Systemic therapy for cervical carcinoma – current status
Krystyna Serkies, Jacek Jassem
Department of Oncology and Radiotherapy, Medical University of Gdańsk, Poland
Introduction
Cervical cancer (CC) is the fourth most common cancer in women, amounting to 528,000 new cases and 266,000 deaths annually worldwide (1). Despite advances in screening and treatment strategies, a significant number of CC patients, especially in less-developed countries, still present with advanced disease; and many others will develop failure after curative primary therapy. For most of these patients, palliative treatments remain the standard of care.Radiation therapy (RT) along with radical surgery (RS) is the mainstay of CC treatment. The efficacy of both methods is similar in early disease [(International Federation of Gynecology and Obstetrics (FIGO) stages I,IIA)] (2), whereas RT is the treatment of choice in locally advanced CC (LACC; stages IB2, IIB to IVA). The efficacy of RT decreases with the tumor size. In consequence,30%—70% of patients with LACC will experience locoregional failure, with or without accompanying distant recurrence. In patients managed with RS, adverse pathological features include the involvement of lymph nodes and parametrium, positive surgical margins, lymphvascular space invasion (LVSI) or large and deep tumor invasion. Depending on various prognostic factors,reported pelvic recurrence rates after RS vary from 7% to 58%, with distant metastasis rates up to 41%. Nearly half of the patients with loco-regional failure will develop extrapelvic recurrence. Various efforts, including incorporation of systemic therapies, have been made to increase the efficacy of curative local therapies for LACC and high-risk early-stage patients. In advanced or recurrent patients who are not amenable to curative treatments,especially surgery (3), systemic therapy plays a major palliative role. Chemotherapy continues to be the established form of systemic therapy for CC. This article reviews the role of systemic therapy including chemotherapy,targeted therapy and immunotherapy for CC patients.
Chemotherapy combined with definitive local therapies
Concomitant chemotherapy and definitive RT
Selected randomized controlled trials (RCTs) addressing the role of chemotherapy combined with definitive RT are presented inTable 1(4-14).
Improved treatment outcomes with concomitant chemoradiotherapy (CCRT) may be due to increased killing of tumor cells, inhibiting repair of cell radiation damage, synchronization of tumor cells, recruiting nonproliferating tumor cells into the cell cycle, and sensitization of hypoxic tumor cells. However, these benefits are achieved at the expense of enhanced toxic effects.
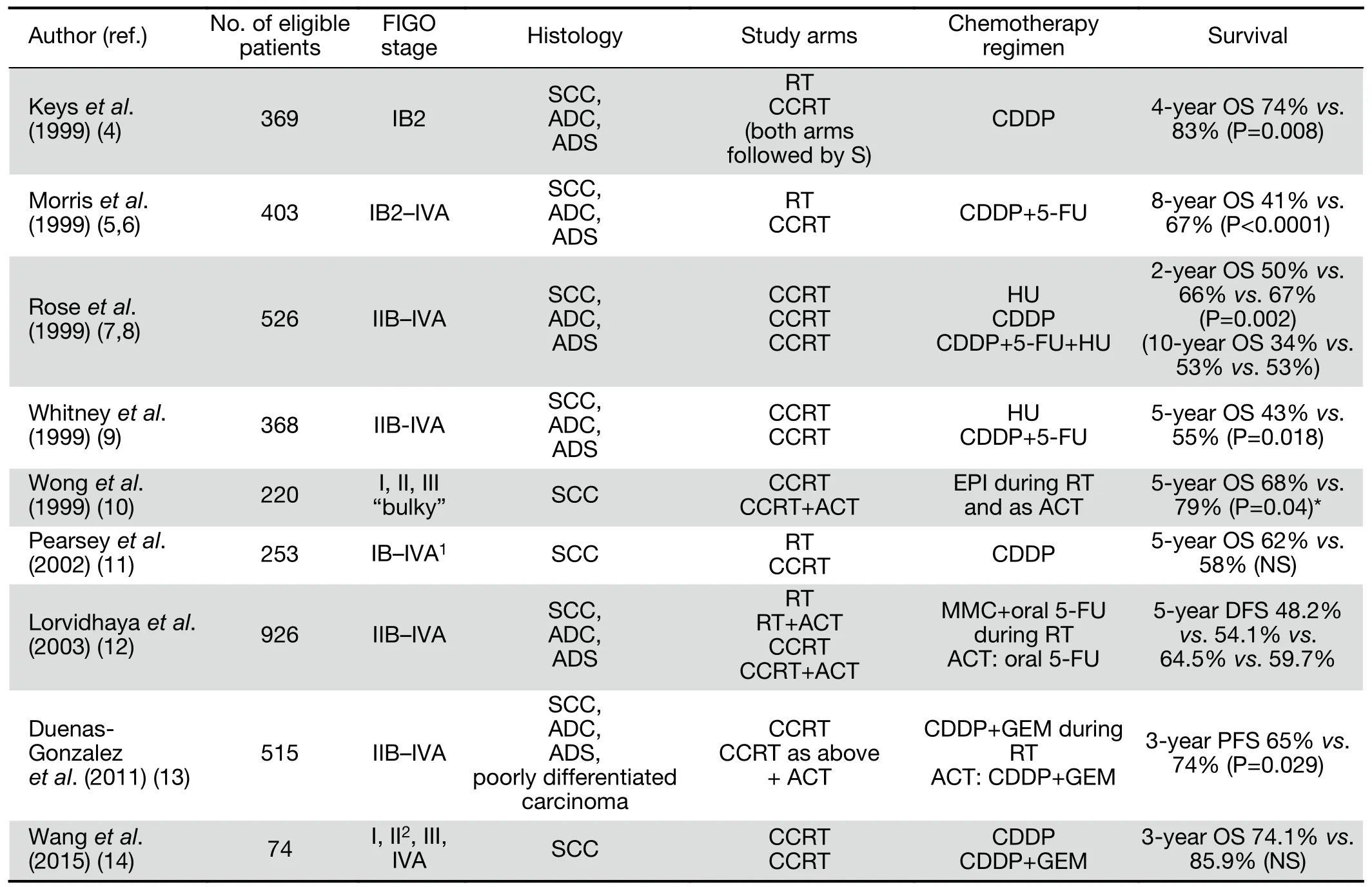
Table 1 Selected phase III trials addressing the role of chemotherapy concomitant and adjunctive to RT
A series of RCTs were performed in the 1990s,comparing cisplatin-based CCRT with RT alone or RT combined with hydroxyurea in various stages of CC managed with definitive or preoperative irradiation(4,5,7,9). These trials, involved women with all stages of CC, and used different inclusion criteria, staging methods to rule out para-aortic node involvement (lymphangiography,computed tomography or surgical exploration),chemotherapy and RT schedules. The absolute survival benefits with the addition of cisplatin-based chemotherapy to RT ranged from 9% to 18%, with a significant reduction in the relative risk of recurrence and death (a mean of 50% and 30%, respectively). In consequence, in 1999 the US National Cancer Institute recommended the addition of cisplatin-based chemotherapy concomitantly with RT in all patients irradiated for CC (15).
