Transcatheter versus surgical aortic valve replacement in severe, symptomatic aortic stenosis
Tsigkas Grigorios, Despotopoulos Stefanos, Makris Athanasios, Koniari Ioanna, Armylagos Stylianos,Davlouros Periklis, Hahalis George
Department of Cardiology, Medical School, University of Patras, Patras, Greece
1 Introduction
One of the most common types of valvular heart disease in the elderly population is aortic stenosis (AS). As the ageing population increases, the prevalence of life-threatening,severe AS will rise.[1]In the US population over 75 year old,12.4% suffer from AS. The current practice regarding severe symptomatic AS is surgical aortic valve replacement(SAVR), but the treatment options has evolved noticeably.[2,3]Transcatheter aortic valve replacement (TAVR) has emerged as a new alternative approach in patients at high surgical risk. Comparison of the two methods is essential for selecting the ideal therapy in each patient. In the current review, we provide all the most recent data regarding the efficacy and safety of TAVR over SAVR, as well as important issues regarding complications’ pattern and incidence of the two techniques.[3]
2 Historical background
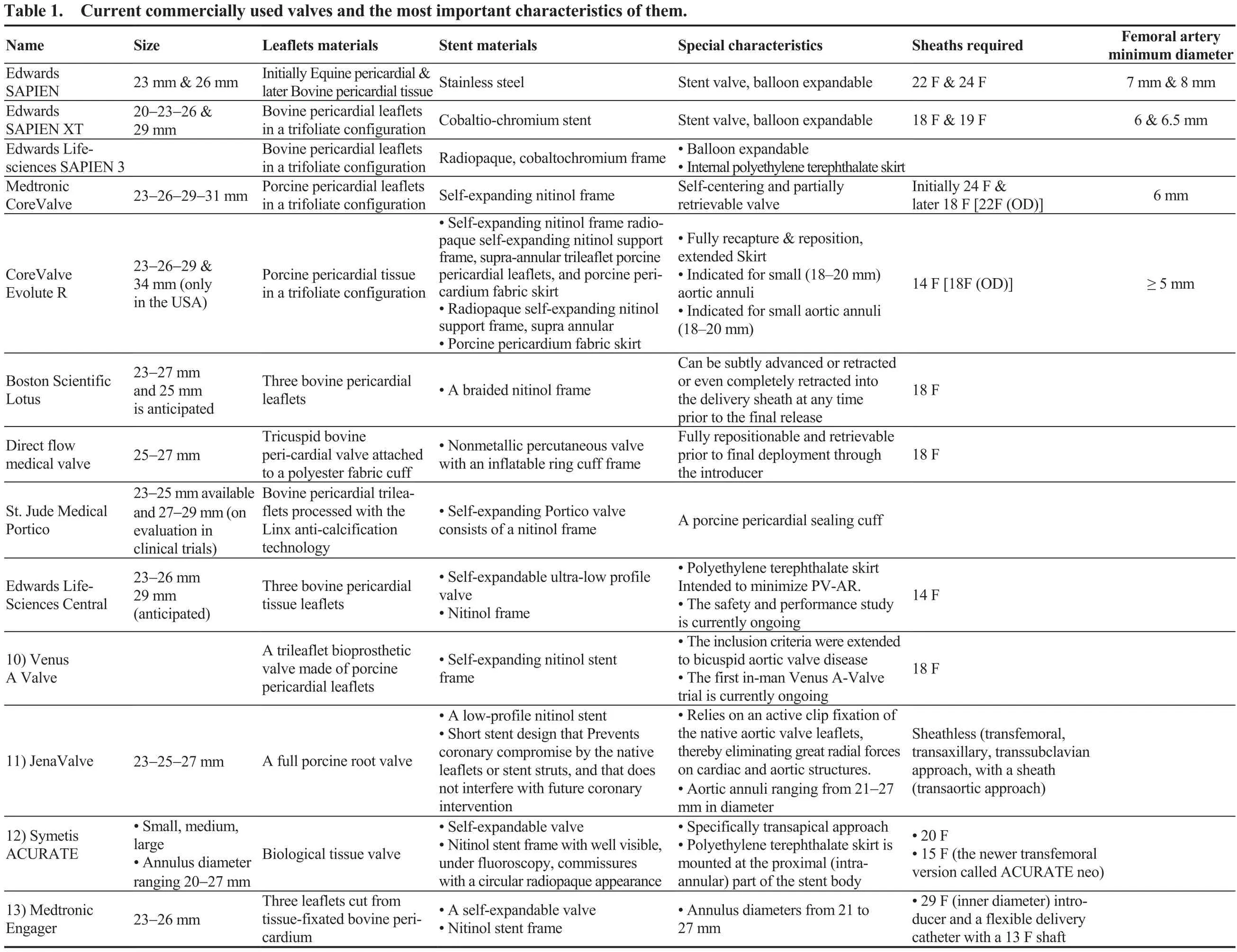
The first transcatheter aortic stent valve in animals was described in 1992, by Andersen, et al.[4]presenting the results of the implantation in pigs. It is notable that the first transcatheter aortic stent valve was implanted in humans, in 2002, using the transeptal approach and femoral vein access due to the bulky characteristics of the first device.[5]In 2005,due to the technology progression, novel techniques and tools led to change the way we approach the patient from the femoral vein to the transfemoral artery position, making the technique easier for the doctor and the patient as well.[6]TAVR, in general, is indicated for high risk patients, using the appropriate risk scores, who suffered from severe symptomatic AS. Currently, FDA expanded the approval for the TAVR technique, for special device, for people with symptomatic AS who are considered to be not only high risk but intermediate as well. The two procedures are completely different since SAVR remains a well standardized technique for most of the patients, but TAVR tends to be the approach for most of them during the following years. The current commercially used valves and the most important characteristics of them are listed in Table 1.
