Improvement of increased cQTd is associated with heart function in patients with ischemic heart failure
Hui GUO, Miao WANG, Juan ZHAO, Jing LIU, Jie-Mei YANG
1Department of Cardiology, the First Affiliated Hospital of Harbin Medical University, Harbin, China
2Department of Cardiology Ultrasound, the First Affiliated Hospital of Harbin Medical University, Harbin, China
1 Introduction
Chronic heart failure (CHF) is the final stage in the development of various cardiovascular diseases and is lifethreatening without timely or effective intervention. A prolonged QT interval and QT dispersion (QTd), which reflect inhomogeneity of ventricular repolarization and asynchronization of ventricular wall motion,[1-3]are associated with malignant ventricular arrhythmias. However, these parameters are affected easily by heart rate.[4-7]
Assessment of the corrected QTd (cQTd) can effectively avoid the influence of heart rate on the QT interval or cQT and more clearly represent the ventricular repolarization heterogeneity and electrical instability. The cQT of the normal myocardium is narrow, while the cQT of the ischemic zone continuously increases. cQTd is defined as the difference between the minimum and maximum cQT durations in any of the 12 ECG leads. An increased cQTd is an important cause for the formation of a reentrant arrhythmia, which seriously deteriorates cardiac function and threatens patient survival.[8-10]
Therefore, a decrease in cQTd may enhance the sensitivity of ECG as an effective indicator of the prognosis of CHF patients. Based on the etiology, CHF is commonly divided into ischemic cardiomyopathy (ICM) and non-ischemic cardiomyopathy, with the main type being dilated cardiomyopathy (DCM). The aims of this study were to examine the dynamic change in cQTd in CHF patients with DCM or ICM during one year of optimized medical treatment and determine its correlation with clinical and echocardiogram parameters.
2 Methods
2.1 Patients and grouping
From January 2013 to December 2015, 240 patients (age range, 40-59 years), who were categorized as New York Heart Association functional class (NYHA) III-IV with a left ventricular ejection fraction (LVEF) < 40%, were con-tinuously enrolled from the Department of Cardiology, the First Affiliated Hospital of Harbin Medical University, China.Patients were excluded from this study if they had a life expectancy ≤ 6 months; had myocardial hypertrophy, restrictive cardiomyopathy, or valvular heart disease; or had bundle branch block, etc.
Based on the etiology, the patients were divided into a non-ischemic dilated cardiomyopathy (DCM) group (n =120) and an ischemic cardiomyopathy (ICM) group (n =120), and then the ICM group was divided into two subgroups: a QS group (cQTd ≤ 60 ms, n = 70) and a QL group(cQTd > 60 ms, n = 50). Demographic and laboratory data were collected at admission. The inclusion criteria for the DCM and ICM groups are listed in Table 1.
All patients were given optimized medical treatment according to the 2012 European guidelines for the treatment of chronic heart failure. Medications included angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers,β-blockers, aldosterone antagonists, or diuretics for at least one year.
2.2 cQTd definition
The QT interval was measured from the start of the QRS complex to the endpoint of the T wave (Figure 1A). When the T wave was merged with the U wave, the endpoint of the T wave was considered the intersection between the maximum slope of the descending limb of the T-wave and the baseline (Figure 1B). During ECG, patients’ heart rates were kept within 60-100 beats/min. Thus, the QT intervals were corrected for RR using Bazett’s formula:[11]cQT =where RR is the interval from the onset of one QRS complex to the onset of the next QRS complex.
cQTd was defined as the difference between the minimum and maximum cQT duration in any of the 12 ECG leads: cQTd = cQTmax- cQTmin. Then two investigators calculated the cQTd from the ECG data.

Table 1. Diagnostic criteria applied in the enrolled patients.
2.3 Measurement of laboratory indicators
All data were extracted from patients’ medical charts in the Department of Cardiology, including age, body mass index (BMI), and histories of hypertension and diabetes mellitus. On the first day after enrollment and also at the end of the study, venous blood samples (4 mL with heparin and 2 mL without anticoagulant) were collected from all patients after fasting. Blood samples without anticoagulant were prepared for N-terminal pro brain natriuretic peptide(NT-proBNP), ion (Mg2+, Ca2+, K+), and serum albumin measurements and renal function tests. Blood samples with heparin were collected for measurement of D-dimer levels.All samples were centrifuged at 3000 r/min for 10 min at 4°C and stored at -70°C until analysis.