The beneficial effect of chemotherapy added to RT in CC was demonstrated in meta-analysis of RCTs carried out between 1981 and 2000 (16). There was an apparent inconsistency among the control group setting of each study though. The overall hazard ratio (HR) of death was 0.71 (P<0.0001) in favor of chemotherapy; 0.70 (P<0.0001)and 0.81 (P=0.20), respectively for trials that did, and did not, use cisplatin. A greater beneficial effect was observed in trials including a high proportion of stage I and II patients. The highly significant reduction in the risk of both local recurrence and distant metastases suggests a systemic cytotoxic effect of chemotherapy.
The superiority of platinum-based [overall survival (OS):HR=0.83, P=0.017] and non-platinum-based CCRT (OS:HR=0.77, P=0.009) over RT alone was supported by metaanalysis conducted in 2010, which included individual patient data (IPD) from 13 trials published before 2005(17). Significantly improved OS and progression-free survival (PFS) in the cisplatin-RT-treated subgroup of high-risk (LACC or bulky tumor) patients was demonstrated in 2016 meta-analysis, including recent data from 8 RCTs and 3 cohort studies (18). The pooled HR for OS and PFS were 0.68 and 0.63, respectively. The benefit of CCRT compared to RT alone seems to diminish in later-stage disease (16,19,20). Estimated absolute 5-year survival benefits are 10% at stages IA—IIA, 7% at stage IIB,and 3% at stages III—IVA (19). The lack of significant benefits of CCRT including cisplatin-based chemotherapy in some trials may be attributed to several factors, such as different study designs, patient characteristics, control settings,regimens used, RT duration, and duration of follow-up(6,11,20). As negative results of CCRT were noted in cohort studies in Asian women, a potential racial difference for different CCRT regimens was also suggested (18).
Increased peak concentration of cisplatin, surgery following CCRT, consolidation chemotherapy following CCRT, and neoadjuvant chemotherapy (NACT) before CCRT, were strategies expected to enhance the therapeutic efficacy of CCRT in CC patients with large tumors and radiologically enlarged lymph nodes (high-risk group) (21).
There is currently no published randomized data comparing definitive CCRT and RT alone in patients with stage IB1/IIA1 CC. More recent large retrospective analysis of stage IB1/IIA1 patients managed without surgery demonstrated an OS improvement with the addition of chemotherapy to RT (22).
Chemotherapy in postoperative setting for early-stage CC
The RCTs addressing the role of chemotherapy in the postoperative adjuvant therapy are presented inTable 2(23-27).
Postoperative platinum-based CCRT or RT alone decreases the risk of loco-regional recurrence and is recommended as the standard management in early-stage CC patients with high- or intermediate-risk factors for recurrence (15,25,28,29). However, a secondary retrospective analysis of the Gynecologic Oncology Group(GOG) trial demonstrated that the addition of postoperative chemotherapy to RT may be relatively less beneficial in patients with small (<2 cm) tumors (26). In patients with intermediate-risk factors the benefit of adjuvant CCRT over adjuvant RT alone remains unclear(30). Moreover, survival improvement with the addition of platinum-based chemotherapy to adjuvant RT in early stage CC (IA2—IIA), was at the expense of an increased risk of severe toxicity (31).
To reduce extrapelvic recurrence, postoperative chemotherapy alone using paclitaxel/cisplatin regimen was suggested as an alternative strategy (32). In a large subgroup of patients with pelvic and/or para-aortic metastasis, postoperative chemotherapy alone as compared to CCRT was associated with a higher local recurrence rate(23%vs. 14%, P=0.001), lower distant recurrence rate(19%vs. 24%, P<0.001) and similar specific CC mortality(33). Similar outcome with better toxicity profile of adjuvant chemotherapy (ACT) compared with CCRT for high-risk CC patients was demonstrated in another retrospective study (34).
Postoperative sequential chemotherapy includingpaclitaxel/carboplatin preceding RT was not superior to standard CCRT with weekly cisplatin in high-risk CC (27).

Table 2 Phase III trials addressing the role of chemotherapy in the postoperative adjuvant therapy for early-stage CC with pathological risk factors
Further improvement in adjuvant therapy should emerge from an improved definition of prognostic risk factors,better patient selection, and refinements in both local and systemic therapies (29).
Consolidation chemotherapy after concurrent chemoradiation
ACT following CCRT is expected to be the most beneficial for LACC. ACT in combination with CCRT was used in a few RCTs (10,12,25). The efficacy of ACT consisting of mitomycin C and oral 5-fluorouracil was directly addressed in the large study that compared RT alone with RT combined with concomitant, adjuvant or concomitant and ACT (12). Despite better loco-regional control in both CCRT arms, the metastatic rates were not significantly different through all four arms. In the Southwest Oncology Group (SWOG) trial, which showed a superiority of combined modality approach over postoperative RT alone,patients in the CCRT arm were additionally administered two cycles of consolidation cisplatin/5-fluorouracil chemotherapy (25). In a single institution study, the addition of epirubicin, administered during and after standard pelvic RT, significantly decreased the incidence of distant failure compared to the same RT, but there was no impact on the incidence of local recurrences (10).
A significant survival benefit for patients who underwent CCRT with weekly cisplatin and gemcitabine followed by 2 cycles of ACT including cisplatin/gemcitabine regimen at higher doses compared to the same CCRT alone was demonstrated (13). However, recent systematic review showed insufficient evidence supporting the use of ACT following CCRT (35).