3 Major trials comparing TAVR versus SAVR
3.1 TAVR in high risk patients
The first large trial which compared the TAVR versus standard treatment in high-risk inoperable patients was the PARTNER 1B study.[7]The trial showed superior outcomes from TAVR versus conservative treatment for death from any cause and death from cardiovascular causes. This was a revolutionary step which changed the way physicians approached inoperable patients with severe symptomatic AS.The next step was the PARTNER 1A trial, where 699 high-risk patients with severe AS were randomly assigned to undergo either TAVR with a balloon-expandable bovine pericardial valve (both either transfemoral or the transapical approach) or SAVR.[8]The authors concluded that in highrisk patients with severe AS, the two procedures were associated with similar rates of death from any cause at 1 year.At 30 days, major vascular complications (11.0% vs. 3.2%,P < 0.001) and rates of stroke (8.3% vs. 4.3%, P < 0.05)were more frequent with TAVR over SAVR. The results were consistent even in the 2-year follow up with mortality rates 33.9% for TAVR vs. 35.0% for SAVR (P = 0.78).Importantly, TAVR was associated with an increased late mortality with a hazard ratio of 2.11 (95% CI: 1.43-3.1; P <0.001) due to more mechanical complications of the valve such as paravalvular leak, which was more common in the TAVR group (6.9% for TAVR vs. 0.9% for SAVR, P <0.001, at 2-year follow-up).[8]
A meta-analysis of five randomized trials and 31 observational studies including 16,638 patients showed no mortality difference of TAVR as compared with SAVR but potential benefit in low-to-intermediate risk patients undergoing transfemoral TAVR over SAVR (OR: 0.67; 95% CI:0.42-1.07).[9]It was confirmed that the incidence of periprocedural myocardial infarction, major bleeding, acute kidney injury or new onset atrial fibrillation was lower with TAVR and the risk of pacemaker implantation, vascular complications, and paravalvular leak increased lower with SAVR.[9]
3.2 TAVR in intermediate risk patients
An Italian observational, multicenter, “real-world” study,in a low-intermediate risk population, revealed comparable mortality, major adverse cardiac and cardiovascular events(MACCE) and rates of rehospitalization between SAVR and TAVR in a propensity-matched population of 1300 patient.[10]
In the PARTNER 2A randomized trial, TAVR was compared with SAVR in 2032 intermediate-risk patients. The primary endpoint of all-cause mortality or disabling stroke at two years was to be similar for both groups (Pnoninferiority=0.001). TAVR resulted in higher rate of major vascular complications and more paravalvular regurgitation; while surgical replacement resulted in higher rates of acute kidney injury, severe bleeding and new-onset atrial fibrillation.[11]
The latest Surgical Replacement and Transcatheter Aortic Valve Implantation (SURTAVI) trial was a multinational,randomized, clinical trial which included 1746 patients at intermediate surgical risk, of whom 1660 underwent TAVR or surgical operation.[12]The incidence of primary endpoint,a composite of death from any cause or disabling stroke at 24 months was 12.6% and 14% in the two groups, respectively. Complications followed the same pattern shown in PARTNER 1A and 2A trials[8,11]and the aforementioned meta-analysis.[9]Based on the results of the SURTAVI trial and summarizing all the data regarding the intermediate risk patients, a new IIa indication was given for the TAVR procedure according to the 2017 AHA/ACC focused update of the 2014 AHA/ACC Guideline for the management of patients with valvular heart disease.[13]
4 TAVR vs. SAVR in low risk patients
Despite the already proven efficacy of TAVR, in highand intermediate-risk patients, an expansion of the indication for TAVR in lower-risk patients is not justified. A recent analysis compared outcomes of TAVR vs. SAVR in patients with EuroSCORE II < 4%. The analysis demonstrated similar 30-day survival (97.4% after TAVR and 97.1% after SAVR, P = 0.82) after the two procedures. The survival at three years was 72.0% after TAVR and 83.4%after SAVR (P = 0.0015) with freedom from MACCE of 67.3% and 80.9% (P < 0.001), respectively. Moreover, incidence of cardiac tamponade (4.3% vs. 1.7%; P = 0.049),permanent pacemaker implantation (12.7% vs. 2.6%; P <0.001), major vascular damage (7.6% vs. 0; P < 0.001), and moderate-to-severe paravalvular regurgitation (48.2% vs.11.3%; P < 0.001) was more frequent after TAVR. On the contrary, the rate of cardiogenic shock, severe bleeding and acute kidney injury was higher after SAVR. The mean transvalvular gradient after TAVR was lower than after SAVR (10.6 vs. 14.4 mmHg; P < 0.001). The clear message was that in this low-risk group for surgery, SAVR appears more advantageous than TAVR. Further studies with newgeneration valve prostheses are necessary before expanding indications of TAVR in lower-risk patients.[14]
5 TAVR in patients at extremely high risk of perioperative mortality
A single-center study, which included 319 patients who underwent transapical or transaortic TAVR, compared patients with extremely high risk of perioperative mortality (EuroSCORE > 40%, n = 90) with those with a lower calculated risk (EuroSCORE < 40%, n = 229). There was not statistically significant difference between the two groups in 30-day mortality, stroke rate or the incidence of acute, stage III kidney injury. Statistically significant lower rates in the lowerrisk group were observed regarding need for cardiopulmonary bypass (3.9% vs. 11.1%, P = 0.02) or sternotomy conversion(1.3% vs. 5.6%, P = 0.04), mean ventilation time and length of intensive care unit stay (2.9 vs. 6.8 days, P < 0.001). In conclusion, the safety of TAVR procedure is established even in patients with excessively high risk of perioperative mortality and MACCE.[15]
