The role of echocardiography and CT angiography in transcatheter aortic valve implantation patients
Emmanouil Chourdakis, Ioanna Koniari, Nicholas G Kounis, Dimitrios Velissaris, Nikolaos Koutsogiannis,Grigorios Tsigkas, Karl Eugen Hauptmann, Bruno Sontag, George Hahalis
1Krankenhaus der Barmherzigen Brüder Trier, Germany
2Department of Cardiology, University Hospital of Patras, Rion, Patras, Greece
3Department of Internal Medicine, University Hospital of Patras, Rion, Patras, Greece
1 Introduction
The transcatheter aortic valve implantation (TAVI) constitutes an alternative treatment in inoperable or high perioperative risk patients with severe aortic stenosis. Multimodality imaging using transthoracic echocardiography(TTE) or transesophageal echocardiography (TOE) and multislice CT (MSCT) are crucial techniques for the quantification of aortic stenosis, appropriate patient selection, annular sizing, selection of access-site, as well as for peri-procedural guidance, follow up and recognition of possible transcatheter heart valve (THV) related complications.[1]
CT systems with at least 64-detector technology and 0.5 to 0.6 mm spatial resolution are sufficient for the anatomic evaluation of the aortic root and the iliofemoral arteries.[2]ECG synchronized imaging with retrospective gating and prospective triggering play critical role to avoid image artifacts due to the motion of the aortic valve, root, and ascending aorta during cardiac cycle. With this acquisition mode, images are acquired only during a limited, pre-specified phase of the cardiac cycle (e.g., systole), whereas the radiation exposure and iodinated contrast medium volume of these high-pitch acquisitions remain relatively low (3-5 mSv, 40-60 mL with 370 mg/mL iodine), fact that particularly benefits TAVI patients.[2,3]
The use of MRI before THV implantation remains controversial. The non-contrast MRI might significantly contribute to the preoperative evaluation in patients with known previous history of severe allergic type reaction to iodinated contrast medium or impaired renal function and in patients with poor acoustic window or low flow /low gradient aortic stenosis (AS) with reduced left ventricular ejection fraction.[4]Multiple breath holds, much longer scan time, calcified plaques and porcelain aorta consist the main limitations of MRI use.[4]
2 Quantification and classification of severity aortic stenosis
TTE demonstrates the amount of calcification, the aortic valvular morpholgy, the left ventricular (LV) function, the presence of left atrium (LA) dilatation and the presence of concominant valvulopathies. The TAVI procedure is indicated in patients with preserved ejection fraction and direct aortic valve area calculation ≤ 1 cm2, aortic valve area(AVA) based on continuity equation or a mean aortic valve gradient of ≥ 40 mmHg.[1]In case of reduced or LV-dysfunction, a further evaluation with low dose dobutamine stress echocardiography is recommended to differentiate the true severe AS from pseudo-severe AS. The TOE offers a better measurement of aortic valve area in the aortic short axis view with the use of orthogonal plane and 3D technique, measured between the endothelial point trisecting the posterior aortic wall, non-coronary cusp hinge and anterior mitral leaflet hinge, and that bisecting the anterior aortic wall and the right coronary cusp hinge.[1]CT can be also useful to classify the severity of AS with direct planimetry aortic valve area measurement correlating well with those derived from TOE.[5]
The determination of the stenosis level either subvalvular,valvular, or supravalvular must be evaluated with TTE and TOE before TAVI.[6]Notably, the presence of significant LV outflow tract (LVOT) obstruction or subvalvular membrane represents a main contraindication for the TAVI procedure (Figure 1A).[1]Furthermore, the existence of thrombotic material in the LV may also delay the implantation time. Concominant secondary mitral regurgitation (MR) is common in TAVI patients and often a decrease of the regurgitation severity is succeeded post TAVI.[1]On the other side, the presence of severe primary MR before THV implantation is associated with poor prognosis, rendering the significance of MR documentation, pro-, peri-, and postprocedural.
3 Aortic root morphology and annular size
The anatomical evaluation of the aortic valve and annular dimensions are critical for TAVI success: the diameter of the ascending aorta, sinotubular junction, sinuses of Valsalva, the AV annulus, the height of the Valsava sinuses and the height of the coronary artery ostia from the AV annulus should be measured meticulously before TAVI.[7]Therefore,aortic annulus is widely acknowledged as a virtual ring, a significant underestimation of aortic annulus size has been reported, in 2D single plane echocardiogram measurements.[7]

Figure 1. Transesophageal echocardiography. (A): Four chamber view, aortic long-axis views. Membranous subvalvular aortic stenosis with concomitant aortic root aneurysm producing high transvalvular gradient. A contraindication for TAVI; (B): 3D view and X-plane aortic view. Bicuspid calcified aortic valve with aortic aneurysm. TAVI: transcatheter aortic valve implantation.