Cytotoxic agents used in combination with RT
Owing to its convenience, favorable toxicity and relative effectiveness, cisplatin 40 mg/m2weekly for 6 weeks concurrent with radiation is widely accepted as the standard regimen of CCRT. Indeed, among the two approaches:weekly cisplatin or cisplatin plus 5-fluorouracil every 3 weeks, the former seems to be less toxic and more feasible.A variety of cisplatin regimens and doses have been combined with RT, however no larger trial addressed the optimal cisplatin scheduling. The substitution of cisplatin by carboplatin, a less nephrotoxic platinum analogue seems appealing and feasible (36), yet its beneficial effect remains to be established. In one study including IIB—IVA CC, triweekly cisplatin (75 mg/m2in 3 cycles) concurrent with RT was found more effective than the conventional weekly dose of 40 mg/m2in 6 cycles (5-year OS: 89%vs. 67%;HR=0.375), with similar compliance (37). Higher peak concentration of cisplatin reduced the percentage of distal failure (17%vs. 26%). However, the most recent meta-analysis that included 5 RCTs demonstrated a similar therapeutic effect of weekly compared to tri-weekly regimen, and lower hematologic toxicity (38).
Since gemcitabine is a potent radiosensitizer, two RCTs have been undertaken to clarify the use of cisplatin and gemcitabine in combination with RT for CC patients(13,14). Duenas-Gonzalezet al. showed improved survival for the treatment of stage IIB—IVA CC by the addition of weekly concurrent gemcitabine and 2 cycles of adjuvant cisplatin/gemcitabine compared to standard single-agent cisplatin (HR=0.68, P=0.0224) (13). Combination chemotherapy showed a statistically significant advantage in distant failure rate (8.1%vs. 16.4%, P=0.005), whereas the difference in local failure rate was not significant(11.2%vs. 16.4%, P=0.097). No improvement from the addition of gemcitabine to weekly cisplatin in stage III, IVA CC was demonstrated in another small trial (14).
Several other agents and drug combinations, including the taxanes, have been investigated as radiation sensitisers in CC. The most recent review of 13 different chemotherapy regimens concomitant to RT in the treatment of LACC indicated that CCRT with cisplatin/docetaxel might be the most effective (39).However, weekly cisplatin remains the least toxic among all chemotherapy regimens in combination with RT.
NACT
The aim of chemotherapy preceding local modalities is to reduce the volume of disease, making the subsequent irradiation or surgery more effective, while controlling micrometastatic disease. The disadvantages of this strategy include the delay in the onset of local treatment, the possibility of accelerated repopulation of tumor cells, and the risk of developing radio-resistant cell clones.Therefore, it is important to select patients who will most likely benefit from NACT (40).
Several studies indicated beneficial effects of chemotherapy preceding RT or surgery (40,41). Surgical series including patients with FIGO stage I—IVA showed an operability rate of 48% to 100% following primary chemotherapy, with no increased surgery-related morbidity. Pathologically confirmed complete responses were achieved in 9%—27% of cases, and the incidence of lymph node metastases seemed to be markedly low,reducing the number of high-risk patients requiring postoperative RT. A survival benefit associated with NACT followed by surgery (NCS) compared to conventional RT was demonstrated in three RCTs (42-44). NACT in two studies consisted of vincristine/bleomycin/cisplatin regimen(42,43). The Italian study compared cisplatin-based NACT in LACC patients (44). Most importantly, in view of the recent CCRT studies, exclusive RT in the control arm in these studies may be viewed as suboptimal management.
Significantly better local control, PFS and OS, beside a significant decrease in adverse pathological findings in the NCS group compared to the surgery group was shown in the Cochrane review conducted in 2012 (45). Another review, however, has not shown an advantage for this approach in stage IB1—IIA CC (46). The benefit of NACT followed by surgery (±RT) versus definitive RT in LACC was demonstrated in systematic review and meta-analysis of IPD from 21 RCTs (47). No increased OS, despite a significant reduction in tumor volume by primary chemotherapy, was demonstrated in meta-analysis of RCTs comparing RT alone with RT preceded by chemotherapy(48). Apart from the limitations of meta-analysis, the results of these analyses should be interpreted in view of the current replacement of RT by CCRT as the standard management.
The efficacy of NACT followed by surgery versus cisplatin-based CCRT is addressed in the ongoing large RCT conducted by the European Organization for Research and Treatment of Cancer (EORTC) in patients with FIGO stage I—IIB CC.
Current studies investigate NACT with modern chemotherapy regimens, including irinotecan/nedaplatin followed by RS (49).
NACT with paclitaxel/carboplatin or cisplatin/gemcitabine before CCRT is another postulated strategy for potentially systemic LACC (21). Two cycles of cisplatin combined with gemcitabine as an upfront treatment for LACC patients managed with cisplatin-based CCRT did not show a meaningful improvement in small series (50).
ACT (but also adjuvant RT), as compared to no further treatment, does not seem to improve the clinical outcomes of patients with extra-cervical residual disease after platinum-based NACT followed by RS (51).
Palliative chemotherapy
Since current palliative systemic therapy offers only the modest gains in OS, it should consider not only the survival benefit, but also minimal treatment toxicity and positive impact on quality of life (QOL). For advanced, persistent or recurrent CC, cisplatin-based chemotherapy remains standard treatment, although its effect is of short duration(52). Currently, recommended regimen is a combination of cisplatin/paclitaxel. Selected RCTs addressing the role of palliative chemotherapy are presented inTable 3(53-58).Significant increase in response rate (RR) (36%vs. 19%)and median PFS but not OS (9.7 and 8.8 months), with sustained QOL for this doublet compared to cisplatin alone was demonstrated in GOG RCT (53). Response was more frequent in patients with disease in non-irradiated sites(79%vs. 23%). Performance status 1 or 2, pelvic recurrence,prior radio-sensitizing chemotherapy, African American race, and first recurrence within 1 year of diagnosis were poor prognostic factors significantly and independently associated with reduced OS (59). The superiority of the doublet with cisplatin plus topotecan in terms of RR (27%vs. 13%), median PFS, but also median OS (9.4vs. 6.5 months) was demonstrated in another GOG trial (54). A subsequent GOG trial compared four cisplatin doublets:cisplatin/paclitaxel (reference arm) against cisplatin/vinorelbine, cisplatin/gemcitabine, and cisplatin/topotecan(55). There were no significant differences in regard to RR,which were 29.5%, 25.9%, 22.3%, and 23.4% for cisplatin combinations with paclitaxel, vinorelbine, gemcitabine, and topotecan, respectively. Noninferiority of the carboplatin/paclitaxel doublet, compared to cisplatin/paclitaxel, was shown in the Japanese study (58). In a subset analysis of patients who had not received prior platinum, the cisplatin/paclitaxel doublet showed superior OS (median of 23.2 monthsvs. 13.0 months, respectively) and was less toxic.