2.4 Evaluation of heart function by 6-min walking test

Figure 1. Measurement of cQTd in 12-lead ECG when (A) the T wave returned to the isoelectric interval, or (B) when the T wave merged with the U wave. cQTd: corrected QT dispersion.
For the 6-min walking test (6MWT), the patients were asked to walk back and forth along a flat 100-foot-long surface and to attempt to cover as much ground as possible; at the same time, two investigators used a stopwatch to measure the distance walked within 6 min. According to their physical ability, the patients were free to stop to rest and then resume walking or to choose to terminate the test.
2.5 Evaluation of heart function by ECG
Heart function was evaluated by two investigators in a randomized double-blind manner using an M-mode, twodimensional and pulsed echocardiogram (Philips HPSonos 7500, MA, USA) with the patients in the supine position.The patients’ data were recorded at admission and after 1, 3,6, and 12 months. All ECG parameters were collected from the average of three consecutive cardiac cycles. During follow-up, patients underwent a comprehensive clinical assessment that included NYHA classification.[12]
2.6 Multi-parameter acquisition of 12-lead ECG
A 12-lead ECG was recorded for all patients at admission and after 1, 3, 6, and 12 months of standard treatment using a standard digital recorder (GE Medical Systems IT, MAC 1200, Germany) at a speed of 50 mm/s. QRS onset and T-wave offset were determined, and then the HR, RR, and QT intervals were measured by two investigators who were double-blinded to each patient’s group. All ECG signals were calculated from the average of three consecutive cardiac cycles.
The cQT and cQTd were determined as described above(shown in Figure 1). The JT interval was also measured from the end of the QRS complex (J point) to the end of the T wave in the ECG. The JT dispersion was defined as the difference between the minimum and maximum JT duration in any of the 12 ECG leads (JTd = JTmax- JTmin).
2.7 Follow-up
The patients were evaluated by three investigators through outpatient visits at 1, 3, 6, and 12 months while receiving standard treatment according to the 2012 ESC Guidelines for 1 year. In the ICM group, five patients withdrew from the study due to disease deterioration or selection of other treatment options, and three patients could not carry out the process of the 6MWT. Seven patients in the DCM group were excluded because of a change in treatment option, and five patients had wheelchair restriction or cognitive impairment. The loss rates were similar between the two groups (6.67% in the ICM group and 10% in the DCM group, P > 0.05). Figure 2 shows a flow chart of the study design.