CT angiography is much more accurate regarding the total definition of the long-axis and short-axis aortic annulus diameter and circumferential area.[8]In comparison to 3D imaging techniques (MSCT, MRI and 3D TOE), 2D echocardiography, underestimates the aortic valve annulus diameter, while 3D TOE imaging provides measurements of the aortic valve annulus similar to those delivered by MSCT(Figure 2).[5]It has been evident that the annulus is a dynamic structure and measurements should ideally be performed at the end systole to achieve the greatest annular stretch. Actually, underestimation of the valve size would occur in 20% of patients if diastolic measurement were used.[9]The basic measurements consist in major and minimal diameters, cross-sectional area and perimeter of the annulus.[9]The perimeter-based valve size presents minimal variation throughout the cardiac cycle and it is not affected by geometric shape changes compared to the other parameters. Current literature supports a perimeter based valve size selection.[9]However, accurate measurements of heavily calcified annulus remains challenging. Moreover, an eccen-tric orifice, a bicuspid aortic valve with asymmetric aortic annulus and eccentric calcification increase the risk of malposition and dislocation of THV (Figure 1B).[7]A 1-2 mm difference in the anatomic measurements of the annulus might change significantly the size and type of prosthesis based on the manufacturer’s recommendations.[5]Therefore,the accurate sizing and prosthesis selection are crucial.
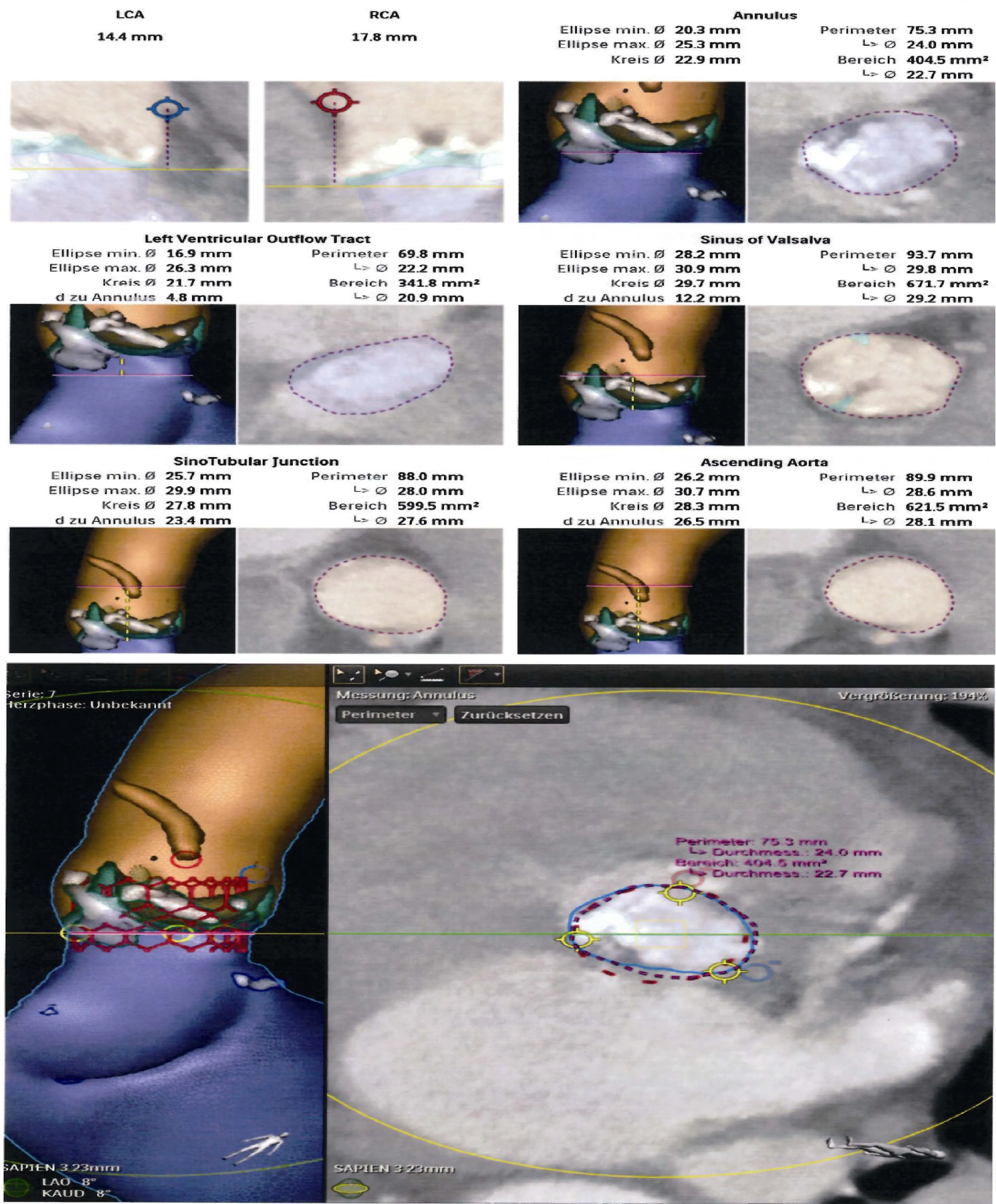
Figure 2. Pre-operative CT angiography data incorporated to Philips 3D Heart Navigator System to produce 3D images. The reconstructed 2D data can be combined with peri-procedural live images to provide a better 3D Cath Lab Navigation. The CT data are automatically segmented to identify the aortic root, coronary ostia, the aortic valve, the left ventricle and the valve plane running through the bottom of the three cusps.