There is currently no consensus on the standard care for second-line systemic treatment of recurrent/metastatic CC(60). There is also no evidence that treatment in the second-line setting improves OS compared to the best supportive care. Available single agents beyond first-line platinum-based therapy, including topoisomerase inhibitors, taxanes, alkylating agents and antimetabolites have limited efficacy in this setting (60,61).
Targeted therapy
Bevacizumab, a humanized monoclonal antibody targetingvascular endothelial growth factor receptor (VEGFR) in combination with chemotherapy was approved as first-line therapy for advanced CC, with a significant OS improvement of 3.5 months (HR=0.77, P=0.007) as compared with chemotherapy alone (56,57). Chemotherapy in this pivotal RCT consisted of cisplatin/paclitaxel or topotecan/paclitaxel, continued until disease progression.Despite the higher rate of severe adverse events including genitourinary fistulas, thromboembolic events and hypertension, the addition of bevacizumab to chemotherapy did not adversely affect health-related QOL. More than 70% of patients in each group had previously received platinum-based CCRT. The role of bevacizumab in the first-line therapy of advanced and recurrent CC remains to be elucidated.
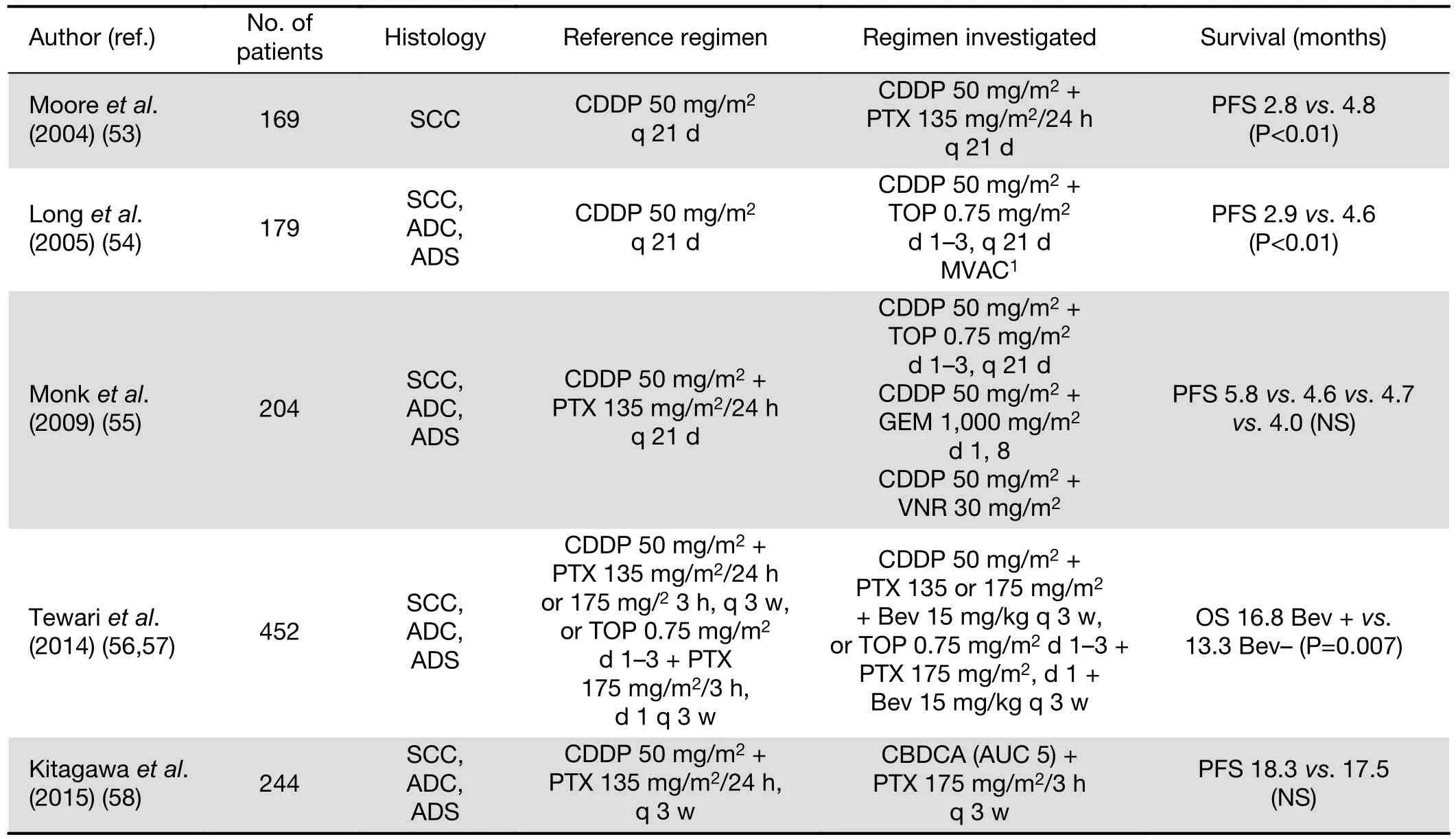
Table 3 Selected phase III trials addressing the role of chemotherapy in stage IVB, recurrent or persistent CC
Cediranib and pazopanib are other anti-angiogenic agents that have shown promising efficacy in advanced CC.The addition of cediranib, a potent tyrosine kinase inhibitor of VEGFR1-3 and stem cell factor receptor (c-KIT), to chemotherapy in patients with metastatic or recurrent CC in the first-line setting was investigated in a randomized, placebo-controlled phase II trial (62). Six cycles of carboplatin/paclitaxel plus daily cediranib,continued until progression resulted in a significant improvement of PFS (8.1vs. 6.7 months; HR=0.58;P=0.032) but not of OS. The RR of 64% in the cediranib group was the highest reported in this setting. Cediranib did not increase the rate of fistulae. In another study using pazopanib, a multi-targeted tyrosine kinase inhibitor (TKI)with activity against the VEGFR1-3, platelet-derived growth factor receptor (PDGFR) α and β and c-KIT, the median PFS was 12.4 months (63,64).
Other fundamental anti-angiogenic (sunitinib, sorafenib,nintedanib) and molecularly targeted agents (cetuximab,erlotinib, sunitinib, gefitinib or imatinib) have failed to show significant benefits in advanced CC (52).
Several targeted therapies, with or without chemotherapy,including non-VEGF-dependent angiogenesis inhibitors(eg. the angiopoietin axis inhibitor trebananib) and vascular-disrupting agents that target existing tumor vasculature are being investigated in CC patients(52,60,65,66). Signal transduction pathways relevant to cervical carcinogenesis that can be targeted include the phosphoinositide 3 kinase B-mammalian target of rapamycin pathway, homologous recombination deficiency pathways that can exploit synthetic lethality, and the Notch binary cell fate decision pathway, which might also represent viable therapeutic options in the future.