2.8 Statistical analysis
Continuous data were expressed as mean ± SD and analyzed by one-way analysis of variance (ANOVA) with the Newman-Keuls post-hoc test. Qualitative data were presented as percentages and then compared using the chisquared test. Pearson’s correlation was used to assess the association between cQTd and the parameters of heart function. A value of P ≤ 0.05 was considered statistically significant. All analyses were performed using SPSS 19.0 software (SPSS, Chicago, IL, USA).
2.9 Ethics
This study was approved by the Ethics Committee of First Affiliated Hospital, Harbin Medical University, China.All enrolled patients signed a written consent form before admission.
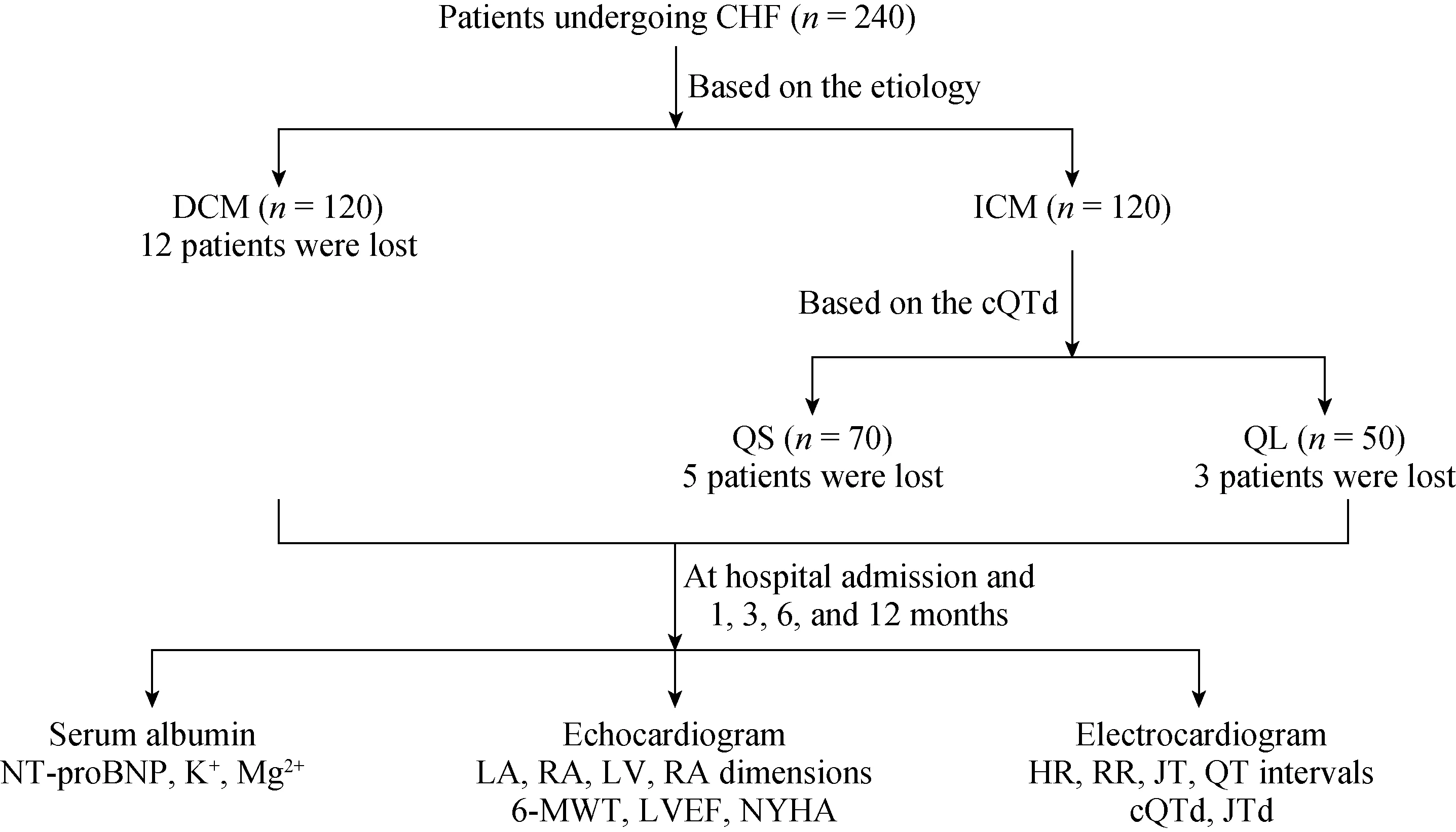
Figure 2. Flow chart of study design. CHF: chronic heart failure; DCM: dilated cardiomyopathy; ICM: ischemic cardiomyopathy; LA:left atrium; LVEDd: left ventricular end-diastolic; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; RA: right atrium; RV: right ventricle; 6MWT: 6-min walking test.

Table 2. Comparison of general characteristics between the two groups at admission.
3 Results
3.1 Comparison of general characteristics
With the exception of history of smoking, the DCM and ICM groups were statistically similar at enrollment (baseline) with regard to demographics (age, gender), BMI, history of diabetes mellitus, hypertension, and some parameters of heart function (P > 0.05; Table 2).
3.2 Changes in laboratory test indicators
At 12 months after enrollment, serum albumin and Mg2+levels were sharply increased from baseline levels, whereas the NT-proBNP level and D-dimer level were strikingly decreased in each group in comparison with baseline values(all P < 0.05; Table 3). Serum albumin levels in the ICM group were even higher than those in the DCM group after 12 months of standard treatment (40.02 ± 0.42 vs. 38.66 ±0.54 g/L; P = 0.049), and the serum albumin level in the ICM group with QL was even higher than that in the DCM group after 12 months of standard treatment (42.25 ± 0.34 vs. 38.66 ± 0.54 g/L; P < 0.05; Table 3).
3.3 Changes in ECG parameters, 6MWT distance, and NYHA classification
At 12 months after enrollment, the following parameters in both groups were remarkably improved compared withthe baseline values: left atrium (LA) dimension, right atrium(RA) dimension, right ventricle (RV) dimension, left ventricular end diastolic diameter (LVEDd), left ventricular ejection fraction (LVEF), 6MWT distance, and NYHA class(P < 0.05; Figure 3). In addition, the patients with ICM had improved heart function including increased LVEF, 6MWT distance, and E/A ratio and a decreased NYHA class as compared with the DCM group at 6 and 12 months (Figure 3H-J; all P < 0.05).