The current recommendation is a prosthesis selection with a cross-sectional area modestly larger than that of the aortic annulus. A target of 10%-15% annular area oversized with upper and lower limits of 20% is suggested.[10]Oversizing the annulus > 20% may increase the risk of annular injury, coronary ostial obstruction, suboptimal stent expansion, impaired leaflet mobility, low transvalvular aortic regurgitation (AR) above the prosthetic tissue rim, or mitral valve dysfunction.[9,11]On the contrary, annular undersizing might increase the paravalvular AR and THV displacement.[9,11]Moreover, significantly low THV-implantation might lead to embolization or impingement on the anterior mitral valve leaflet,[7,11]whereas much higher provoke an upwards migration into the aorta, coronary ostial occlusion and also paravalvular regurgitation.[7]
CT provides a detailed evaluation of the aortic valve, the annulus, and its relationship to the coronary arteries. In addition, measurement of the aortic root, sinotubular junction(STJ), and sinus of Valsalva (SOV) height are critical for proper positioning of the device and ensuring there is no obstruction on the coronary ostia. The measurement of the height of the coronary ostia relative to the aortic annulus from an oblique sagittal or coronal projection is an important requirement before TAVI and should be at least ≥ 10-11 mm.[12,13]The combination of a shallow sinus of Valsava <30 mm and low coronary ostia take off < 10-12 mm increases the risk for coronary occlussion.[14,15]TOE modified mid-oesophageal long-axis view using 3D techniques with reconstruction of the coronal plane allows accurate measurement of the AV annulus-left coronary ostial height.[7]
The aortic root is well visualized in the mid-oesophageal long-axis view between using 2D- and 3D-TOE.[13]The SOV diameter is also perpendicular to the long axis of the root and typically parallel to the aortic valve area. It is measured as the widest intraluminal distance within the sinuses. The STJ diameter is the intraluminal diameter,where the sinuses narrow and join the ascending aorta. The ascending aorta diameter is measured at the widest diameter visible by TOE in the long-axis view.[12,13]
For the Edwards-Sapien prosthesis, there are no SOV or STJ requirements.[11]Notably, the presence of significant aneurismal aortic root dilatation is a contraindication for the use of CoreValve. The proper supra-annular position of CoreValve require a minimum trans-sinus dimension of 27 mm and an ascending aorta diameter less < 43 mm.[13]Furthermore, heavy calcification in the STJ may cause restriction during balloon expansion at the aortic end and further resulting in ventricular displacement of the device at the time of deployment.
The angulation of LVOT with aortic root is performed by a coronal oblique projection and is defined as the angle between the axis of the first portion of the ascending aorta and the LVOT axis corresponding to the upper part and the distal portion or landing zone of the bioprosthesis, respectively.Patients present with an LVOT-angulation > 90 degrees should not be considered as TAVI candidates.[12,14]
In conclusion, the precise evaluation of aortic valve anatomy and location of calcification by echocardiography and MSCT may help to improve procedure planning and avoid potential complications.