Immunotherapy
Immunotherapy with immune-checkpoint inhibitors has recently shown spectacular antitumor efficacy with durable responses in a variety of tumors (melanoma, renal and lung carcinomas). Given the presence of a virus in CC oncogenesis leading to antigen production, there is a strong rationale supporting the development of immunotherapy in this tumor. Significant programmed-cell-death protein 1 ligand (PD-L1) expression, a putative predictive marker for PD-1/PD-L1 inhibitors, was demonstrated in 38%—54%,12%—29% and 14% of primary tumor samples of squamous cell carcinoma (SCC), adenosquamous carcinoma (ADS)and adenocarcinoma (ADC), respectively (67,68). Other studies showed PD-L1 expression of various degrees in all LACC and recurrent tumors, with no apparent difference between SCC and ADC (69,70). One study reported a positive correlation between HPV16E7 and PD-L1 expression (71).
There are multiple ongoing studies to evaluate the role of immune checkpoint inhibitors including pembrolizumab,durvalumab, tremelimumab, atezolizumab, nivolumab and ipilimumab in the upfront treatment, in combination with RT, after CCRT, as neoadjuvant therapy, and in recurrent setting (66,72). There are currently only four case reports on the use of pembrolizumab and nivolumab in the metastatic setting, including chemo-refractory and PD-L1-negative tumors (73-75). A rapid response or response following transit increase of lesions was reported. Of those,there was a case of neuroendocrine cervical carcinoma with near-complete systemic resolution of disease, ongoing at 10+ months after nivolumab, sandostatin and stereotactic body radiation therapy (SBRT; 20 Gy/4 fractions)including abdominal mass (76). The response seen outside the field of radiation, termed the “abscopal” effect, was probably mediated by radiation-induced cross presentation of tumor antigens resulting in immune activation. Tissue rebiopsy and comprehensive genomic profiling confirmed a high tumor mutational burden: 53 mutations per megabase with >19 considered high, multiple other alterations including a mismatch repair gene (MSH2gene) defect, and high microsatellite instability status.
Beside checkpoint inhibitors, therapeutic vaccines have been intensively studied in CC (65).
Role of systemic therapy in non-squamous histologies
Numerous studies have shown different outcomes following primary RT or RS in particular histotypes of cervical tumors: but no RCT assessed the treatment efficacy in relation to CC cell types. Moreover, in some studies evaluating the combination of chemotherapy and local modalities, the accrual was restricted to SCC. The incidence of invasive ADC and its variants has increased over last decades; this type now amounts to about 20%—25% of all invasive CCs. Several retrospective studies and large national databases demonstrated poorer OS for ADC compared to similarly staged SCC (77). This was due to more aggressive behaviour of this type — frequent lymph node involvement, distant organ metastasis, or the relative radio- and chemoresistance of ADC (78). No survival improvement in non-SCC IIB, or more advanced CC was achieved with NACT (79). Similar outcomes of LACC with ADC or ADS histology compared to SCC were reported by others (80).
Significant differences in molecular and immunological profiles, between the two most common CC histotypes were recently shown (67,81). Notably, HER2 overexpression found in a half of ADC/ADS constituted an independent prognostic marker (82).
Only a few studies have reported separate outcomes for ADC. The role of tumor histology was evaluated in SWOG RCT of postoperative adjuvant CCRT for women with positive nodes, parametria or margins (25). This study demonstrated an apparently poorer 5-year PFS (40%vs.65%) with RT alone, but similar outcomes with CCRT(80%vs. 77%), compared to ACT. Similar results were demonstrated in the large retrospective analysis of prospectively collected data on CCRT from the GOG trials that enrolled 1,671 patients (83). This analysis demonstrated that both main histotypes respond well to CCRT. Poorer outcome for advanced ADC/ADS as compared to SCC was noted for RT alone, and the use of cisplatin-based CCRT nullified this difference.
A recent study showed the therapeutic effect of both neoadjuvant and consolidation chemotherapy with CCRT in advanced ADC/ADS (84). In this study, 880 Chinese patients were randomly assigned to CCRT or CCRT preceded by one cycle of NACT and supplemented by two tri-weekly cycles of ACT with paclitaxel/cisplatin (135 mg/m2and 75 mg/m2). Patients treated with NACT/ACT regimen showed a significantly longer DFS and OS. They had also decreased rates of both long-term local control and distant failure (P<0.05).
Small cell neuroendocrine carcinoma of the cervix, a rare histologic entity, is considered to confer a poor prognosis because of its early metastatic potential to both regional lymph nodes and distant sites. Due to the aggressive behaviour of this tumor, multimodality treatment including chemotherapy is advocated even in early-stage disease (85-87). The use of ACT alone or combined with RT was independent factors for improved survival in the largest series of small cell CC patients (85). The worse outcome with NACT was revealed. Cisplatin combined with etoposide appears to be the most commonly used regimen.Postoperative CCRT did not improve survival compared with ACT alone in another relatively large retrospective series (86).
Conclusions
Definitive CCRT is considered the standard treatment for stage IB2—IVA CC. The role of chemotherapy in addition to definitive RT for stage IB1/IIA1 CC patients remains undefined. The addition of cisplatin-based chemotherapy to postoperative RT significantly improves the outcome of patients with a high-risk for recurrence. The superiority of CCRT over RT alone in patients with intermediate-risk remains unknown. The most common chemotherapy schedule for a concomitant approach includes single-agent cisplatin administered at a weekly dose of 40 mg/m2. Even though there is no convicting data on its superiority to other regimens, cisplatin alone is considered standard therapy due to its proven efficacy, ease of administration,and relatively low toxicity. In contrast to the concomitant approach, chemotherapy preceding RT does not seem to improve the outcome. A series of RCTs including stage IB to IIB patients, demonstrated the superiority of preoperative chemotherapy over RS or definitive irradiation alone. Currently, CCRT and chemotherapy followed by radical surgery are being compared in a large RCT performed by the EORTC. The combination of cisplatin and paclitaxel is considered a standard palliative regimen. Other cisplatin doublets were not superior to cisplatin/paclitaxel, whereas substituting carboplatin for cisplatin and topotecan, or gemcitabine for paclitaxel might be helpful for some patients, considering different toxicity profiles. Bevacizumab combined with chemotherapy was shown to significantly improve the outcome in advanced disease setting. Available data with immune checkpoint inhibitors in CC are scarce, but owing to the causative role of HPV, this tumor deserves further study. The optimal management including systemic therapy for non-squamous cervical tumors has not been determined. Ongoing accumulation of data on genomic and proteomic characteristics provide insight into the molecular heterogeneity of CC, and may pave the way for developing distinct molecularly targeted therapies (88).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
1.GLOBOCAN Cancer Fact Sheets: Cervical cancer 2012. Available online: http://globocan.iarc.fr/old/FactSheets/cancers/cervix-new.asp
2.Landoni F, Colombo A, Milani R, et al. Randomised study between radical surgery and radiotherapy for the treatment of stage IB-IIA cervical cancer: 20-year update. J Gynecol Oncol 2017;28:e34.
