Effect of boron addition on the MoO3/CeO2–Al2O3 catalyst in the sulfur-resistant methanation☆
Baowei Wang*,Wenxia Yu,Weihan Wang,Zhenhua Li,Yan Xu,Xinbin Ma
Key Laboratory for Green Chemical Technology of the Ministry of Education,School of Chemical Engineering and Technology,Tianjin University,Tianjin 300072,China
1.Introduction
In recentyears,the crises of energy and environment problems have been frequently mentioned.Many efforts were made to improve the present situation,including the effective utilization of the extant resources,researching the reproducible,clean energy and so on.Combined with the energy reserves of China(rich coal,meager oil and little gas)[1],put into action to improve utilization efficiency of coal.It has been proved in many excellent studies that synthetic natural gas(SNG)is a source of clean energy which is synthesized from the coal.This process includes the coal gasification,the purification of gas,and methanation.Methanation is a critical step for the synthesis of SNG[2].It notonly takes full advantage of coal butalso reduces the emission of greenhouse gas.Nowadays,there are mainly two kinds of catalysts for methanation including the Ni catalysts and Mo catalysts.The Nibased catalysts have been applied commercially in industry because of their higher CO conversion and CH4selectivity.However,the feed gas has to have a higher H2/CO(e.g.H2/CO=3)ratio and the content of H2S needs to be under 0.1 μl·L−1with the traditional Ni based catalysts.In this point,the novel catalysts should be developed to overcoming the above-mentioned limits.Recently,Mo-based catalysts have attracted more and more attention due to their good property of sulfur resistance and developed catalytic activity of methanation.It is mentioned that MoS2catalyst has been used in hydrotreating reaction,such as HDS and water-shift-gas[3–5].In the sulfur-resistant methanation,the CO hydrogenation reaction on Mo-based catalysts was carried out according to the following reaction(1),described as directmethanation[6].

It is important to highlight that MoO3instead of MoS2is welldispersed on the supports in our study,and molybdenum trioxide has no catalytic activity for methanation.Hence,it is necessary for MoO3catalysts to be presul fided with the H2S/H2before the evaluation of catalysts.The catalytic activity in the sulfur-tolerant methanation is lower than that of Ni catalysts,which is the mainly challenge for the Mo-based catalysts.Therefore,great efforts have been focused on improving the catalytic activity,including the employment of CeO2–Al2O3composite supports[7],diversification of the Mo precursors[8],and using some promoters like Co[9,10]and citric acid[11].Currently,the catalysts modified with boron has been demonstrated in plenty of study that the addition of boron could improve the monolayer loading over the alumina and weaken the interaction between the active component and supports[12].Morihige and Akai[13]have reported the effect of boron on MoO3/Al2O3catalysts,indicating that the boron addition actually promoted the Mo dispersion on the surface of γ-Al2O3and reduced the amount of the crystalline MoO3.In addition,the suitable contents ofB2O3would cause the little changes of morphological parameters on the supported-Mo catalysts[14],as wellas NiMo/Al2O3catalysts[15,16].It was proposed that the addition of boron could weaken the interactions between the molybdenum oxides and Al2O3[14,17].The intrinsic activity of cobalt–molybdenum catalysts improved in the TOF on CoMoS phase when the catalysts presulfided at 673 K for the hydro desulfurization of thiophene.Similarly,Chen et al.[18]studied the supported molybdenum catalysts modified by boron acid in the HDS,and the results suggested the acidity on the alumina surface had changed with the means of infrared spectroscopy,which has a positive influence on the active phase,and these are in agreement with Yu et al.[19].There were also numerous studies about the boron modified catalysts in the hydrodenitrogenation[20],hydrogenation[21,22],dry reforming of methane[23],and the oxygen-free conversion of methane[24].The addition of B2O3could improve actually the catalytic activity.
There are few studies on the impact of B2O3on the sulfur-resistant methanation so far.This study would try to modify the MoO3/CeO2–Al2O3catalysts with B2O3.In the present work,it was devoted to the influence of the B2O3modified catalysts on the dispersion of active component,acidic sites on the surface,and catalytic properties.