4 Appropriate angle of deployment using CT
Correct coaxial alignment of the THV along the centerline of the native aortic valve and aortic root is important during deployment. Inappropriate position and absence of the device perpendicularity is associated with increased risk of procedural complication.[14,15]Aortic root orientation,using catheter aortograms in 1or 2 orthogonal planes when starting the procedure has been rendered inaccurate. Typically a caudal angulation is chosen when in a right anterior oblique projection and cranial angulation when in the left anterior oblique projection. However, CT angiography using double oblique transverse multiplanar reconstructions at the level of the root is more powerful than aortogram and can provide an accurate prediction of the perpendicular valve view/angle, rendering the positioning of the THV and the likelihood of coaxial implantation easier, quicker and more accurate.[2,3,13]Pre-procedural angle prediction with MSCT is associated with reduced number of aortograms,shortening both procedure time and contrast usage.[2,5,13]
The appropriate angle of implantation is easier to estimate with MSCT particularly in patients with musculoskeletal abnormalities, kyphoscoliosis, and markedly unfolded aortas.[5,13]
5 Access approach
5.1 Transfemoral approach

Figure 3. A transapical TAVI was performed in chronic type B aortic dissection. TAVI: transcatheter aortic valve implantation.
MSCT with reconstruction technique depicts the anatomy and calcification of the entire aorta and the iliofemoral arteries, contributing to the best access route selection and avoidance of site complications. It allows the evaluation of peripheral vascular tree, considering calibre, tortuosity, and calcification.[1,13,14]The minimum luminal diameter defined by the implantable THV type, the size of the THV/sheath and generally a common femoral artery with at least 6 mm of diameter is required.[12]The combination of eccentric,extensive and circumferential calcification/plaque, small caliber vessels or significant stenotic segments constitute a contraindication for a transfemoral artery approach, as arterial dissection or perforation might be caused,[1,12,13,16,17]contributing to a further increase on bleeding, transfusions,and mortality.[17]
Vascular complications might not be only access site-related, but can be also involved dissection of the ascending or descending aorta, induced by catheter injury or aortic valvuloplasty. Consequently, the entire aorta must be careful investigated before TAVI (Figure 3).[14,17]
The transfemoral approach is considered as the default vascular access. In case of infeasible transfemoral approach,alternative access should be carefully selected.[1,14,17]
5.2 Transapical approach
The transapical approach (TA) approach is much more invasive. Patients with severe pulmonary disease, chest wall deformity, obesity, severe LV dysfunction, previous LV patch,significant pericardial calcification, apical scar/prior infarct,or intracavitary thrombus should be better excluded from a transapical implantation.[4,16]The above conditions as well as the intercostal space and the distance of this access site from the midline can be measured by axial non-gated assessed with MSCT.[4,14]
5.3 Transoartic approach
MSCT-analysis of the ascending aorta is essential for the transoartic approach (Tao) patient selection. The TAo zone(where the purse-string sutures are placed) should be not calcified. The minimum distance from the aortic annulus to the TAo zone should be 5-7 cm for the Edwards SAPIENXT valve and 6-7 cm for the CoreValve, respectively, to allow complete valve deployment.[16]
Mini-sternotomy is performed if the aorta is on the left side and its distance from sternum is above 5 cm or if the aorta is on right side and its distance from sternum is more than 7 cm.[16]On the other hand, right thoracotomy should be considered if the distance of aorta from sternum is < 6 cm and > 50% aorta is to the right.[16]
5.4 Subclavian approach
The left subclavian artery is preferred over the right access site, because of its better implantation angle and more straightforward course. Assessment of anatomic characteristics of the subclavian artery (vessel diameter, tortuosity and calcification) as well as exclusion of relevant stenoses prior to the procedure are performed by MSCT. Regarding the subclavian approach, the presence of a patent left internal mammary coronary artery bypass graft is a relative contraindication, because the cannulation of the left subclavian artery can cause transient flow obstruction and ischaemia during the procedure.[4,14,16]
6 Non-cardiovascular CT incidental findings