3.Sardain H, Lavoue V, Redpath M, et al. Curative pelvic exenteration for recurrent cervical carcinoma in the era of concurrent chemotherapy and radiation therapy. A systematic review. Eur J Surg Oncol 2015;41:975-85.
4.Keys HM, Bundy BN, Stehman FB, et al. Cisplatin,radiation and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med 1999;340:1154-61.
5.Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para aortic radiation for high-risk cervical cancer. N Engl J Med 1999;340:1137-43.
6.Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and paraaortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial(RTOG) 90-01. J Clin Oncol 2004;22:872-80.
7.Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med 1999;340:1144-53.
8.Rose PG, Ali S, Watkins E, et al. Long-term follow-up of a randomized trial comparing concurrent single agent cisplatin, cisplatin-based combination chemotherapy, or hydroxyurea during pelvic irradiation for locally advanced cervical cancer: a Gynecologic Oncology Group Study. J Clin Oncol 2007;25:2804-10.
9.Whitney C, Sause W, Bundy BN, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: A Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol 1999;17:1339-48.
10.Wong LC, Ngan HY, Cheung AN, et al.Chemoradiation and adjuvant chemoradiotherapy in cervical cancer. J Clin Oncol 1999;17:2055-60.
11.Pearsey R, Brundage M, Drouin P, et al. Phase III trial comparing radical radiotherapy with and without cisplatin chemotherapy in patients with advanced squamous cell cancer of the cervix. J Clin Oncol 2002;20:966-72.
12.Lorvidhaya V, Chitapanarux I, Sangruchi S, et al.Concurrent mitomycin C, 5-fluorouracil, and radiotherapy in the treatment of locally advanced carcinoma of the cervix: a randomized trial. Int J Radiat Oncol Biol Phys 2003;55:1226-32.
13.Duenas-Gonzales A, Zarba JJ, Patel F, et al. Phase III,open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J Clin Oncol 2011;29:1678-85.
14.Wang CC, Chou HH, Yang LY, et al. A randomized trial comparing concurrent chemoradiotherapy with single-agent cisplatin versus cisplatin plus gemcitabine in patients with advanced cervical cancer: An asian Gynecologic Oncology Group study. Gynecol Oncol 2015;137:462-7.
15.McNeil C. New standard of care for cervical cancer sets stage for next questions. J Natl Cancer Inst 1999;91:500-1.
16.Green JA, Kirwan JM, Tierney JF, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet 2001;358:781-6.
17.Chemoradiotherapy for Cervical Cancer Metaanalysis Collaboration (CCCMAC). Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: individual patient data metaanalysis. Cochrane Database Syst Rev 2010:CD008285.
18.Meng XY, Liao Y, Liu XP, et al. Concurrent cisplatin-based chemoradiotherapy versus exclusive radiotherapy in high-risk cervical cancer: a metaanalysis. Onco Targets Ther 2016;9:1875-88.
19.Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol 2008;26:5802-12.
20.Zuliani AC, Esteves SC, Teixeira LC, et al.Concomitant cisplatin plus radiotherapy and highdose-rate brachytherapy versus radiotherapy alone for stage IIIB epidermoid cervical cancer: a randomized controlled trial. J Clin Oncol 2014;32:542-7.
21.Todo Y, Watari H. Concurrent chemoradiotherapy for cervical cancer: background including evidencebased data, pitfalls of the data, limitation of treatment in certain groups. Chin J Cancer Res 2016;28:221-7.
22.Haque W, Verma V, Fakhreddine M, et al. Addition of chemotherapy to definitive radiotherapy for IB1 and IIA1 cervical cancer: Analysis of the National Cancer Data Base. Gynecol Oncol 2017;144:28-33.
23.Curtin JP, Hoskins WJ, Venkatraman ES, et al.Adjuvant chemotherapy versus chemotherapy plus pelvic irradiation for high-risk cervical cancer patients after radical hysterectomy and pelvic lymphadenectomy(RH-PLND): a randomized phase III trial. Gynecol Oncol 1996;61:3-10.
24.Lahousen M, Haas J, Pickel H, et al. Chemotherapy versus radiotherapy versus observation for high-risk cervical carcinoma after radical hysterectomy: A randomized, prospective, multicenter trial. Gynecol Oncol 1999;73:196-201.
25.Peters WA 3rd, Liu PY, Barrett RJ, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol 2000;18:1606-13.
26.Monk BJ, Wang J, Im S, et al. Rethinking the use of radiation and chemotherapy after radical hysterectomy:a clinical-pathologic analysis of a Gynaecologic Oncology Group/Southwest Oncology Group/Radiation Therapy Oncology Group Trial. Gynecol Oncol 2005;96:721-8.
27.Sehouli J, Runnebaum IB, Fotopoulou C, et al. A randomized phase III adjuvant study in high-risk cervical cancer: simultaneous radiochemotherapy with cisplatin (S-RC) versus systemic paclitaxel and carboplatin followed by percutaneous radiation (PCR): a NOGGO-AGO Intergroup Study. Ann Oncol 2012;23:2259-64.
28.Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynaecologic oncology group study. Int J Radiat Oncol Biol Phys 2006;65:169-76.
29.Takekuma M, Kasamatsu Y, Kado N, et al. The issues regarding postoperative adjuvant therapy and prognostic risk factors for patients with stage I-II cervical cancer: A review. J Obstet Gyaecol Res 2017;43:617-26.
30.Mahmound O, Hathout L, Shaaban SG, et al. Can chemotherapy boost the survival benefit of adjuvant radiotherapy in early stage cervical cancer with intermediate risk factors? A population based study.Gynecol Oncol 2016;143:539-44.
31.Falcetta FS, Medeiros LR, Edelweiss MI, et al.Adjuvant platinum-based chemotherapy for early stage cervical cancer. Cochrane Database Syst Rev 2016;11:CD005342.