6 TAVR in nonagenarians
The nonagenarian are frequently an exclusion criterion when designing a randomized study. There is no enough data regarding this under-represented group of patients despite the dramatic increase of the average life expectancy.Meanwhile, the existing data and evidence has not yet confirmed the feasibility and safety of TAVR in this special group of patients. A recent study compared carefully selected patients > 90 years old, without many co-morbidities,versus younger patients who underwent TAVR. Major complications were similar, and all-cause mortality at 30 days and 1 year was not statistically different (2.9% and 12.5% in patients aged ≥ 90 vs. 2.8% and 12.3% in patients aged < 90, respectively). Only minor vascular complications(13.2% vs. 7.7%; P = 0.04) were more frequent among nonagenarians. Thus, clinicians should not deter, judiciously selected, nonagenarian patients with severe, symptomatic AS for TAVR.[16]
7 Low ejection fraction and TAVR
Severe reduced left ventricular ejection fraction (LVEF)is associated with worse outcomes in SAVR, especially when there is no contractile reserve. In a study including low-flow/low-gradient AS patients without contractile reserve undergoing TAVR, perioperative mortality was 22%and the main cause was cardiogenic shock (83.4%).[17]In the German TAVI Registry, 1432 patients who underwent TAVR were separated into two groups (A: LVEF ≤ 30%, n= 169, mean age 79.9 ± 6.7 years, logistic EUROSCORE 34.2% ± 17.8%; B: LVEF > 30%, n = 1263, age 82.0 ± 6.1 years, logistic EUROSCORE 18.9% ± 12.0%). High procedural success rates [95.9% (A) and 97.6% (B)] and excellent outcomes in survival and NYHA classification at 30 days were observed in both groups. Moreover, the quality of life improvement, according to the self-assessment in health condition (scale 0-100) was significant, with a larger benefit for patients of group A (28 vs. 19 patients, P < 0.0001).However, group A was associated with higher rates of mortality at 30 days (14.3% vs. 7.2%) and 1 year (33.7% vs.18.1%, P < 0.001), low cardiac output syndrome (P < 0.01)and need for resuscitation (P < 0.05).[18]
8 TAVR in patients with or without previous coronary artery by-pass graft
The perioperative risk in patients with history of previous coronary artery by-pass graft (CABG) is increased in SAVR.A recent study, based on data from the FRANCE-2 registry,compared the outcome and the overall survival in patients who underwent TAVR with and without history of CABG in 683 patients. On multivariate analysis, CABG was not associated with greater 1-year post-TAVR mortality. Finally,this study concluded that previous CABG not only does not adversely affect outcome in patients who underwent TAVR,but also it could be an alternative procedure to surgery, especially in high-risk patients with history of CABG.[19]
A meta-analysis based on data from five cohort studies evaluated the relative perioperative and long-term survival of patients with previous CABG who underwent TAVR vs.those who underwent SAVR. It was demonstrated that both procedures were equally safe and effective with 1-year allcause-mortality of 17.2% vs. 16.4%, thereby making TAVR an alternative therapy to SAVR in CABG patients.[20]
9 TAVR and previous chest radiation
The patients with severe AS undergoing SAVR and history of mediastinal radiation therapy (XRT) have significantly worse longer-term survival versus a matched cohort group of patients (mortality rate 48% in XRT group vs. 7%in the comparison group at a 6 ± 3 years follow-up), as shown in a recent study.[21]In another study, the authors compared the outcomes in post chest radiation patients with matched control patients undergoing TAVR and tried to identify predictive factors of survival. It was demonstrated that 30-day survival was 92% in both groups, but patients in the radiation group displayed lower, albeit not statistically significant survival rates compared to the controls at 5-year follow-up(33% ± 10% vs. 42% ± 11%; P = NS). Moreover, the main cause of death in the radiation group was respiratory failure. Nevertheless, in this population, it was shown a sustained post-procedural improvement in functional results after TAVR.[22]
10 Redo SAVR or valve-in-valve with TAVR
During the last years, there is an increased rate of bio-prosthetic aortic valves implantations compared with mechanical valves, resulting in an increased need of a future reoperation in case of bioprosthetic valve malfunction.Given the frailty of this sub-group population and their comorbidities, the pending dilemma is redo aortic valve surgery (SAV-in-SAV) or transcatheter valve-in-valve (TAV-in-SAV) implantation. A recent single-centre study with 102 patients showed similar, good early clinical outcomes of both SAV-in-SAV and TAV-in-SAV procedures. In particular, thirty-day mortality was not significantly different between the two groups [TAV-in-SAV vs. SAV-in-SAV: 2(4%) vs. 0, P = 0.238]. However, 1-year survival was statistically significant lower in the TAV-in-SAV than in the SAV-in-SAV group (83% vs. 96%, P < 0.001). It is notable that the patients in the TAV-in-SAV group were significantly older, had a higher mean logistic EuroSCORE and exhibited a lower mean LVEF than patients in the SAV-in-SAV group. Nonetheless, redo surgery still remains the standard of care in cases of failing of surgical bioprosthetic valves.[23]
11 Effectiveness and safety of different TAVR devices versus SAVR
A network meta-analysis which included four trials(CHOICE trial, PARTNER Cohort A trial, STACCATO trial, US CoreValve trial) evaluated the all-cause mortality in patients underwent TAVR (either transapical Sapien,transfemoral Sapien, CoreValve) vs. SAVR. Both procedures appeared similar regarding safety and efficacy and each procedure followed the same pattern of complications,as previously described. Within the same study, a comparison between the transcatheter devices demonstrated less strokes with CoreValve, than with transfemoral or transpical Sapien, whereas pacemaker implantation was more common with the Core-Valve.[24]