According to current expert recommendations, TAVI should not be performed in patients with severe co-morbidities and life expectancy of less than 12 months. TAVI cancellation or postponement based on MSCT findings,play an important role.[18]The average prevalence of overall extracardiac findings has been reported up to 41%, clinically significant and life-threatening or malignant features ranging between 16% and 2.2%.[18]Malignancies causing procedure cancellation were documented in 4.4%.[18]
6.1 Guiding the procedure
The fluoroscopy still remains the cornerstone for the TAVI-procedure guidance. There is interest in fusion imaging modalities (echocardiography, MSCT, or CMR with fluoroscopy) to generate a 3D model, containing all relevant anatomical landmarks. TOE is not mandatory during TAVI,while its main disadvantage is the general anaesthesia requirement. 3D TOE can have an important role in guiding all stages and specifically aortic valve crossing, balloon dilatation, prosthesis deployment,[7]as well as to confirm prosthesis function evaluating the normal leaflet mobility,the presence of AR, transvalvular gradients and potential complications, immediately after implantation. The midoesophagus long-axis view enables visualization of the guide wire/THV through the aortic valve.[12,18]In many centers, only TTE is performed but it is often suboptimal, due to supine position, chest wall incisions, hyperinflation of lungs and other deformities.[7,19]
The optimal position concerning the ventricular side of the prosthesis can be identified. In general, it should be located 2-4 mm below the annulus for Edwards Sapien and 5-10 mm for the CoreValve.[7]
The presence of cardiogenic shock caused from pericardial effusion/tamponade, coronary occlusion, aortic dissection/rupture or hypovolemia should be immediately documented with fluoroscopy and echocardiography during periprocedural management. TOE could define accurately the complication and to optimize the fluid management (transgastric views) and vasopressors administration.[1,7]
6.2 Complications
6.2.1 Aortic regurgitation
Various complications have been described during or after the procedure. The most frequent aetiology of transcatheter heart valve failure is the paravalvular regurgitation.[20]The preprocedural MSCT constitutes the gold standard modality minimizing the presence of paravalvular AR. Protruding annular calcifications > 4 mm and adherent calcification > 6 mm (particularly left-sided) or calcifications with an Agatston score of > 3000 has been recognized as predictors of paravalvular leaks after TAVI.[4]The AR based on the origin of the jet, is classified as transvalvular, paravalvular and supra-annular, occurring as a result of incomplete expansion, incorrect positioning, restricted cusp motion, or inappropriate prosthetic size, location or extent of calcification.[1,21,22]Mild paravalvular leaks are trivial without significant clinical consequences and might reach up to 70%-75%. The exact estimation of severity of AR is crucial for the prognostic outcome and may be more challenging in comparison with native AR due to shadowing effect and reflectance.[1,4-6]In clinical practice, the established criteria for the AR measurement should be used with caution. According to the Valve Academic Research Consortium recommendations, the proportion of the sewing ring circumference occupied by the jet suggests a semi-quantitative guide to severity, 10% of the sewing ring classified as mild,10%-29% classified as moderate, and ≥ 30% classified as severe.[1,13,21,22]The occurrence and degree of paravalvular AR must be assessed by angiography immediately post TAVI.[1,21]Finally, a multimodal approach for the evaluation of AR with use of peri-procedural haemodynamic measurements and imaging modalities is further recommended to quantify the severity of AS after TAVI.[1,21,22]
“Aortic regurgitation index”, is the ratio of the end-diastolic, transvalvular gradient between diastolic blood pressure (RRdia) in the aorta minus left ventricular end-diastolic pressure (LVEDP) to systolic blood pressure (RRsys) in the aorta: [(RRdia- LVEDP)/RRsys] × 100, constituting a strong predisponing factor for prediction 1-year mortality after TAVI( cut-off value 25) and for evaluation additional interventional maneuver.[1,21-23]In the context of residue moderate to severe AR with additional AR index < 25, an additional intervention with post-dilation, valve-in-valve implantation,reposition the prosthesis with snare technique or transcatheter plug device closure is required for better outcomes.[1,13,21,22]
6.2.2 Migration/Embolisation
The migration and embolisation of the valve prosthesis constitute two different entities that should be distinguished with imaging techniques. The upward or downward movement within the aortic annulus compared to its initial position is defined as migration.[20]THV embolisation is the loss of contact between the THV with aortic annulus, occurring frequently into the LVOT (89%).[20]Notably, the prosthesis dislocation towards the LVOT can result in MR, but the displacement at the opposite site towards (aorta) can cause coronary ostial occlusion.