32.Asano H, Todo Y, Watari H. Adjuvant chemotherapy for early-stage cervical cancer. Chin J Cancer Res 2016;28:228-34.
33.Matsuo K, Shimada M, Aoki Y, et al. Comparison of adjuvant therapy for node-positive clinical stage IBIIB cervical cancer: Systemic chemotherapy versus pelvic irradiation. Int J Cancer 2017;141:1042-51.
34.Takekuma M, Kasamatsu Y, Kado N, et al. Adjuvant chemotherapy versus concurrent chemoradiotherapy for high-risk cervical cancer after radical hysterectomy and systematic lymphadenectomy. Int J Clin Oncol 2016;21:741-7.
35.Tangjitgamol S, Katanyoo K, Laopaiboon M, et al.Adjuvant chemotherapy after concurrent chemo-radiation for locally advanced cervical cancer.Cochrane Database Syst Rev 2014:CD010401.
36.Nam EJ, Lee M, Yim GW, et al. Comparison of carboplatin- and cisplatin-based concurrent chemoradiotherapy in locally advanced cervical cancer patients with morbidity risks. Oncologist 2013;18:843-9.
37.Ryu SY, Lee WM, Kim K, et al. Randomized clinical trial of weekly vs. triweekly cisplatin-based chemotherapy concurrent with radiotherapy in the treatment of locally advanced cervical cancer. Int J Radiat Oncol Biol Phys 2011;81:e577-81.
38.Chen X, Zou H, Li H, et al. Weekly versus triweekly cisplatin-based chemotherapy concurrent with radiotherapy in the treatment of cervical cancer. A meta-analysis. Int J Gynecol Cancer 2017;27:344-9.
39.Fu ZZ, Li K, Peng Y, et al. Efficacy and toxicity of different concurrent chemoradiotherapy regimens in the treatment of advanced cervical cancer. A network meta-analysis. Medicine 2017;96:e5853.
40.Iwata T, Miyauchi A, Suga Y, et al. Neoadjuvant chemotherapy for locally advanced cervical cancer.Chin J Cancer Res 2016;28:235-40.
41.Serkies K, Jassem J. Chemotherapy in the primary treatment of cervical carcinoma. Crit Rev Oncol Hematol 2005;54:197-208.
42.Sardi J, Giaroli A, Sananes C, et al. Long-term follow-up of the first randomized trial using neoadjuvant chemotherapy in stage Ib squamous carcinoma of the cervix: The final results. Gynecol Oncol 1997;67:61-9.
43.Sardi J, Sananes C, Giaroli A, et al. Neoadjuvant chemotherapy in cervical carcinoma stage IIb: a randomized controlled trial. Int J Gynecol Cancer 1998;8:441-50.
44.Benedetti-Panici P, Greggi S, Colombo A, et al.Neoadjuvant chemotherapy and radical surgery versus exclusive radiotherapy in locally advanced squamous cell cervical cancer: results from the Italian multicenter randomized study. J Clin Oncol 2002;20:179-88.
45.Rydzewska L, Tierney J, Vale CL, et al. Neoadjuvant chemotherapy plus surgery versus surgery for cervical cancer. Cochrane Database Syst Rev 2010:CD007406.
46.Kim HS, Sardi JE, Katsumata N, et al. Efficacy of neoadjuvant chemotherapy in patients with FIGO stage IB1 to IIA cervical cancer: an international collaborative meta-analysis. Eur J Surg Oncol 2013;39:115-24.
47.Neoadjuvant Chemotherapy for Cervical Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy for locally advanced cervix cancer: a systematic review and meta-analysis of individual patient data from 21 randomised trials. Eur J Cancer 2003;39:2470-86.
48.Tierney JF, Stewart LA, Parmar MK. Can the published data tell us about the effectiveness of neoadjuvant chemotherapy for locally advanced cancer of the uterine cervix? Eur J Cancer 1999;354:406-9.
49.Yamaguchi S, Nishimura R, Yaegashi N, et al. Phase II study of neoadjuvant chemotherapy with irinotecan hydrochloride and nedaplatin followed by radical hysterectomy for bulky stage Ib2 to IIb, cervical squamous cell carcinoma: Japanese Gynecologic Oncology Group study (JGOG 1065). Oncol Rep 2012;28:487-93.
50.de Azevedo CRAS, Thuler LCS, de Mello MJG, et al.Phase II trial of neoadjuvant chemotherapy followed by chemoradiation in locally advanced cervical cancer.Gynecol Oncol 2017;146:560-5.
51.Landoni F, Sartori E, Maggino T, et al. Is there a role for postoperative treatment in patients with stage Ib2-IIb cervical cancer treated with neo-adjuvant chemotherapy and radical surgery? An Italian multicenter retrospective study. Gynecol Oncol 2014;132:611-7.
52.Tsuda N, Watari H, Ushijima K. Chemotherapy and molecular targeting therapy for recurrent cervical cancer. Chin J Cancer Res 2016;28:241-53.
53.Moore DH, Blessing JA, McQuellon RP, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol 2004;22:3113-9.
54.Long HJ 3rd, Bundy BN, Grendys EC Jr., et al Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix:a Gynecologic Oncology Group study. J Clin Oncol 2005;23:4626-33.
55.Monk BJ, Sill MW, McMeekin DS, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol 2009;27:4649-55.
56.Tewari KS, Sill MW, Long HJ 3rd, et al. Improved survival with bevacizumab in advanced cervical cancer.N Engl J Med 2014;370:734-43.
57.Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled,open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet 2017;390:1654-63.
58.Kitagawa R, Katsumata K, Shibata T, et al. Paclitaxel plus carboplatin versus paclitaxel plus cisplatin in metastatic or recurrent cervical cancer: The openlabel randomized phase III trial (JCOG0505). J Clin Oncol 2015;33:2129-35.
59.Moore DH, Tian C, Monk BJ, et al. Prognostic factors for response to cisplatin-based chemotherapy in advanced cervical carcinoma: a Gynecologic Oncology Group Study. Gynecol Oncol 2010;116:44-9.
60.McLachlan J, Boussios S, Okines A, et al. The impact of systemic therapy beyond first-line treatment for advanced cervical cancer. Clin Oncol 2017;29:153-60.
61.Boussios S, Seraj E, Zarkavelis G, et al. Management of patients with recurrent/advanced cervical cancer beyond first line platinum regimens: Where do we stand? A literature review. Crit Rev Oncol/Hematol 2016;108:164-74.