Table 3. Comparison of laboratory testing indicators between the two groups at admission and after 12 months of CHF therapy.

Figure 3. Changes in the ECG parameters in the two groups at baseline and after 1, 3, 6, and 12 months of standard treatment. (A):heart rate; (B): left atrium dimension; (C): right atrium dimension; (D): right ventricle dimension; (E): IVs, interventricular septal thickness at diastolic; (F): LVEDd; (G): 6MWT distance; (H): LVEF; (I): E/A ratio; (J): NYHA classification. Panels 3K to 3N show the changes in the ECG parameters in the two subgroups of ICM patients from baseline to 12 months of standard treatment. (K) 6MWT distance; (L) LVEF;(M) E/A ratio; (N) NYHA classification. *P < 0.05 compared with the same group at admission baseline; #P < 0.05 compared with the DCM group at the same timepoint. DCM: dilated cardiomyopathy; ICM: ischemic cardiomyopathy; LVEDd: left ventricular end-diastolic; LVEF:left ventricular ejection fraction; NYHA: New York Heart Association; 6MWT: 6-min walking test.
At the same time, the 6-WMT distance, LVEF, E/A ratio,and NYHA in the ICM group with QL showed more significant improvement than those in the QS group at each timepoint (Figure 3K-N; all P < 0.05).
3.4 Electrical changes in 12-lead ECG
A lower HR interval and longer RR interval were detected in the ICM group compared with baselines values and these values for the DCM group at 1 month (all P < 0.05;Figure 4A and 4B). After standard treatment, the JT interval in the ICM group was strikingly shorter as compared with that at baseline (Figure 4F; all P < 0.05); however, the JTd did not differ between any two timepoints (Figure 4G).During the 1-year follow-up, only the cQTd among the ECG parameters differed significantly between the two groups (except at 6 months), and the cQTd in the ICM group was significantly shorter after 1 year compared with that at baseline (all P < 0.05; Figure 4E).
3.5 Correlation between cQTd and heart function parameters in the ICM group

Figure 4. ECG parameters in the two groups at baseline and after 1, 3, 6, and 12 months of CHF treatment. (A): Heart rate; (B): RR interval; (C): QRS wave; (D): cQT; (E): cQTd; (F): JT; and (G): JTd. *P < 0.05 compared with the same group at admission; #P < 0.05 compared with the DCM group at the same timepoint. cQTd: corrected QT dispersion; DCM: dilated cardiomyopathy; HR: heart rate; ICM:ischemic cardiomyopathy.

Table 4. Pearson’s correlation analysis between cQTd and UCG variables or other variables in the ICM group.
We performed Pearson’s correlation analysis to identify any associations between the cQTd and other parameters that reflect left ventricular function in ICM patients, which included the ECG parameters and other continuous data. In the ICM group, the cQTd was negatively correlated with LVEF and 6MWT (both P < 0.05) and positively correlated with NYHA class (P < 0.05; Table 4). Even in the ICM group with QL, cQTd was also negatively correlated with LVEF and positively correlated with 6MWT distance and NYHA class (all P < 0.05). No association was evident between the cQTd and NT-proBNP or albumin level (r =-0.052, P = 0.721, and r = 0.087, P = 0.497, respectively).
4 Discussion
In this study, while receiving standard treatment for one year, all patients experienced improved heart function as reflected by 6MWT distance, LVEF, and NYHA class.However, significant shortening of the cQTd, especially with QL, in the ICM group was observed compared with baseline data and compared with that in the DCM group after one month. These findings reveal that recovery of electrical activity precedes improvement of cardiac function,which can possibly be attributed to the effect of standard treatment on myocardial blood supply in ICM patients.
Under ischemic and hypoxic conditions, some myocardium will reduce its function by self-adjustment in order to meet the demand for blood supply. Clinical studies have shown that this phenomenon of myocardial stunning occurs in approximately 50% of the myocardium of ICM patients.[13]The greater the number of coronary arteries involved, the more severe the myocardial ischemia and injury will be, and then the longer the prolongation of QTd. Even if myocardial blood flow is restored in a short time, recovery from myocardial dysfunction can take several hours,weeks, or even months.
After the ICM patients in our study had received standard treatment for 1 year, myocardial perfusion was effectively improved and the function of the myocardium was also partially recovered. Improved heart function further contributed to cardiac electrical stability and synchronization,[14,15]and then the improvement of increased cQTd occurred. Similarly, after 1 year of standard treatment, the DCM patients experienced certain improvement in heart function, but the shorten cQTd was not evident. This is likely because DCM is characterized by diffuse myocardial fibrosis, which does not support increased myocardial blood supply to reverse myocardial injury.