2.Experimental
2.1.Catalyst preparation
2.1.1.The preparation of composite supports
Several CeO2–Al2O3composite supports with different concentrations of B2O3were prepared by deposition precipitation,as described in our previous study[7].The concentration of the CeO2in the support was 25 wt%and the loading of MoO3was 20 wt%.The high CO conversion and selectivity of CH4were confirmed by a pre-experiment for sulfur-resistant methanation.First,Al(NO3)3·9H2O and Ce(NO3)3·9H2O(Kemiou Chemical Reagent Co.Ltd.,Tianjin,China)[C(Ce+3+Al+3)=1 mol·L−1]were dissolved in deionized water.Then,15 wt%ammonia solution was added with continuous stirring.First,the suspension was agitated for about 30 min as adding the desired boron acid,followed the mixture standed for 60 min.The products were filtrated,washed with deionized water until the pH of the filtrates was close to 7.These products were first dried at room temperature for 24 h.After drying at 120 °C overnight,the solids were heated to 600 °C,and kept for 4 h in a muffle furnace.Finally,the samples were crushed and sieved to granules(≤180 meshes)for synthesis of catalysts.
2.1.2.Preparation of catalysts
MoO3/CeO2–Al2O3catalysts were prepared with impregnation of the composite support with a solution of ammonium heptamolybdate((NH4)6Mo7O24·4H2O)(Kemiou Chemical Reagent Co.Lit.,Tianjin,China),as the precursor of MoO3.The impregnated samples were dried at room temperature,dried at 120 °C for 4 h,and calcined at 600 °C for 4 h.The 20%MoO3/25%CeO2–Al2O3catalysts with different contents of B2O3were denoted as Mo/CeAl-x,where x was the B2O3content in mass percent.
2.2.Catalyst characterization
The prepared catalysts were characterized by N2-physisorption analysis,X-ray diffraction patterns(XRD),NH3-TPD,and H2-TPR.
N2-physisorption analysis was performed on a Tristar-3000 apparatus(Micromeritics,USA),in order to acquire the textural properties,such the BET surface and pore size.The trace water and the foreign gas were removed out from the samples(about 0.1–0.2 g)at 300 °C for 3 h,before any measurement was carried out.
XRD analysis was characterized by a RigakuD/max-2500 X-ray diffractometer(Rigaku,Japan),which was with a Ni- filtered Cu-Kαradiation source(λ=0.154 nm).The sweep angle was from 5(°)to 90(°).The crystallite sizes of all catalysts were calculated by the Scherrer equation and the values of the full-width in the XRD spectra.
The temperature programmed reduction(TPR)analysis was conducted by an Auto Chem 2910 analyzer(Micromeritics,USA).Before the measurements were collected,the sample(approximately 200 mg)was swept with nitrogen at 200°C,aimed to remove the water traces and some foreign gas absorbed on the surface of catalysts,then cooled to 60°C.The reduction temperature of samples was increased up to nearly 1000 °C at the heating rate of 10 °C·min−1,this process was kept in a gas mixture of 10 vol%H2/Ar.
NH3-TPD analysis was performed using a TP-5076 TPD/TPR dynamic adsorption instrument(Xianquan,Tianjin,China).With the same goals as the pretreatment,the samples with N2-physisorption analysis were swept with argon(99.999%)at 400°C for 60 min and cooled to 100°C,before the measurements were taken out.Then,the flow of ammonia was passed through the product at 100°C for 60 min,and the gas was replaced by argon,in order to get rid of the adsorbed NH3.The process of desorption was performed by heating the products from 100°C to 700 °C at the rate of 10 °C·min−1.The quantity of desorbed ammonia was tested by a TCD detector.
The Raman analysis of all spent Mo/CeAl-x catalysts was performed by a DXR Raman spectrometer(Thermo Fisher,USA)and the emission line at 532 nm was used,in order to obtain information about the MoS2species after the methanation reaction.
2.3.Evaluation of catalytic activity
The catalytic activity for sulfur-methanation had been evaluated in a fixed-bed continuous- flow reactor,whose inner diameter was 12 mm and length was 700 mm.The loading of catalysts was 3 ml[pellets,830–380 μm(20–40 meshes)].Before catalytic evaluation,all catalysts were firstly sulfided with 3 vol%H2S/H2,which was described by Jiang et al.[25].And then the sul fide gas was switched into the syngas.The catalytic activity measurements were carried out under the following conditions:syngas(H2/CO ratio=1.0)including 0.27 vol%H2S and 10 vol%N2,P=3.0 MPa,GHSV=5000 h−1,T=550 °C.The out gas was analyzed on-line using a GC equipped with a thermal conductivity detector(TCD)and flame ionization detector(FID).