62.Symonds RP, Gourley C, Davidson S, et al. Cediranib combined with carboplatin and paclitaxel in patients with metastatic or recurrent cervical cancer (CIRCCa):a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Oncol 2015;16:1515-24.
63.Monk BJ, Mas Lopez L, Zarba JJ, et al. Phase II,open-label study of pazopanib or lapatinib monotherapy compared with pazopanib plus lapatinib combination therapy in patients with advanced and recurrent cervical cancer. J Clin Oncol 2010;28:3562-9.
64.Monk BJ, Pandite LN. Survival data from a phase II,open-label study of pazopanib or lapatinib monotherapy in patients with advanced and recurrent cervical cancer. J Clin Oncol 2011;29:4845.
65.Crafton SM, Salani R. Beyond chemotherapy. An overview and review of targeted therapy in cervical cancer. Clin Ther 2016;38:449-58.
66.Luvero D, Plotti F, Lopez S, et al. Antiangiogenics and immunotherapies in cervical cancer: an update and future’s view. Med Oncol 2017;34:115.
67.Heeren AM, Punt S, Blker MC, et al. Prognostic effect of different PD-L1 expression patterns in squamous cell carcinoma and adenocarcinoma of the cervix. Mod Pathol 2016;29:753-63.
68.Reddy OL, Shintaku PI, Moatamed NA. Programmed death-ligand 1 (PD-L1) is expressed in a significant number of the uterine cervical carcinomas. Diagn Pathol 2017;12:45.
69.Enwere EK, Kornaga EN, Dean M, et al. Expression of PD-L1 and presence of CD-positive T cells in pretreatment specimens of locally advanced cervical cancer. Mod Pathol 2017;30:577-86.
70.Ring KL, Yemelyanova AV, Soliman PT, et al.Potential immunotherapy targets in recurrent cervical cancer. Gynecol Oncol 2017;145:462-8.
71.Liu C, Lu J, Tian H, et al. Increased expression of PD-L1 by the human papillomavirus 16 E7 oncoprotein inhibits anticancer immunity. Mol Med Rep 2017;15:1063-70.
72.Borcoman E, Le Touneau C. Pembrolizumab in cervical cancer: latest evidence and clinical usefulness.Ther Adv Med Oncol 2017;9:431-9.
73.Martinez P, Del Campo JM. Pembrolizumab in recurrent advanced cervical squamous carcinoma.Immunotherapy 2017;9:467-70.
74.Miyoshi T, Kataoka T, Asahi A, et al. A transient increase and subsequent sharp decrease of chemorefractory liver-metastasized uterine cervical small cell carcinoma to autologous formalin-fixed tumor vaccine plus anti-PD-1 antibody. Clin Case Rep 2016;4:687-91.
75.Paraghamian SE, Longoria TC, Eskander RN.Metastatic small cell neuroendocrine carcinoma of the cervix treated with the PD-1 inhibitor, nivolumab: a case report. Gynecol Oncol Res Pract 2017;4:3.
76.Sharabi A, Kim SS, Kato S, et al. Exceptional response to nivolumab and stereotactic body radiation therapy (SBRT) in neuroendocrine cervical carcinoma with high tumor mutational burden: management considerations from the center for personalized cancer therapy at UC San Diego Moores Cancer Center.Oncologist 2017;22:631-7.
77.Galic V, Herzog TJ, Lewin SN, et al. Prognostic significance of adenocarcinoma histology in women with cervical cancer. Gynecol Oncol 2012;125:287-91.
78.Takeuchi S. Biology and treatment of cervical adenocarcinoma. Chin J Cancer Res 2016;28:254-62.
79.He L, Wu L, Su G, et al. The efficacy of neoadjuvant chemotherapy in different histological types of cervical cancer. Gynecol Oncol 2014;134:419-25.
80.Katanyoo K, Sanguanrungsirikul S, Manusirivithaya S. Comparison of treatment outcomes between squamous cell carcinoma and adenocarcinoma in locally advanced cervical cancer. Gynecol Oncol 2012;125:292-6.
81.Wright AA, Howitt BE, Myers AP, et al. Oncogenic mutations in cervical cancer: Genomic differences between adenocarcinoma and squamous carcinoma of the cervix. Cancer 2013;119:3776-83.
82.Martinho O, Silva-Oliveira R, Cury FP, et al. HER family receptors are important theranostic biomarkers for cervical cancer: blocking glucose metabolism enhances the therapeutic effect of HER inhibitors.Theranostics 2017;7:717-32.
83.Rose PG, Java JJ, Whitney CW, et al. Locally advanced adenocarcinoma and adenosquamous carcinomas of the cervix compared to squamous cell carcinomas of the cervix in gynecologic oncology group trials of cisplatin-based chemoradiation.Gynecol Oncol 2014;135:208-12.
84.Tang J, Tang Y, Yang J, et al. Chemoradiation and adjuvant chemotherapy in advanced cervical adenocarcinoma. Gynecol Oncol 2012;125:297-302.
85.Cohen JG, Kapp DS, Shin JY, et al. Small cell carcinoma of the cervix: treatment and survival outcomes of 188 patients. Am J Obstet Gynecol 2010;203:347.e1-6.
86.Lee JM, Lee KB, Nam JH, et al. Prognostic factors in FIGO stage IB-IIA small cell neuroendocrine carcinoma of the uterine cervix treated surgically:results of a multi-center retrospective Korean study.Ann Oncol 2008;19:321-6.
87.Xie S, Song L, Yang F, et al. Enhanced efficacy of adjuvant chemotherapy and radiotherapy in selected cases of surgically resected neuroendocrine carcinoma of the uterine cervix. A retrospective cohort study.Medicine 2017;96:e6361.
88.The Cancer Genome Atlas Research Network.Integrated genomic and molecular characterization of cervical cancer. Nature 2017;543:378-84.
 Chinese Journal of Cancer Research2018年2期
Chinese Journal of Cancer Research2018年2期
- Chinese Journal of Cancer Research的其它文章
- Future of anti-PD-1/PD-L1 applications: Combinations with other therapeutic regimens
- Tumor immunotherapy: New aspects of natural killer cells
- Impact of 5-Fu/oxaliplatin on mouse dendritic cells and synergetic effect with a colon cancer vaccine
- A nomogram to predict adjuvant chemotherapy recommendation in breast cancer patients with intermediate recurrence score
- A multicenter hospital-based diagnosis study of automated breast ultrasound system in detecting breast cancer among Chinese women
- Health-related quality of life among rural residents aged 45−69 years in Hua County, Henan Province, China: Results of ESECC Trial for esophageal cancer screening with endoscopy