12 Peri-procedural bleeding after TAVR versus SAVR
According to a recent paper from Genereux, et al.,[25]bleeding complications after SAVR is about 22.7%. The authors concluded that high-risk patients with AS who enrolled in the PARTNER I randomized trial, bleeding complications occurred more frequently after SAVR than after TAVR and were also related with a worse long-term prognosis (1-year mortality). The implication of bleeding complications on one year clinical outcomes were confirmed in another study with 129 patients who underwent TAVR.[26]The serious bleeding events according to the Valve Academic Research Consortium 2 (VARC-2) criteria were 19.4%,of which 7% were life-threatening/disabling and 12.4%were major bleedings. Even if TAVR seems to be a safer procedure in comparison to SAVR regarding bleeding complications, the latter remain a frequent event and is associated with decreased short and mid-term survival (4.0% vs.20.0%, P = 0.02) and 1-year mortality (11.1% vs. 40.0%, P< 0.002). Moreover, according to the study results, diabetes mellitus (OR: 2.93, 95% CI: 1.08-7.93; P = 0.03) and transsubclavian access (OR: 4.38, 95% CI: 2.13-14.29; P = 0.01)are independent risk factors for serious bleeding events.
13 Antithrombotic therapy following TAVR and SAVR
The administration of antiplatelet treatment after TAVR is not well established and is based on expert opinion (level of evidence C). According to the recently published American Heart Association (AHA) guidelines, clopidogrel 75 mg daily for the first 6 months and life-long aspirin 75-100 mg daily are recommended.[13]
In a pooled analysis of individual patient data from 672 participants who underwent TAVR, single antiplatelet treatment was compared to dual antiplatelet therapy (DAPT). No difference was observed between the two groups in the primary endpoint that was a composite of net adverse clinical and cerebral events (NACE) including MACCE and major bleeding at 30-days. In the aspirin-only group, a NACE rate of 13% was observed vs. 15% in the DAPT group (OR: 0.83,95% CI: 0.48-1.43; P = 0.50). Additionally, there was not statistically significant difference between the two groups in all-cause mortality, ACS, or stroke, with a tendency of less life-threatening and major bleeding events in the aspirin-only group (P = 0.09). Further investigation is recommended by the authors for the value of clopidogrel administration when added to aspirin after TAVR.[27]
In contrast, life-long anticoagulation is not required in patients with aortic bioprostheses. Nevertheless, the risk of ischemic stroke early postoperatively, especially in the first 90 to 180 days after a bioprosthetic AV replacement (AVR),is increased. In a large observational Danish registry, a lower risk of stroke and death with vitamin K antagonists(VKA) therapy extending up to six months was observed,without significantly increase in bleeding events. Thus, the recent AHA/ACC guidelines recommend that anticoagulation with VKA may be reasonable for at least 3 months and perhaps for as long as 6 months after bioprosthetic AVR in patients at low bleeding risk.[28]
Oral anticoagulation with VKA is universally indicated in patients with mechanic aortic valves life-long. In a nationwide population-based study that aimed to demonstrate rates of stroke/thromboembolism and major bleeding in specific age categories and identify risk factors for adverse events in patients with mechanic aortic prostheses, the observation was that the rate of first major bleeding was 2.6 per 100 patient-years with AVR (P < 0.001). Additionally,increasing age and history of major bleeding were identified as independent risk factors for major bleeding incidents.[29]
An observational study that included 890 patients from two registries (RESOLVE and SAVORY) demonstrated data for the prevalence of subclinical leaflet thrombosis in bioprosthetic valves as depicted in 4D computer tomography (CT) imaging after TAVR and SAVR. Additionally, the effect of novel oral anticoagulants (NOACs) on the subclinical leaflet thrombosis was analyzed. Higher rates of subclinical leaflet thrombosis were observed in TAVR than in SAVR group (13% vs. 4%) and in patients on DAPT than in patients on oral anticoagulants (15% vs. 4%); NOACS and warfarin were equally effective. Moreover, subclinical leaflet thrombosis resolved in 100% of patients treated with oral anticoagulants (NOACs or warfarin), but it remained or even progressed in 91% of patients that did not receive anticoagulants (P < 0.0001). The comments from the authors according to these results were that it seems more reasonable to prescribe oral anticoagulation especially for the younger patients.[30]
14 Impact of anesthesia type on outcomes
There is still no wide-ranging consensus concerning the use of general instead of local anesthesia with sedation throughout the TAVR procedure. Data from the multicenter ADVANCE study showed clearly that both types are equally safe without significant differences between them, regarding all-cause mortality (25.4% vs. 23.9%, P = 0.78), cardiovascular mortality or stroke through 2-year follow-up. As expected, major vascular complications were more likely to happened, in the local anesthesia group, due to inappropriate movements of the patient. Moreover, the total hospital stay was comparable between the two types of anesthesia, concluding that the final decision has to be individualized.[31]
The results of another recent retrospective study confirmed the above data. The authors concluded that there was no significant difference between the two types of anesthesia regarding the mid-term survival.[32]Importantly, considering the access route for TAVR (transapical or transfemoral), no significant difference could be found in the mid-term survival.[32]