Finally,the CO conversion and selectivity to hydrocarbon were calculated with the following equation:
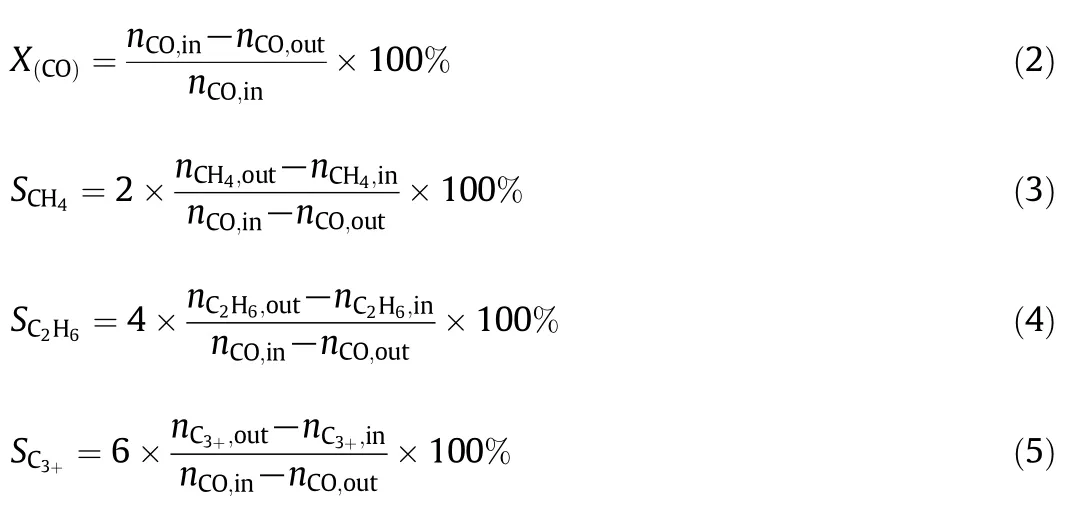
3.Results
3.1.Textural properties of the Mo/CeAl-x catalysts
As shown in Table 1,the information about the BET surface areas,pore volume and pore size of all the samples can be obtained.Thetextural properties of all catalyst samples were changed by adding boron into CeO2–Al2O3composite supports.It can be seen from Table 1 that the BET surface area slightly changed from 103 m2·g−1to 123 m2·g−1with the loading of B2O3under 0.5 wt%,but decreased significantly to 61 m2·g−1with a further increase of B2O3to 2.0 wt%.The pore size of Mo/CeAl-2.0 catalysts was largerthan that of other catalysts,and it was mainly caused by penetrations of the dispersed super fluous B2O3into the pores of support,resulting in passage through some hole and partial collapse of structure.
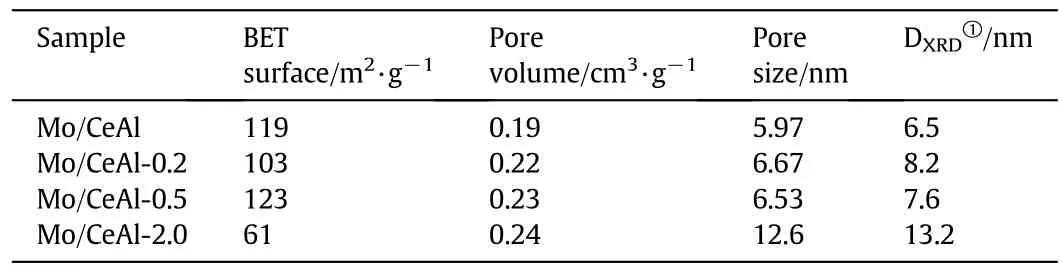
Table 1 Textural properties and DXRD for the Mo/CeAl-x catalysts
3.2.XRD
The XRD spectra of the Mo/CeAl-x catalysts are shown in Fig.1.All catalysts revealed the diffraction peaks at 2θ =28.5°,33.3°,47.6°,and 56.5°,which were assigned to CeO2species[25],and one peak of γ-Al2O3was found at 67.1°(PDF:08-0384).Compared to MoO3/CeO2–Al2O3,the diffraction peak of crystalline MoO3was not detected at 23.1°.Thus,we could consider that the molybdenum species were dispersed easily on the CeO2–Al2O3supports after adding B2O3.It should be pointed out that the diffraction peak of B2O3at 45.6°(PDF:80-0338)was detected when the B2O3loading was 2.0 wt%.This indicated that the addition of B2O3was presented as highly dispersed on the surface of support when B2O3content was below 2.0 wt%.The crystalline B2O3was found in Mo/CeAl-2.0 catalysts.This suggested that 2.0 wt%boron was super fluous,which was in agreement with BET analysis.
