable Pd–graphene catalyst:Mechanistic insights into heterogeneous and homogeneous catalysis,RSC Adv.2(2012)9380–9382.
[32]L.M.Pastrana-Martínez,A.T.Silva,N.N.C.Fonseca,J.R.Vaz,J.L.Figueiredo,J.L.Faria,Photocatalytic reduction of CO2with water into methanol and ethanol using graphene derivative–TiO2composites:Effect of pH and copper(I)oxide,Top.Catal.59(2016)1279–1291.
[33]G.D.Jiang,Q.Chang,F.F.Yang,X.Y.Hu,H.Q.Tang,Sono-assisted preparation of magnetic ferroferric oxide/graphene oxide nanoparticles and application on dye removal,Chin.J.Chem.Eng.23(2015)510–515.
[34]W.S.Hummers,R.E.Offeman,Preparation of graphitic oxide,J.Am.Chem.Soc.80(1958)1939.
[35]Y.X.Zhang,H.P.Li,X.L.Cui,Y.H.Lin,Graphene/TiO2nanocomposites:synthesis,characterization and application in hydrogen evolution from water photocatalytic splitting,J.Mater.Chem.A 20(2010)2801–2806.
[36]H.Zhang,X.J.Lv,Y.M.Li,Y.Wang,J.H.Li,P25-graphene composite as a high performance photocatalyst,ACS Nano 4(1)(2010)380–386.
[37]Y.H.Zhang,Z.R.Tang,X.Z.Fu,Y.Xu,TiO2graphene nanocomposites for gas-phase photocatalytic degradation of volatile aromatic pollutant is TiO2graphene truly different from other TiO2carbon composite materials,ACS Nano 4(12)(2010)7303–7314.
[38]C.D.Wagner,W.M.Riggs,L.E.Davis,J.F.Moulder,in:G.E.Muilenberg(Ed.)Handbook of X-ray Photoelectron Spectroscopy,110,Perkin-Elmer,Minnesota,1979.
[39]D.D.Zhou,L.Ding,H.Cui,H.An,J.P.Zhao,Q.Li,Fabrication of Pd/TiO2-multi wall carbon nanotubes catalyst and investigation of its electrocatalytic activity for formic acid oxidation,J.Power Sources 222(2013)510–517.
[40]R.Tu,S.Y.Chen,W.Cao,S.Y.Zhang,L.C.Li,T.Ji,J.Li,X.H.Lu,The effect of H2O2desorption on achieving improved selectivity for direct synthesis of H2O2over TiO2(B)/anatase supported Pd catalyst,Catal.Commun.89(2017)69–72.
 Chinese Journal of Chemical Engineering2018年3期
Chinese Journal of Chemical Engineering2018年3期
- Chinese Journal of Chemical Engineering的其它文章
- Numerical investigation on flow and heat transfer characteristics of corrugated tubes with non-uniform corrugation in turbulent flow
- Investigations on pool boiling critical heat flux,transient characteristics and bonding strength of heater wire with aqua based reduced graphene oxide nano fluids
- Heavy metals adsorption by banana peels micro-powder:Equilibrium modeling by non-linear models
- Potential aspect of rice husk biomass in Australia for nanocrystalline cellulose production
- Fouling evaluation on membrane distillation used for reducing solvent in polyphenol rich propolis extract
- Investigation on a vertical radial flow adsorber designed by a novel parallel connection method☆