15 Transfemoral TAVR vs. Transapical TAVR
An important issue regarding high risk patients who are unable to proceed with SAVR, is safety and efficacy between the transfemoral transcatheter aortic valve replacement (TF-TAVR) as compared with the transapical transcatheter aortic valve replacement (TA-TAVR). There are controversial results in the recent literature. In the observational study of effectiveness of avR-TAVI procedures for severeaortic stenosistreatment study (OBSERVANT study),patients who underwent TF-TAVR and TA-TAVR were evaluated on the immediate and intermediate outcome with propensity score matching. The 30-days frequencies after TA-TAVR over TF-TAVR in the 199 matched pairs were as follows: 8.0% and 4.0% for mortality (P = 0.102), postoperative stroke 2.0% and 1.0% (P = 0.414), cardiac tamponade 4.1% and 1.5% (P = 0.131), pacemaker implantation 8.7% and 13.3%, P = 0.414) and infections 6.7% and 3.6% (P = 0.180). Furthermore, TA-TAVR was associated with significant more red blood cell transfusion (P = 0.0002)and acute kidney injury (P < 0.0001) compared with TFTAVR. Three-year survival rate was 69.1% after TF-TAVR and 57.0% after TA-TAVR (P = 0.006), whereas freedom from MACCEs was 61.9% after TF-TAVR and 50.4% after TA-TAVR (P = 0.011). Therefore, the transfemoral approach whenever feasible, should be considered the preferable access route for TAVR.[33]
A confirming retrospective review from Mayo Clinic showed that the access did not influence treatment-related mortality rates. The transapical access was chosen in patients with more comorbidities [higher Society of Thoracic Surgeons score (STS), more peripheral vascular disease, older patients, more previous CABG]. Despite differences in baseline patients’ risk, statistically significant difference was only shown in the rate of paravalvular regurgitation grade(transapical, 2.9%; transfemoral, 10.4%; P = 0.001). The conclusion was in line with the previous demonstrated results, confirming that access does not have impact on treatment-related mortality rates.[34]
A meta-analysis of 14 published studies showed that the 30-day mortality was 7.5% versus 11.6% respectively with similarly higher rates for stroke and MACCE favoring the transfemoral access.[35]
16 TF-TAVR vs. TA-TAVR in sub-groups without difference in Logistic Euroscore
Given the imbalance of the logistic EuroSCOREs between TA and TF patients, a recent Chinese meta-analysis assessed the rates in mortality and other complications among patients undergoing either TF-TAVR (n = 666) or TA-TAVR (n = 457) without significant between-group differences regarding the logistic EuroSCORE. This analysis revealed that TF-TAVR has a higher risk of vascular complication (14.7% vs. 7.1%, P = 0.01) and postoperative heart block (13.4% vs. 4.6%, P = 0.03), but a similar incidence of stroke (4.7% vs. 2.6%, P = 0.21) and mortality at 30 days (9.2% vs. 11.4%; P = 0.14) as well as beyond one year. According to these results, TA approach is an alternative and feasible choice for patients with severe AS.[36]
17 Implantation of permanent pacemaker in patients after TAVR
Atrioventricular conduction disturbances needing permanent pacemaker implantation (PPMI) in patients after TAVR, is one of the most common complications of this procedure.[37]On the other hand, the need for PPMI subsequently to SAVR is uncommon. In a recent meta analysis, the overall prevalence of PPMI after SAVR is in the order of 5.0% [SAVR alone, 4.8%; SAVR + CABG, 4.6%; SAVR +MVR (mitral valve replacement), 7.7%; SAVR + MVR:10%].[38]The incidence of PPMI depends on the design and the ability of precise delivery of the prosthesis. According to the FRANCE-2 registry, the developments of delivery devices of the CoreValve system failed to translate into a significant decrease in the incidence of PPMI after TAVR,with PPMI occurring in about 28%-30% of the patients and all-cause mortality being similar between the groups that received or not received PPM (16.3 vs. 16.9%, P = 0.82).Moreover, PPMI does not necessarily imply long-term pacing dependence, thereby supporting the view of the need for new valve and delivery system technologies in order to reduce PPMI rates.[39]In the aforementioned SURTAVI trial,the need of PPMI was 25.9% after TAVR, with similar rates among patients who received either CoreValve (25.5%) or EvolutR valve (26.7%).[12]In a multicenter study that aimed to evaluate whether TAVR with SAPIEN-3 is a viable alternative to SAVR in intermediate or high risk patients, the rate of new pacemaker implantation was 13.3% at 30 days,a rate lower than that referred with self-expanding valves.[40]
18 Pulmonary hypertension in patients undergoing TAVR
Pulmonary hypertension (PH) is a very strong predictive risk factor of perioperative mortality in patients undergoing TAVR and is included both in the STS and EuroSCORE logistic calculation. A recent study assessed the impact of PH on prognosis. A pre-procedural right-heart catheterization determined pre-capillary or post-capillary PH categories and demonstrated that pre-capillary PH did not improve after TAVR (49.0 ± 12.6 vs. 51.6 ± 14.3 mmHg; P = 0.36),while post-capillary PH improved (57.8 ± 14.1 vs. 50.4 ±17.3 mmHg; P = 0.015). Nevertheless, both PH groups had by two to three-fold higher 1-year mortality rate, when compared with patients without preexistent PH.[41]
19 Obesity paradox and TAVR
It is common knowledge that elevated body mass index(BMI) is a predictor of mortality after surgery.[42]An Italian meta-analysis with 10,196 patients found that greater BMI is associated with significantly less in-hospital and 30-day early (P = 0.001) less mid-term as well as long-term post-TAVR mortality (P = 0.04). The authors concluded that obviously there is an obesity paradox in TAVR procedures.[43]