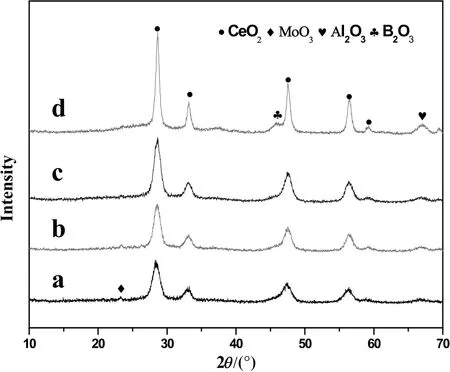
Fig.1.XRD spectra of MoO3/CeO2–Al2O3 with and without boron:Mo/CeAl(a),Mo/CeAl-0.2(b),Mo/CeAl-0.5(c),Mo/CeAl-2.0(d).
The CeO2crystallite sizes of the series of Mo/CeAl catalysts were calculated from their XRD FWHMs,which were summarized in Table 1.
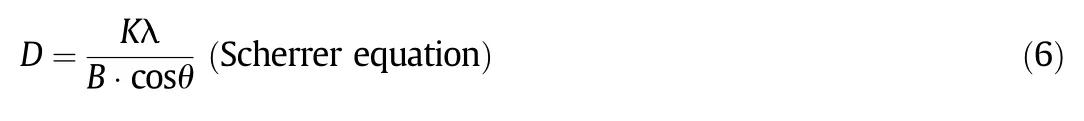
As shown in Table 1,the CeO2crystallite size of catalysts was larger than that of the Mo/CeAl-x(x=0,0.2,0.5 wt%)catalysts.This indicated that the 2.0 wt%loading of B2O3may cause the increased aggregation of CeO2,compared with the catalysts with the addition of B2O3less than 0.5 wt%.And the CeO2particles were dispersed unevenly resulting from the growth of CeO2crystallite size.
3.3.H2-TPR
TPR measurements were performed in order to investigate the dispersion and state of Mo species on the surface of composite supports,which were modified with boron addition.Fig.2 shows the TPR patterns of the MoO3/CeO2–Al2O3with various B2O3contents.Generally,the TPR patterns of Mo catalysts showed the lower temperature peak at 490°C or the higher temperature peak at about 600°C.For the reduction of Mo species,the H2consumption peaks contained the reduction of octahedrally coordinated Mo+6to Mo+4,which were well-dispersed on the surface of supports between 350 and 560°C,whereas the reduction of crystalline of MoO3occurred atabout600°C[25].The reason for the migration of H2absorption peak was the stronger interaction of crystalline MoO3than that of amorphous form.As for Mo/CeAl catalysts,there was only one peak at 600°C,indicating that large amounts of crystalline MoO3existed on the surface of catalysts without B2O3.The temperature of H2consumption peak decreased from 600 °C to 490 °C with an increase in B2O3concentration from 0.0 to 0.5 wt%,which indicated that the higher amounts of amorphous Mo species were dispersed on the supports in the absence of boron.It also suggested that the addition of boron in the MoO3/Al2O3catalysts weakened the interaction between active component and the supports[12].As for the Mo/CeAl-2.0 catalysts,the H2consumption peak was at 570°C which was higher than that of Mo/CeAl-0.5 catalysts.In combination with the results of XRD,the crystallite size of CeO2sharply increased for Mo/CeAl-2.0 catalysts,resulting in uneven distribution ofCeO2on the Al2O3surface.This result led to the formation of Al2(MoO4)3,which caused the stronger interaction between active component and the supports[26].
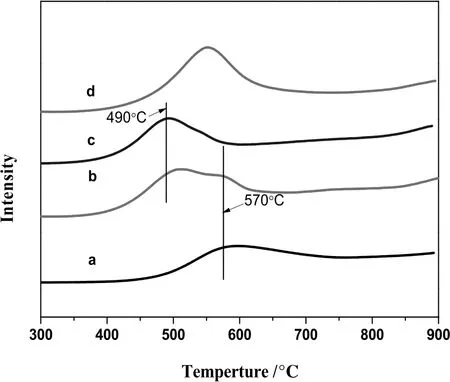
Fig.2.TPR profiles of Mo/CeAl-x catalysts:Mo/CeAl(a),Mo/CeAl-0.2(b),Mo/CeAl-0.5(c),Mo/CeAl-2.0(d).