The dispersion of repolarization (expressed as the QT variability index) reflects myocardial structural damage and chronic neuro-humoral activation that characterizes heart failure.[16]Pearson’s correlation analysis revealed that reduction of the cQTd was closely correlated with the changes in heart function in ICM patients, which is partly consistent with a previous study.[17]The improvement of increased cQTd in the ICM patients reflects the recovery of myocardial cell repolarization and heterogeneity, which may delay heart enlargement and improve heart function.[18-21]
Previous research[22]showed that JT and JTd measurements better reflect the risk of arrhythmia compared with cQTd, because these parameters are less dependent on ventricular depolarization. In the present study, significant changes in the JT were also observed in the ICM group at the different timepoints, which is consistent with previous results.[23-25]However, we found no association between the JT and cQTd or heart function in the ICM group during the follow-up.
The present study confirms that the serum NT-proBNP level was remarkably decreased while the albumin level was greatly increased in the two groups after 1 year of standard treatment in comparison with the levels at admission, and changes are associated with the improvement of heart function.[25-27]However, as heart failure indicators, serum NT-proBNP and albumin levels were not found to accurately reflect the changes in heart function at the different timepoints.
Some limitations of this study are that it was conducted at a single center and had a relatively small number of patients and short-term follow-up. Data from Holter monitoring and electrophysiological evaluation would be useful for supporting our observations; however, these initiatives would likely increase the economic burden and pain incurred by the patients. Recent studies show that delayed gadolinium enhancement in cardiovascular magnetic resonance imaging further help diagnose the areas with myocardial fibrosis, however, it will take a long time to apply the technology in China. In comparison, 12-lead ECG is relatively low cost and easy to perform in most medical institutions.[28]
In conclusion, during a 1-year observation, the ICM patients receiving standard treatment showed a more significant improvement in heart function than did patients with DCM, and a progressively shortened cQTd was observed on ECG of ICM patients. Furthermore, we found a strong association between the reduction of the increased cQTd and heart function in the follow-up of ICM patients. These findings reveal that the cQTd may be very meaningful for assessing cardiac function in the follow-up of patients with ICM. Further analysis in different clinical situations is recommended to provide further valuable information for treatment of ICM.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81301276) and the Heilongjiang Provincial Department of Education (No. 12541544).The authors declare that there are no competing interests.
References
1 Spargias KS, Lindsay SJ, Kawar GI, et al. QT dispersion as a predictor of long-term mortality in patients with acute myocardial infarctionand clinical evidence of heart failure.Eur Heart J 1999; 20: 1158-1165.
2 Pan KL, Hsu JT, Chang ST, et al. Prognostic value of QT dispersion change following primary percutaneous coronary intervention in acute ST elevation myocardial infarction. Int Heart J 2011; 52: 207-211.
3 Galetta F, Franzoni F, Fallahi P, et al. Effect of telmisartan on QT interval variability and autonomic control in hypertensive patients with left ventricular hypertrophy. Biomed Pharmacother 2010; 64: 516-520.
4 Harada T, Abe J, Shiotani M, et al. Effect of autonomic nervous function on QT interval in dogs. J T oxicol Sci 2005; 30:229-237.
5 Ahnve S, Vallin H. Influence of heart rate and inhibition of autonomic tone on the QT interval. Circulation 1982; 65:435-439.
6 Baumert M, Schlaich MP, Nalivaiko E, et al. Relation between QT interval variability and cardiac sympathetic activity in hypertension. Am J Physiol Heart Circ Physiol 2011; 300:H1412-1417.
7 Huang CL. Computational analysis of the electromechanical consequences of short QT syndrome. Front Physiol 2015;6: 44.
8 Hombach V. Electrocardiography of the failing heart. Cardiol Clin 2006; 24: 413-426.
9 Brooksby P, Batin PD, Nolan J, et al. The relationship between QT intervals and mortality in ambulant patients with chronic heart failure. The United Kingdom heart failure evaluation and assessment of risk trial (UK-HEART). Eur Heart J 1999; 20: 1335-1341.
10 Kutyifa V, Stockburger M, Daubert JP, et a l. PR interval identifies clinical response in patients with non-left bundle branch block: a multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy sub-study. Circ Arrhythm Electrophysiol 2014; 7: 645-651.