6.2.3 Pericardial effusion
The presence of relevant pericardial effusion or cardiac tamponade occurs secondary to wire perforation of the left or right ventricle as well as tear or ruptures of the aortic root.This situation may be obtained after balloon valvuloplasty or prosthesis deployment, especially in the presence of ex-tensive annular/sub-annular calcification or prosthesis oversizing.[7,11,14]
6.3 Pacemaker implantation
TOE/TTE and MSCT performance could predict the possibility of pacemaker implantation post-procedural. Narrow LVOT, LVOT/annulus ratio and depth of the implantation correlates with increased frequency of intraventricular conduction disturbances and necessity for pacing.[24]Furthermore, noncoronary cusp thickness > 8 mm as well as the severity/extension of mitral annular calcification has been identified also as predictor factors.[24]The use of the Core-Valve system in comparison with Edwards Sapien leads to higher rate of conduction disturbances, probably due to the compression from the longer stent frame and the deeper implantation of the THV in the LVOT.[12-14]
6.4 Rupture/Tear
Rupture of the aortic root is a rare major vascular non access site related.[9]The aortic rupture based on the anatomical location is classified as intra-annular, sub-annular,supra-annular or combined.[15,25,26]The most frequently recognized echocardiographic rupture types are documented in the annulus-area (67.7%) and sinus of valsalva (16.1%)and less often in the LVOT (9.7%) or sinotubular junction(6.4%).[27]The pre-procedural MSCT has been established as the gold standard for identification of anatomical predisposing factors such as severe LVOT, intraventricular septum or valve calcification (size and shape), severe asymmetric sub-aortic LV hypertrophy or aortic annulus,[15,25,26]avoiding more than 20% of oversizing. A tear created at the level of the valve inflow can result a left to right atrial shunt.[15,26]The surgical or conservative therapy lies on the localization,the size of defect, the hemodynamic status of patient in the acute phase and the individual decision of the heart team.
6.5 Mitral regurgitation
The presence of a new transient or persisting MR can be assessed well by TOE. MR might be induced during catheter advancement, and could be catastrophic in case of mitral apparatus injury or anterior mitral leaflet motion impedance by the ventricular end of a transcatheter prosthesis.[7,14]
6.6 New left ventricular abnormal contractility
The rapid echocardiographic detection of left ventricular dysfunction is crucial in the context of complete or partial coronary occlusion caused by the valve frame, the sealing cuff and displacement of the bulky, calcified native leaflet,requiring further appropriate intervention.[14]Other possible reasons for regional deterioration of LV systolic function are a new left bundle branch block or rapid pacing and balloon aortic valvuloplasty.[7,11,12]
6.7 Acute structural THV failure
Acute valve failure causing AR has been reported very rarely and further requires implantation of a second valve.Acute THV failure occurs primarily due to the peri-procedural maneuver (manufacturing defects, leaflet damage during crimping or implantation).[14]
6.8 Follow up
Multimodality imaging for the adequate follow up of the TAVI patients is important, and allows the early detection and management of transcatheter aortic valve dysfunction.Both TTE and TOE, consist initially the basic examination for post TAVI evaluation. In case of THV-failure or unidentified THV-dysfunction, the MSCT could be used as additional imaging technique. MSCT offers better understanding of post-procedural AR underlying mechanisms providing additional information for a prosthetic aortic valve malfunction cause.[4,13,16]This allows a better visualization in comparison with TTE/TOE of the bioprosthetic valve expansion,circularity (eccentricity index of 0.1) and apposition as well as the implantation depth assessment.[13]There is no established role for the routine clinical use of CT for TAVI follow-up in patients without evidence of paravalvular regurgitation on echocardiography.[13]
6.9 THV degeneration
The impact of the durability of the transcatheter aortic valve is unknown.[12]A significant increase in degeneration rate has been observed after 5-7 years. Structural valve deterioration/dysfunction defined as a new increase of mean aortic valve gradient ≥ 20 mmHg, decrease of the indexed effective orifice area (EOA) ≤ 0.9-1.1 cm2and/or doppler velocity index (DVI) 0.35 m/s, and/or moderate or severe prosthetic valve regurgitation requiring re intervention.[2]The early degeneration could be attributed to several predisposing factors as the characteristics of the tissue, valve design, asymmetric and suboptimal leaflet coaptation, leaflet trauma during catheter/balloon inflation, transvalvular gradients, residual paravalvular AR as well as multiple clinical factors.[11,28]In the Partner trial and Canadian multicentre registry, significant changes in valve gradients/AVA/AR were not documented during the 2-year and 4-year follow-up, respectively.[11]