3.4.NH3-TPD
The NH3-TPD profiles of the Mo/CeAl catalysts with various B2O3contents were shown in Fig.3,which were divided into the low temperature and high-temperature regions,respectively.These corresponded to weak and strong acid sites.According to previous studies[27,28],ammonia could be adsorbed on the acid sties of catalysts,and the peaks of NH3desorption increased by increasing the number of acid sites on the surface of catalysts.It can be seen in Fig.3 and Table 2 that the total acidities of Mo/CeAl-x catalysts with B2O3were higher than those of catalysts without B2O3.The Mo/CeAl catalysts exhibited two desorption peaks,one at the range of 200–400 °C and another at above 500°C which indicated that the weak and strong acid centers existed on the surface of Mo/CeAl catalysts.As for the catalysts with the loading of B2O3under 0.5 wt%,the higher temperature peak ascribed to the strong acid center almost disappeared and the amount of weak acid centers increased obviously,which was revealed in Table 2.However,increasing the addition of B2O3,the amount of weak acid sites of the Mo/CeAl-2.0 catalysts sharply decreased and the strong acid sites were found again.These indicated that the addition of B2O3could actually change the proportion of weak and strong acid sites.
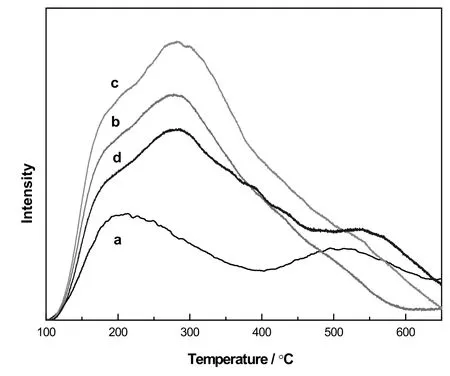
Fig.3.NH3-TPD profiles of Mo/CeAl-x catalysts:Mo/CeAl(a),Mo/CeAl-0.2(b),Mo/CeAl-0.5(c),Mo/CeAl-2.0(d).
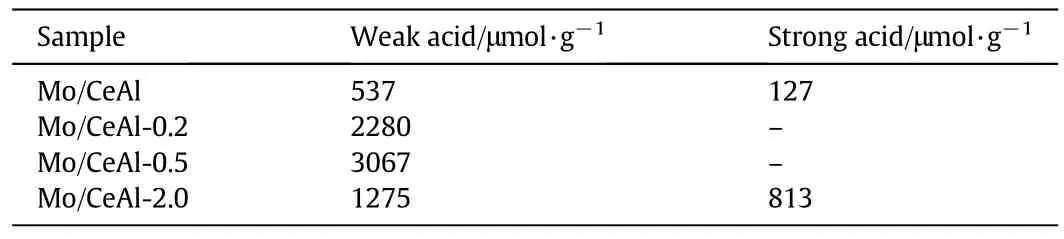
Table 2 Acidity amount of Mo/CeAl-x catalysts
It was proved that the acidity on the catalysts had a direct influence on the properties of molybdenum sulfide[18],which was in favor for hydrogenation activity of the active phase.Thus in the sulfur-resistant methanation,the number of weak acid of Mo/CeAl catalysts with B2O3loading under 0.5 wt%was greater in those of the Mo/CeAl catalysts,resulting in better dispersion of active Mo species which was prove by the results of H2-TPR and better catalytic performance.Although the number of strong acid sites of the Mo/CeAl-2.0 catalysts and crystalline size of CeO2were more than those of the Mo/CeAl-x(x=0.2,0.5)catalysts,suggesting the superfluous boron was harmful to the catalytic performance,the correlation between the contents of acidity and catalytic performance was reported in study[20,28].The complete details of the catalytic activity caused by boron would be discussed below.
3.5.Raman
The Raman profiles of spent Mo/CeAl-x catalysts with various B2O3contents were displayed in Fig.4.It was seen that all spent catalysts with and without boron revealed peaks found at 379 cm−1and 402 cm−1,which were assigned to MoS2species according to the literature[29].In addition,the intensity of peaks of sulfide Mo changed significantly as the catalysts were added into boron and these Raman peak intensities of Mo/CeAl-0.5 catalysts were higher than those of other catalysts in Fig.4,it was indicated that the amount of MoS2on the surface of Mo/CeAl-x catalysts increased clearly when the contents of B2O3increased up to 0.5 wt%,followed by a decrease with a further increase of B2O3.Compared with the results of XRD,H2-TPR and NH3-TPD,the addition of boron had a positive effect on the dispersion of active catalytic precursor and interaction between support and Mo species which could improve the formation of disulfide Mo.