20 Arterial hypertension after TAVR
It is accepted that morbidity and mortality after SAVR increase when arterial hypertension (HT) coexists. The same applies in patients with uncontrolled HT (blood pressure ≥ 140/90 mmHg) undergoing TAVR. In one study, all-cause (6.5% vs. 16%; P = 0.04) and cardiovascular(3.7% vs. 11.6%, P = 0.035) mortality was significantly higher among hypertensive as compared with normotensive patients. The uncontrolled HT at the time of discharge may also contribute to delayed, post-procedural symptomatic improvement as assessed either with New York Heart Association (NYHA) functional class (class increase of: 1.4 ± 0.8 vs. 0.8 ± 1.0; P = 0.002), or with the six-minute walk test(6-MWT) (distance increase: 100 ± 71 vs. 30 ± 64 m; P <0.001).[44]
21 Minimally invasive strategy versus conventional strategy
A retrospective study assessed the safety and efficacy of a minimally invasive strategy (MIS) versus conventional strategy (CS) during transfemoral TAVR cases. MIS is based mostly on local anesthesia and conscious sedation,performed in the cath laboratory without transesophageal echocardiography guidance. They used all commercially available valves. Even though the baseline characteristics were similar and procedural success was comparable (99.1%in MIS and 98.9% in CS, P = NS), Minimally invasive strategy was associated with notable cost-saving, thanks to shorter postprocedural hospital stay [median, 3.0 (2.0-5.0)days for MIS vs. 6.0 (3.5-8.0) days for CS] and lower cost.Moreover, both procedures showed similar survival rates at a median follow-up of 230 days.[45]
22 Access-related vascular complications
The ideal method of access for transfemoral TAVR is not yet well documented. One study compared the surgical cut-down approach, with the complete percutaneous access.Surgical cut-down technique provided less access and bleeding complications (20.6% vs. 8.1%, P = 0.04 and 18.1% vs. 4.4%, P = 0.03, respectively), but did not differ with the percutaneous approach regarding major complications (P = NS). It appears that both techniques are complementary and necessary for an individualized arterial access depending on patient’s characteristics and anatomy, expertise of the interventional cardiologists and the availability of vascular surgeons.[46]
23 First versus second generation selfexpandable transcatheter heart valves
A case-matched comparison of new type, nitinol-based second generation (SymetisAcurate Neo TF™) heart valves and first generation, self-expandable valves (CoreValve™),showed superiority regarding the post-interventional pressure gradients (7.0 ± 2.8 vs. 8.8 ± 4.0 mmHg, P = 0.006),the effective orifice area (EOA) (1.9 ± 0.3 vs. 1.8 ± 0.2 cm2,P = 0.015), the severity of residual paravalvular leakage(PVL, 2.9% vs. 15.94%, P = 0.013) and the rate of PPM implantation (8.7% vs. 44.9%, P < 0.001). However, there was not shown statistically significant differences in the all-cause 30-day mortality rate (5.8% vs. 10.14%, P = 0.36)and in the disabling stroke (2.9% vs. 5.8%, P = 0.41), respectively. Thus, further clinical evaluation through larger patient cohorts is needed, but the results were really promising.[47]
24 Cost-effectiveness of TAVR vs. SAVR
TAVR as compared with SAVR is a similarly safe, effective and efficacious alternative treatment option for high risk patients with severe, symptomatic AS. A number of studies have addressed the cost-effectiveness of the procedure. A more detailed analysis on this issue can be found in the paper of Kourkoveli, et al.[48]in this issue of the Journal Geriatric of Cardiology. In-hospital cost, length of stay and outcomes between TAVR and SAVR were studied in a trial which compared 595 TAVR patients matched with 1785 SAVR patients in a 1: 3 ratio. The results showed no difference in mean ($181,912 vs. $196,298) or median ($152,993 vs. $155,974) hospital cost between TAVR and SAVR (P =0.60), while the TAVR patients had significant shorter duration of hospital stay (mean 9.76 vs. 12.01 days, P < 0.001,respectively). Post procedural in-hospital death or stroke did not show any significant difference. The authors concluded that TAVR is an equal alternative to SAVR regarding financial data and leads in an approximately 2-day shorter duration of hospitalization.[49]
25 Future considerations
Because pharmacological therapy is not effective in severe, symptomatic AS and sometimes could be harmful, the only effective treatment is TAVR. TAVR is now approved mostly for those patients who are not surgical candidates due to advanced age and comorbid conditions. Following the increasing experience in the real life and given the fact that the logistics are becoming less prohibitive in favor of the less invasive approach, TAVR came here to stay as a treatment of choice in inoperable, high-risk and intermediate risk patients. This is an important modification of the way the medical community has faced so far a severe and frequent medical situation such as severe AS. The expansion of the indications for TAVR in intermediate-risk patients is viewed as an evolutionary therapy to the TAVR revolution.The expansion of the indication for the TAVR is based on positive clinical data from the Nordic Aortic Valve Intervention (NOTION) trial, from a subset analysis from the CoreValve U.S. High Risk Pivotal trial and more recently incorporating the results of the SURTAVI trial. The results revealed that similar clinical outcomes to SAVR can be accomplished by using the TAVR technique in patients who are mostly surgical candidates, including low rates of allcause mortality and major stroke, low incidences of procedural complications and excellent blood flow with the newer TAVR technique, mostly with the CoreValve Evolut R bioprosthesis valve.