7 Endocarditis
Generally, three of four diagnostic echocardiographic Duke criteria (“abscess”, “new dehiscence of prosthetic valve” and “new valvular regurgitation”) cannot be easily used for the detection TAVI-endocarditis, thus complicating the correct and early diagnosis. In the literature, the vegetations are located more frequently on the transcatheter valve leaflets (39%), on the valve stent frame (17%), and or in both structures (9.2%).[29,30]Abscesses and fistulae have been observed in 47%, 9% of patients, respectively. Satellite endocarditis of the mitral valve as result of the direct contact the low-lying aortic THV with the mitral apparatus has been identified in 24% of prosthetic valve endocarditis cases,while secondary mitral valve involvement estimated in 10%of patients.[29,30]
8 Thrombosis
Subclinical leaflet thrombosis might not be detected by TTE as it may be silent from haemodynamic and clinical point of view.[31,32]The most common thrombus morphological characteristics on echocardiography were thickened leaflets or thrombotic apposition of leaflets (n = 20; 77%),whereas only in a minority of cases, a thrombotic formation was identified on the leaflets (n = 6; 23%).[21]The performance of 2D (axial cross-section assessment) and 3D volume-rendered CT-imaging is more sensitive for the detection of hypo-attenuated leaflet thickening of the valve leaflets and assessment of the reduced leaflet motion at maximal leaflet opening during systole with at least reduction of the motion > 50%.[31-33]The above criteria are essential to define the diagnosis excluding other responsible causes for the THV-failure such as: pannus, calcification, underexpansion or trauma, mal-sizing, endocarditis, native leaflet prolapse impeding prosthetic leaflet motion, malapposition,leaflet tear perforation, prolapse, or retraction, and suture breakage or disruption.[31-33]
A significant elevation of transvalvular aortic gradient(mean gradient of > 20 mmHg or rise in gradient of > 10 mmHg) has been demonstrated only in 14% of patients with reduced leaflet motion and signs of THV-thrombosis.[31-33]
9 Mismatch
Prosthesis-patient mismatch occurs in case of discordance between the effective orifice area of a normally functioning prosthetic valve (too small) and the patient’s body size.[12]Non-randomized studies as well as in post hoc analysis of Partner cohort A trial showed that TAVI in comparison with SAVR might be associated with a lower incidence of PPM, particularly, in patients with a small aortic annulus.[16]In case of a low indexed EOA (moderate between 0.65 and 0.85 cm2/m2and severe < 0.65 cm2/m2)and normal DVI, a prosthesis―patient mismatch must be strongly suspected.[16,25]
References
1 Zamorano JL, Gonçalves A, Lang R, et al. Imaging to select and guide transcatheter aortic valve implantation. Eur Heart J 2014; 35: 1578-1587.
2 Schoenhagen P, Hausleiter J, Achenbach S, et al. Computed tomography in the evaluation for transcatheter aortic valve implantation (TAVI). Cardiovasc Diagn Ther 2011; 1: 44-56.
3 Storz C, Geisler T, Notohamiprodjo M, et al. Role of imaging in transcatheter aortic valve replacement. Curr Treat Options Cardiovasc Med 2016; 18: 59.
4 Chaturvedi A, Hobbs SK, Ling FS, et al. MRI evaluation prior to transcatheter aortic valve implantation (TAVI): when to acquire and how to interpret. Insights Imag ing 2016; 7:245-254.
5 De Heer LM, Kluin J, Stella PR, et al. Multimodality imaging throughout transcatheter aortic valve implantation. Future Cardiol 2012; 8: 413-424.
6 Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr 2009; 22: 1-23.
7 Smith LA, Monaghan MJ, et al. Monitoring of procedures:peri-interventional echo assessment for transcatheter aortic valve implantation. Eur Heart J Cardiovasc Imaging 2013; 14:840-850.
8 Little SH, Shah DJ, Mahmarian JJ, et al. Multimodality noninvasice imaging for transcatheter aortic valve implantation: a primer. Methodist Debakey Cardiovasc J 2012; 8: 29-37.
9 Nguyen G, Leipsic J. Cardiac computed tomography and computed tomography angiography in the evaluation of patients prior to transcatheter aortic valve implantation. Curr Opin Cardiol 2013; 28: 497-504.
10 Willson AB, Webb JG, Freeman M, et al. Computed tomography-based sizing recommendations for transcatheter aortic valve replacement with balloon-expandable valves: Comparison with transesophageal echocardiography and rationale for implementation in a prospective trial. J Cardiovasc Comput Tomogr 2012; 6: 406-414.
11 Chin D. Echocardiography for transcatheter aortic valve implantation. Eur J Echocardiogr 2009; 10: 21-29
12 Zamorano JL, Gonçalves A, Lang R. Imaging to select and guide transcatheter aortic valve implantation. Eur He art J 2014; 35: 1578-1587.
13 Leipsic J, Gurvitch R, Troy M, et al. Multidetector computed tomography in transcatheter aortic valve implantation. JACC Cardiovasc Imaging 2011; 4: 416-429.
14 Masson JB, Kovac J, Schuler G, et al. Transcatheter aortic valve implantation review of the nature, management, and avoidance of procedural complications. JACC Cardiovasc Interv 2009; 2: 811-820.