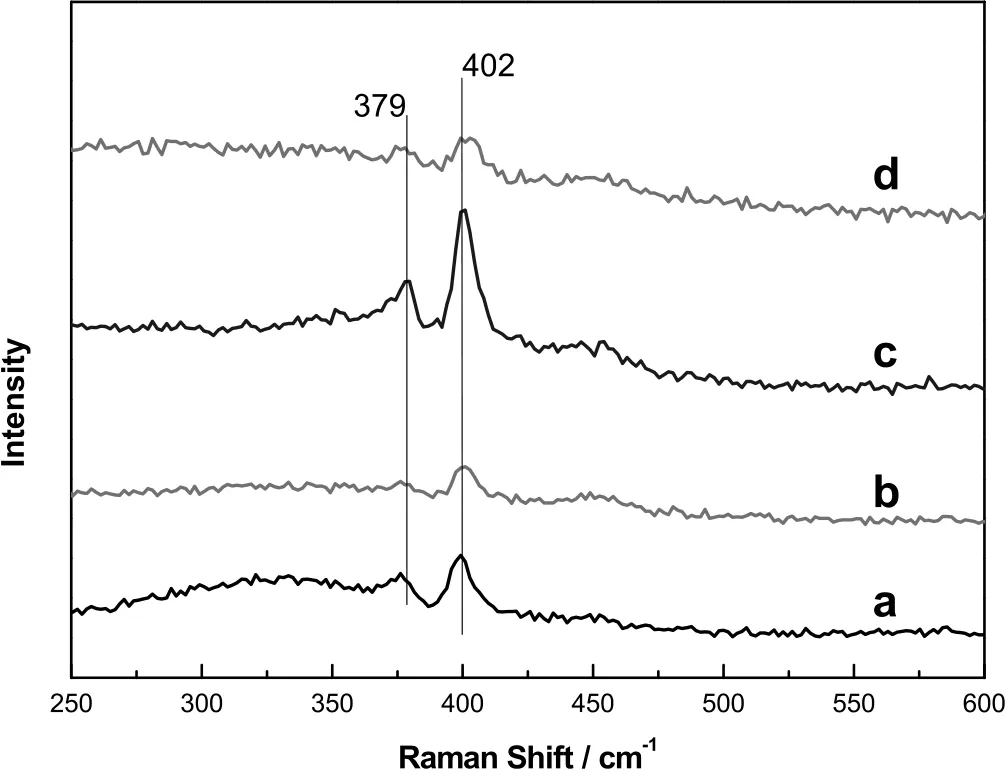
Fig.4.Raman profiles of spent Mo/CeAl-x catalysts:Mo/CeAl(a),Mo/CeAl-0.2(b),Mo/CeAl-0.5(c),Mo/CeAl-2.0(d).

Fig.5.CO conversion and selectivity to hydrocarbon of Mo/CeAl-x catalysts:Mo/CeAl(a),Mo/CeAl-0.2(b),Mo/CeAl-0.5(c),Mo/CeAl-2.0(d).
3.6.Effect on the catalytic performances of Mo/CeAl catalysts
The effect of boron on the CO conversion and selectivity to hydrocarbon over the Mo/CeAl-x catalysts is displayed in Fig.5.As shown in Fig.5,the catalytic activity on the sulfur-resistant methanation increased from 47%to 62%as the B2O3contents rose up to 0.5 wt%,however the conversion of CO decreased with increasing boron addition to 2.0 wt%.In other words,the catalysts modified by 0.5 wt%B2O3showed the highest catalytic activity.It was noted that the catalysts with 0 wt%and 0.2 wt%B2O3exhibited fairly similar activity toward methanation.However,the catalysts modified by 2.0 wt%showed a drastic decline as seen from Fig.5,which showed the lowest CO conversion,about 35%.This indicated that the addition of boron can improve the CO conversion because of the satisfactory dispersion of Mo oxides and the weak interaction between active phase and supports.The reason for the sharp decrease on the catalytic activity was that the interaction between the Mo species and supports was enhanced and the crystalline MoO3was formed.The CeO2layer had not been formed,maybe resulting from the formation of Al2(MoO4)3.This was in agreement with the results of H2-TPR and XRD.Further,it was shown that the addition of B2O3could make no significant effect on the selectivity to hydrocarbons.As for all Mo/CeAl-x catalysts,the selectivity of CH4maintained a high level about 95%while trace of C2H6and C3+was formed.
4.Conclusions
MoO3/CeO2–Al2O3catalysts with various contents of B2O3in the sulfur-resistant methanation were investigated.The study found that the addition of B2O3could change the percentage of acid sites on the surface of catalysts.As for sulfur-resistant methanation,the Mo/CeAl-0.5 catalysts showed the highestcatalytic activity and the COconversion could increase up to 62%.The catalysts with the B2O3loading under 0.5 wt%increased the proportion of weak acid sites while the strong acid sites nearly disappeared,resulting in the weak interaction between the active components and CeAl supports.However for Mo/CeAl-2.0 catalysts,the strong acid sites on the surface of catalysts were found again and the largestcrystalline size of CeO2led to the uneven distribution of CeO2and the formation of Al2(MoO4)3.Thus,these indicated that a small number of B2O3could increase the percentage of weak acid site strength which was in favor of the higher sulfur-resistant methanation activity,and strong acid sites were disadvantageous to the sulfur-resistant methanation.