26 Conclusions
TAVR revolutionized the landscape of structural heart disease by halving mortality over medically-treated patients and showing at least the same good results as SAVR both in high-risk and intermediate-risk patients with severe symptomatic aortic stenosis. Future technical developments and randomized tials will probably broaden the spectrum of indications for TAVR toward lower-risk patients with severe AS.
References
1 Osnabrugge RL, Mylotte D, Head SJ, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol 2013; 62: 1002-1012.
2 Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63: e57-e185.
3 Bonow RO, Carabello BA, Chatterjee K, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008; 52: e1-e142.
4 Andersen HR, Knudsen LL, Hasenkam JM, et al. Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur Heart J 1992; 13:704-708.
5 Cribier AG. The odyssey of TAVR from concept to clinical reality. Tex Heart Inst J 2014; 41: 125-130.
6 Webb JG, Binder RK. Transcatheter aortic valve implantation:the evolution of prostheses, delivery systems and approaches.Arch Cardiovasc Dis 2012; 105: 153-159.
7 Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364: 2187-2198.
8 Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012; 366: 1686-1695.
9 Gargiulo G, Sannino A, Capodanno D, et al. Transcatheter aortic valve implantation versus surgical aortic valve replacement: a systematic review and meta-analysis. Ann Intern Med 2016; 165:334-344.
10 Tamburino C, Barbanti M, D'Errigo P, et al. One-Year Outcomes after transfemoral transcatheter or surgical aortic valve replacement: results from the Italian OBSERVANT Study. J Am Coll Cardiol 2015; 66: 804-812.
11 Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016; 374: 1609-1620.
12 Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med 2017; 376: 1321-1331.
13 Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation 2017;135:e1159-e1195.
14 Rosato S, Santini F, Barbanti M, et al. Transcatheter aortic valve implantation compared with surgical aortic valve replacement in low-risk patients. Circ Cardiovasc Interv 2016; 9: e003326.
15 Goebel N, Ahad S, Schaeufele T, et al. Transcatheter aortic valve implantation in patients at extremely high risk of perioperative mortality. J Heart Valve Dis 2015; 24: 635-639.
16 Abramowitz Y, Chakravarty T, Jilaihawi H, et al. Comparison of outcomes of transcatheter aortic valve implantation in patients ≥ 90 years versus < 90 years. Am J Cardiol 2015; 116:1110-1115.
17 Tribouilloy C, Lévy F, Rusinaru D, et al. Outcome after aortic valve replacement for low-flow/low-gradient aortic stenosis without contractile reserve on dobutamine stress echocardiography. J Am CollCardiol 2009; 53: 1865-1873.
18 Schaefer U, Zahn R, Abdel-Wahab M, et al. Comparison of outcomes of patients with left ventricular ejection fractions ≤30% versus ≥ 30% having transcatheter aortic valve implantation (from the German Transcatheter Aortic Valve Interventions Registry). Am J Cardiol 2015; 115: 656-663.
19 Castellant P, Didier R, Bezon E, et al. Comparison of outcome of transcatheter aortic valve implantation with versus without previous coronary artery bypass grafting (from the FRANCE 2 Registry). Am J Cardiol 2015; 116: 420-425.
20 Ando T, Briasoulis A, Holmes AA, et al. Transcatheter aortic valve replacement versus surgical aortic valve replacement in patients with previous coronary artery bypass surgery: a systematic review and meta-analysis. Int J Cardiol 2016; 215: 14-19.
21 Donnellan E, Masri A, Johnston DR, et al. Long-term outcomes of patients with mediastinal radiation-associated severe aortic stenosis and subsequent surgical aortic valve replacement: a matched cohort study. J Am H eart Assoc 2017; 6:e005396.
22 Bouleti C, Amsallem M, Touati A, et al. Early and late outcomes after trans-catheter aortic valve implantation in patients with previous chest radiation. Heart 2016; 102: 1044-1051.
23 Erlebach M, Wottke M, Deutsch MA, et al. Redo aortic valve surgery versus transcatheter valve-in-valve implantation for failing surgical bioprosthetic valves: consecutive patients in a singlecenter setting. J Thorac Dis 2015; 7: 1494-1500.
24 Biondi-Zoccai G, Peruzzi M, Abbate A, et al. Network metaanalysis on the comparative effectiveness and safety of transcatheter aortic valve implantation with CoreValve or Sapien devices versus surgical replacement. Heart Lung Vessel 2014; 6:232-243.
25 Généreux P, Cohen DJ, Williams MR, et al. Bleeding complications after surgical aortic valve replacement compared with transcatheter aortic valve replacement: insights from the PARTNER I trial (Placement of Aortic Transcatheter Valve). J Am Coll Cardiol 2014; 63: 1100-1109.
26 Kochman J, Rymuza B, Huczek Z, et al. Incidence, predictors and impact of severe periprocedural bleeding according to VARC-2 criteria on 1-year clinical outcomes in patients after transcatheter aortic valve implantation. Int He art J 2016; 57:35-40.