15 Barbanti M. Avoiding coronary occlusion and root rupture in TAVI―the role of pre-procedural imaging and prosthesis selection. Interventional Cardiology Review 2015; 10: 94-97.
16 Bax JJ, Delgado V, Bapat V, et al. Part 2: procedural issues and outcomes after transcatheter aortic valve implantation open issues in transcatheter aortic valve implantation. Eur Heart J 2014; 35: 2639-2654.
17 Toggweiler S, Leipsic J, Binder RK, et al. Management of vascular access in transcatheter aortic valve replacement part 1: basic anatomy, imaging, sheaths, wires, and access routes.JACC Cardiovasc Interv 2013; 6: 643-653.
18 Goitein O, Di Segni E, Eshet Y, et al. Non-valvular findings before trans-catheter aortic valve implantation and their impact on the procedure. Isr Med Assoc J 2015; 17: 764-767.
19 Kronzon I, Jelnin V, Ruiz CE, et al. Debates in imaging for guiding TAVR: transesophageal or transthoracic echocardiography, or just fluoroscopy? JACC Cardio vasc Imagin g 2015; 8: 361-370.
20 Mylotte D, Andalib A, Thériault-Lauzier P, et al. Transcatheter heart valve failure: a systematic review. Eur Heart J 2015;36: 1306-1327.
21 Sinning JM, Werner N, Nickenig G, et al. Challenges in transcatheter valve treatment: aortic regurgitation after transcatheter aortic valve implantation. EuroIntervention 2013; 9:S72-S76.
22 Sinning JM, Christoph H, Vasa-Nicotera M, et al. Aortic regurgitation index defines severity of peri-prosthetic regurgitation and predicts outcome in patients after transcatheter aortic valve implantation. J Am Coll Cardiol 2012; 59: 1134-1141.
23 Abdelghani M, Soliman OI, Schultz C, et al. Adjudicating paravalvular leaks of transcatheter aortic valves: a critical appraisal. Eur Heart J 2016; 37: 2627-2644.
24 Wilczek K, Reguła R, Bujak K, et al. Conduction disturbances after transcatheter aortic valve implantation procedures―predictors and management. Postepy Kardiol Interwe ncyjnej 2016; 12: 203-211.
25 Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the valve academic research consortium-2 consensus document. Eur J Cardiothorac Surg 2012; 42: S45-S60.
26 Pasic M, Unbehaun A, Buz S, et al. Annular rupture during transcatheter aortic valve replacement classification, pathophysiology, diagnostics, treatment approaches, and prevention.JACC Cardiovasc Interv 2015; 8: 1-9.
27 Barbanti M, Yang TH, Rodès Cabau J, et al. Anatomical and procedural features associated with aortic root rupture during balloon-expandable transcatheter aortic valve replacement.Circulation 2013; 128: 244-253.
28 Webb JG, Dvir D. Is transcatheter aortic valve replacement a durable therapeutic strategy? JACC Cardiovasc Interv 2015; 8:1092-1094.
29 Amat-Santos IJ, Ribeiro HB, Urena M et al. Prosthetic valve endocarditis after transcatheter valve replacement: a systematic review. JACC Cardiovasc Interv 2015; 8: 334-346.
30 Amat-Santos IJ, Messika-Zeitoun D, Eltchaninoff H, et al.Infective endocarditis following transcatheter aortic valve implantation: results from a large multicenter registry. Circulation 2015; 131: 1566-1574.
31 Latib A, Naganuma T, Abdel-Wahab M, et al. Treatment and clinical outcomes of transcatheter heart valve thrombosis. Circ Cardiovasc Interv 2015; 8: e001779.
32 Chakravarty T, Søndergaard L, Friedman J, et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet 2017; 389:2383-2392.
33 Jilaihawi H, Asch FM, Manasse E, et al. Systematic CT methodology for the evaluation of subclinical leaflet thrombosis.JACC Cardiovasc Imaging 2017; 10: 461-470.
 Journal of Geriatric Cardiology2018年1期
Journal of Geriatric Cardiology2018年1期
- Journal of Geriatric Cardiology的其它文章
- 2016 Chinese guidelines for the management of dyslipidemia in adults
- Appendix
- Chinese expert consensus on the non-invasive imaging examination pathways of stable coronary artery disease
- Improvement of increased cQTd is associated with heart function in patients with ischemic heart failure
- TAVR in 2017―What we know? What to expect?
- Endocarditis after transcatheter aortic valve implantation: a current assessment