[1]J.Kopyscinski,J.T.Schildhauer,M.A.Biollaz,Production of synthetic natural gas(SNG)from coal and dry biomass—a technology review from 1950 to 2009,Fuel 89(8)(2010)1763–1783.
[2]M.Tao,Z.Xin,X.Meng,Z.C.Bian,Y.H.Lv,Highly dispersed nickel within mesochannels of SBA-15 for CO methanation with enhanced activity and excellent thermostability,Fuel 188(2017)267–276.
[3]Y.Okamoto,S.Y.Kawano,M.Satoh,T.Kubota,Preparation of Co–Mo/Al2O3model sul fide catalysts for hydrodesulfurization and their application to the study of the effects of catalyst preparation,J.Catal.217(1)(2003)12–22.
[4]M.Nagai,K.Matsuda,Low-temperature water–gas shift reaction over cobalt–molybdenum carbide catalyst,J.Catal.238(2)(2006)489–496.
[5]P.Afanasiev,The in fluence of reducing and sul fiding conditions on the properties of unsupported.MoS2-based catalysts,J.Catal.269(2)(2010)269–280.
[6]B.W.Wang,G.Z.Ding,Y.G.Shang,J.Lv,H.Y.Wang,E.D.Wang,Z.H.Li,X.B.Ma,S.D.Qin,Q.Sun,Effects of MoO3loading and calcination temperature on the activity of the sulphur-resistant methanation catalyst MoO3/γ-Al2O3,Appl.Catal.A Gen.431–432(2012)144–150.
[7]B.W.Wang,Y.G.Shang,G.Z.Ding,J.Lv,H.Y.Wang,E.D.Wang,Z.H.Li,X.B.Ma,S.D.Qin,Q.Sun,Effect of the ceria–alumina composite support on the Mo-based catalyst's sulfur-resistant activity for the synthetic natural gas process,React.Kinet.Mech.Catal.106(2)(2012)495–506.
[8]H.Y.Wang,Z.H.L.,B.W.Wang,X.B.Ma,S.D.Qin,S.L.Sun,Q.Sun,Precursor effect on catalytic properties of Mo-based catalyst for sulfur-resistant methanation,Korean J.Chem.Eng.31(12)(2014)2157–2161.
[9]J.L.Dubois,S.Fujieda,Effects of boron in Co-Mo/B-Al2O3hydrotreatment catalysts,Catal.Today 29(1–4)(1996)191–195.
[10]B.W.Wang,Y.Q.Yao,M.H.Jiang,Z.H.Li,X.B.Ma,S.D.Qin,Q.Sun,Effect of cobalt and its adding sequence on the catalytic performance of MoO3/Al2O3toward boron methanation,J.Energy Chem.23(1)(2014)35–42.
[11]B.W.Wang,D.J.Meng,W.H.Wang,Z.H.Li,X.B.Ma,Effect of citric acid addition on the MoO3/CeO2–Al2O3catalyst for sulfur-resistant methanation,J.Fuel Chem.Technol.44(12)(2016)1479–1484.
[12]U.Usman,M.Takaki,T.Kubota,Y.Okamoto,Effect of boron addition on a MoO3/Al2O3catalyst,Appl.Catal.A Gen.286(1)(2005)148–154.
[13]H.Morihige,Y.Akai,Effect of boron addition on the state and dispersion of Mo supported on alumina,Bull.Soc.Chim.Belg.104(4–5)(1995)253–257.
[14]Usman,T.Kubota,Y.Araki,K.Ishida,Y.Okamoto,The effect of boron addition on the hydrodesulfurization activity of MoS2/Al2O3and Co–MoS2/Al2O3catalysts,J.Catal.227(2)(2004)523–529.
[15]D.Ferdous,A.K.Dalai,J.Adjaye,A series of NiMo/Al2O3catalysts containing boron and phosphorus:part II.Hydrodenitrogenation and hydrodesulfurization using heavy gas oil derived from Athabasca bitumen,Appl.Catal.A Gen.260(2)(2004)153–162.
[16]R.Palcheva,L.Kaluža,A.Spojakina,Jirátová,G.Tyuliev,NiMo/gamma-Al2O3catalysts from Ni heteropolyoxomolybdate and effect of alumina modification by B,Co,or Ni,Chin.J.Catal.33(6)(2012)952–961.