27 Hassell ME, Hildick-Smith D, Durand E, et al. Antiplatelet therapy following transcatheter aortic valve implantation. Heart 2015; 101: 1118-1125.
28 Mérie C, Køber L, Skov Olsen P, et al. Association of warfarin therapy duration after bioprosthetic aortic valve replacement with risk of mortality, thromboembolic complications, and bleeding. JAMA 2012; 308: 2118-2125.
29 Labaf A, Svensson PJ, Renlund H, et al. Incidence and risk factors for thromboembolism and major bleeding in patients with mechanical valve prosthesis: a nationwide populationbased study. Am Heart J 2016; 181: 1-9.
30 Chakravarty T, Søndergaard L, Friedman J, et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet 2017; 389: 2383-2392.
31 Brecker SJ, Bleiziffer S, Bosmans J, et al. Impact of anesthesia type on outcomes of transcatheter aortic valve implantation(from the Multicenter ADVANCE Study). Am J Cardiol 2016;117: 1332-1338.
32 Gauthier C, Astarci P, Baele P, et al. Mid-term survival after transcatheter aortic valve implantation: results with respect to the anesthetic management and to the access route (transfemoral versus transapical). Ann Card Anaesth 2015; 18: 343-351.
33 Biancari F, Rosato S, D'Errigo P, et al. Immediate and intermediate outcome after transapical versus transfemoral transcatheter aortic valve replacement. Am J Cardiol 2016; 117: 245-251.
34 Murashita T, Greason KL, Pochettino A, et al. Clinical outcomes after transapical and transfemoral transcatheter aortic valve insertion: an evolving experience. Ann Thorac Surg 2016;102: 56-61.
35 Zhao A, Minhui H, Li X, et al. A meta-analysis of transfemoral versus transapical transcatheter aortic valve implantation on 30-day and 1-year outcomes. Heart Surg Forum 2015; 18: E161-E166.
36 Liu Z, He R, Wu C, et al. Transfemoral versus transapical aortic implantation for aortic stenosis based on no significant difference in logistic EuroSCORE: A meta-analysis. Thorac Cardiovasc Surg 2016; 64: 374-381.
37 Sharma PS, Subzposh FA, Ellenbogen KA, et al. Permanent His-bundle pacing in patients with prosthetic cardiac valves.Heart Rhythm 2017; 14: 59-64.
38 Robich MP, Schiltz NK, Johnston DR, et al. Risk factors and outcomes of patients requiring a permanent pacemaker after aortic valve replacement in the United States. J Card Surg 2016; 31:476-485.
39 Ramazzina C, Knecht S, Jeger R, et al. Pacemaker implantation and need for ventricular pacing during follow-up after transcatheter aortic valve implantation. Pacing Clin Electrophysiol 2014; 37: 1592-1601.
40 Webb J, Gerosa J, Lefevre T, et al. Multicenter evaluation of a next-generation balloon-expandable transcatheter aortic valve. J Am Coll Cardiol 2014; 64: 2235-2243.
41 O'Sullivan CJ, Wenaweser P, Ceylan O, et al. Effect of pulmonary hypertension hemodynamic presentation on clinical outcomes in patients with severe symptomatic aortic valve stenosis undergoing transcatheter aortic valve implantation: insights from the new proposed pulmonary hypertension classification. Circ Cardiovasc Interv 2015; 8: e002358.
42 Turrentine FE, Hanks JB, Schirmer BD, et al. The relationship between body mass index and 30-day mortality risk, by principal surgical procedure. Arch Surg 2012; 147: 236-242.
43 Takagi H, Umemoto T. “Obesity paradox” in transcatheter aortic valve implantation. J C ardiovasc Surg (Torino) 2017; 58:113-120.
44 Reinthaler M, Stähli BE, Gopalamurugan AB, et al. Post-procedural arterial hypertension: implications for clinical outcome after transcatheter aortic valve implantation. J Heart V alve Dis 2014; 23: 675-682.
45 Attizzani GF, Alkhalil A, Padaliya B, et al. Comparison of outcomes of transfemoral transcatheter aortic valve implantation using a minimally invasive versus conventional strategy. Am J Cardiol 2015; 116: 1731-1736.
46 Spitzer SG, Wilbring M, Alexiou K, et al. Surgical cut-down or percutaneous access-which is best for less vascular access complications in transfemoral TAVI? Catheter Cardiovasc Interv 2016; 88: e52-e58.
47 Schaefer A, Treede H, Schoen G, et al. Improving outcomes:case-matched comparison of novel second-generation versus first-generation self-expandable transcatheter heart valves. Eur J Cardiothorac Surg 2016; 50: 368-373.
48 Kourkoveli P, Spargias K, Hahalis G. TAVR in 2017―What we know? What to expect? J Geriatr Cardiol; 2018; 15: 55-60.
49 Minutello RM, Wong SC, Swaminathan RV, et al. Costs and in-hospital outcomes of transcatheter aortic valve implantation versus surgical aortic valve replacement in commercial cases using a propensity score matched model. Am J Cardiol 2015;115: 1443-1447.
 Journal of Geriatric Cardiology2018年1期
Journal of Geriatric Cardiology2018年1期
- Journal of Geriatric Cardiology的其它文章
- 2016 Chinese guidelines for the management of dyslipidemia in adults
- Appendix
- Chinese expert consensus on the non-invasive imaging examination pathways of stable coronary artery disease
- Improvement of increased cQTd is associated with heart function in patients with ischemic heart failure
- TAVR in 2017―What we know? What to expect?
- Endocarditis after transcatheter aortic valve implantation: a current assessment