11 Bazett HC. An analysis of the time-relationships of electrocardiograms. Heart 1920; 7: 353-370.
12 Raphael C, Briscoe C, Davies J, et al. Limitations of the New York Heart Association functional classification system and self-reported walking distances in chronic heart failure. Heart 2007; 93: 476-482.
13 Abbas A, Matthews GH, Brown IW, et al. Cardiac MR assessment of microvascular obstruction. Br J Radiol 2015; 88:20140470.
14 Schmidt S, Hürlimann D, Starck CT, et al. Treatment with higher dosages of heart failure medication is associated with improved outcome following cardiac resynchronization therapy. Eur Heart J 2014; 35: 1051-1060.
15 Pouleur AC, Knappe D, Shah AM, et al. MADIT-CHF Investigators. Relationship between improvement in left ventricular dyssynchrony and contractile function and clinical outcome with cardiac resynchronization therapy: the MADIT-CHF trial.Eur Heart J 2011; 32: 1720-1729.
16 Timineri S, Mulè M, Puzzangara E, et al. Selection of patient for cardiac resynchronization therapy: role of QT corrected dispersion. Pacing Clin Electrophysiol 2012; 35: 850-855.
17 Piccirillo G, Magrì D, Ogawa M, et al. Autonomic nervous system activity measured directly and QT interval variability in normal and pacing-induced tachycardia heart failure dogs. J Am Coll Cardiol 2009; 54: 840-850.
18 Davey PP, Barlow C, Hart G. Prolongation of the QT interval in heart failure occurs at low but not at high heart rates. Clin Sci (Lond) 2000; 98: 603-610.
19 Barr CS, Naas A, Freeman M, et al. QT dispersion and sudden unexpected death in chronic heart failure. Lancet 1994;343: 327-329.
20 Davey PP. QT interval lengthening in cardiac disease relates more to left ventricular systolic dysfunction than to autonomic function. Eur J Heart Fail 2000; 2: 265-271.
21 St John Sutton MG, Plappert T, Abraham WT, et al. Multicenter InSync Randomized Clinical Evaluation (MIRACLE)Study Group. Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation 2003; 107: 1985-1990.
22 Vassilikos VP, Karagounis LA, Psichogios A, et al. Correction for heart rate is not necessary for QT dispersion in individuals without structural heart disease and patients with ventricular tachycardia. Ann Noninvasive Electrocardiol 2002; 7:47-52.
23 Lutfi MF. QT interval derived measurements in patients with cardiac syndrome X compared to coronary artery disease.Front Physiol 2016; 7: 422.
24 Braunschweig F, Pfizenmayer H, Rubulis A, et al. Transient repolarization instability following the initiation of cardiac resynchronization therapy. Europace 2011; 13: 1327-1334.
25 Fruhwald FM, Fahrleitner-Pammer A, Berger R, et al. Early and sustained effects of cardiac resynchronization therapy on N-terminal pro-B-type natriuretic peptide in patients with moderate to severe heart failure and cardiac dyssynchrony.Eur Heart J 2007; 28:1592-1597.
26 Berger R, Shankar A, Fruhwald F, et al. Relationships between cardiac resynchronization therapy and N-terminal probrain natriuretic peptide in patients with heart failure and markers of cardiac dyssynchrony: an analysis from the Cardiac Resynchronization in Heart Failure (CARE-HF) study.Eur Heart J 2009; 30: 2109-2116.
27 Uchikawa T, Shimano M, Inden Y, et al. Serum albumin levels predict clinical outcomes in chronic kidney disease (CKD)patients undergoing cardiac resynchronization therapy. Intern Med 2014; 53: 555-561.
28 Punn R, Hanisch D, Motonaga KS, et al. A Pilot Study Assessing ECG versus ECHO Ventriculo ventricular Optimization in Pediatric Resynchronization Patients. J Cardiov asc Electrophysiol 2016; 27: 210-216.
 Journal of Geriatric Cardiology2018年1期
Journal of Geriatric Cardiology2018年1期
- Journal of Geriatric Cardiology的其它文章
- 2016 Chinese guidelines for the management of dyslipidemia in adults
- Appendix
- Chinese expert consensus on the non-invasive imaging examination pathways of stable coronary artery disease
- TAVR in 2017―What we know? What to expect?
- Endocarditis after transcatheter aortic valve implantation: a current assessment
- Antithrombotic therapy in TAVI