[17]Usman,T.Kubota,Y.Okamoto,Effect of boron addition on the intrinsic activity of Al2O3-supported cobalt–tungsten and cobalt–molybdenum sul fide catalysts for the hydrodesulfurization of thiophene,Bull.Chem.Soc.Jpn.79(4)(2016)637–643.
[18]W.B.Chen,F.Mauge,J.V.Gestl,H.Nie,D.D.Li,X.Y.Long,Effect of modification of the alumina acidity on the properties of supported Mo and CoMo sul fide catalysts,J.Catal.304(4)(2013)47–62.
[19]Y.V.Vatutina,O.V.Klimov,A.Nadeina,I.G.Danilova,E.Y.Gerasimov,I.P.Prosvirin,Influence of boron addition to alumina support by kneading on morphology and activity of HDS catalysts,Appl.Catal.B Environ.199(2016)23–32.
[20]M.Lewandowski,Z.Sarbak,The effect of boron addition on hydrodesulfurization and hydrodenitrogenation activity of NiMo/Al2O3catalysts,Fuel 79(5)(1999)487–495.
[21]D.W.Zhuang,Q.Kang,S.S.Muir,X.D.Yao,H.B.Dai,G.L.Ma,P.Wang,Evaluation of a cobalt–molybdenum–boron catalyst for hydrogen generation of alkaline sodium borohydride solution–aluminum powder system,J.Power Sources 224(4)(2013)304–311.
[22]D.W.Zhuang,J.J.Zhang,i H.B.Da,P.Wang,Hydrogen generation from hydrolysis of solid sodium borohydride promoted by a cobalt–molybdenum–boron catalyst and aluminum powder,Int.J.Hydrog.Energy 38(25)(2013)10845–10850.
[23]J.Ni,L.W.Chen,J.Y.Lin,S.Kawi,Carbon deposition on borated alumina supported nano-sized Ni catalysts for dry reforming of CH4,Nano Energy 1(5)(2012)674–686.
[24]M.W.Ngobeni,A.F.Carley,M.S.Scurrell,C.P.Nicolaides,The effects of boron and silver on the oxygen-free conversion of methane over Mo/H-ZSM-5 catalysts,J.Mol.Catal.A Chem.305(1–2)(2009)40–46.
[25]M.H.Jiang,B.W.Wang,J.Lv,H.Y.Wang,Z.H.Li,X.B.Ma,S.D.Qin,Q.Sun,Effect of sul fidation temperature on the catalytic activity of MoO3/CeO2–Al2O3toward sulfur-resistant methanation,Appl.Catal.A Gen.466(10)(2013)224–232.
[26]M.H.Jiang,B.W.Wang,Y.Q.Yao,Z.H.Li,X.B.Ma,S.D.Qin,Q.Sun,A comparative study of CeO2–Al2O3support prepared with different methods and its application on MoO3/CeO2–Al2O3catalyst for sulfur-resistant methanation,Appl.Surf.Sci.285(1)(2013)267–277.
[27]P.Berteau,B.Delmon,Modified aluminas relationship between activity in 1-butanol dehydration and acidity measured by NH3-TPD,Catal.Today 5(2)(1989)121–137.
[28]A.N.Pour,A.M.Rashidi,K.J.Jozani,A.Mohajri,P.Khorami,Support effects on the chemical property and catalytic activity of Co–Mo HDS catalyst in sulfur recovery,J.Nat.Gas Chem.19(1)(2010)91–95.
[29]S.L.González-Cortés,T.C.Xiao,P.M.F.J.Costa,B.Fontal,M.L.H.Green,Urea–organic matrix method:an alternative approach to prepare Co–MoS2/γ-Al2O3HDS catalyst,Appl.Catal.A Gen.270(1)(2004)209–222.
 Chinese Journal of Chemical Engineering2018年3期
Chinese Journal of Chemical Engineering2018年3期
- Chinese Journal of Chemical Engineering的其它文章
- Numerical investigation on flow and heat transfer characteristics of corrugated tubes with non-uniform corrugation in turbulent flow
- Investigations on pool boiling critical heat flux,transient characteristics and bonding strength of heater wire with aqua based reduced graphene oxide nano fluids
- Heavy metals adsorption by banana peels micro-powder:Equilibrium modeling by non-linear models
- Potential aspect of rice husk biomass in Australia for nanocrystalline cellulose production
- Fouling evaluation on membrane distillation used for reducing solvent in polyphenol rich propolis extract
- Investigation on a vertical radial flow adsorber designed by a novel parallel connection method☆
