Coinoculation of bioinoculants improve Acacia auriculiformis seedling growth and quality in a tropical Al fisol soil
Thangavelu Muthukumar•Karuthamuthu Udaiyan
Introduction
Natural forests that cover about 31%of the land area worldwide are declining due to anthropogenic pressures.Worldforestcoverdeclinedby6.8% from 282.16×106km2in 1990 to 262.97×106km2in 2011(World Bank 2011).In contrast,forest cover in India increased by 7.5%from 0.639×106km2in 1990 to 0.687×106km2in 2011(World Bank 2011).This was possible because of an increase in different types of plantations,including the introduction of exotic fast-growing trees likeAcacia,CasuarinaandEucalyptusthroughout India(Ravindranath et al.2014).Many of these exotic tree species can grow on marginal soils and also bene fit the ecosystem by improving soil fertility especiallyAcaciaandCasuarinabecause they fix nitrogen.However,the raising of plantations on marginal soils is hampered not only by poor nutrient and water availability but also because of decreased abundance and activity of microorganisms(Dumroese et al.2009).
Among the organisms that inhabit soils,arbuscular mycorrhizal(AM)fungi are the most frequent and ubiquitous.These fungi associate with plant roots and aid plants in their uptake of water and nutrients from stressed soils.In addition to improving soil structure,AM fungi also impart resistance in plants against different types of biotic and abiotic stresses(Smith and Read 2008).Like AM fungi,many bacteria inhabiting the root zone of plants also make available soil nutrients to plants.Some of the bacteria in the soil that are capable of solubilizing bound phosphorus(P)and making them available to plants are referred to as phosphate solubilizing bacteria(PSB)(Rodrı´guez and Fraga 1999).In addition to P solubilization,PSB are also capable of producing phytohormones,hydrolytic enzymes and antibiotics(Rodrı´guez and Fraga 1999).PSB can also stimulate mycorrhizal formation and nodulation in leguminous and actinorhizal tree species(Barea et al.2005).
Acacia auriculiformisA.Cunn.ex Benth.,Fabaceae,is an evergreen fast growing tree indigenous to Australia,Papua New Guinea,and Indonesia.In India,this tree was introduced in 1946 and is a popular afforestation species with many uses(Kushalapa 1991).A.auriculiformisis primarily used for fuel wood due to its high calori fic value(4800–4900 kcal/kg)and in Southeast Asia,plantations ofA.auriculiformisare raised chie fly for fuel wood(Grif fin et al.2011).It is also an excellent source of wood for charcoal making(Shukla et al.1990).The wood is used for making furniture,tools,and in the pulp and paper industry.It is also used as an ornamental and as a shade tree.The oil extracted from seeds possesses some therapeutic values like spermicidal and anti-HIV activities(Girjashankar 2011).Ecosystem services rendered byA.auriculiformisinclude improved water-holding capacity of the soil,enhanced nitrogen(N),potassium(K)and organic carbon concentrations through litter inputs thereby conserving soil and water resources.A.auriculiformiscan also serve as a nurse species to facilitate the growth and survival of other plant species(Yang et al.2009).
Application of inorganic fertilizers improves the growth ofA.auriculiformisunder nursery and field conditions(Lamani et al.2004;Uddin et al.2008).However,the use of synthetic fertilizers is not only expensive but also environmentally damaging.A viable alternative is the use ofsoilmicroorganismswhose activitiescan make unavailable nutrients in the soil available to plants.Inoculation with commercial preparations of effective bacteria,and nonmycorrhizal fungi improvedA.auriculiformisseedling growth and vigour under nursery conditions(Khan et al.2014).Acaciaspecies are excellent for revegetation of mine spoils and wastelands because they form associations with mycorrhizal fungi and nodulating bacteria,enabling survival in inhospitable sites.AlthoughA.auriculiformisis capable of associating with both ectomycorrhiza(EM)and AM fungi,association with the latter appears to be more common(Duponnois et al.2000;Diouf et al.2005).Studies of the effects of AM fungi onA.auriculiformishave tested single AM fungus or the combined inoculation of EM fungi and a single AM fungus on plant growth.
The in fluence of the AM fungiRhizophagus fasciculatus(Thaxt.) C. Walker & A. Schu¨ßler (=Glomusfasciculatum)andGlomus macrocarpumTul.&C.Tul.,individually or in combination onA.auriculiformisseedlings in degraded wasteland soils under nursery conditions was examined by Giri et al.(2004).This study was conducted in sterile soil,thus it eliminated the in fluence of native AM fungi and other indigenous soil microbes.The roles of the nitrogen- fixing bacterial symbiontBradyrhizobiumspp.mycorrhizal fungi[AM:Rhizophagus intraradices(N.C.Schenck&G.S.Sm.)C.Walker&A.Schuessler(=Glomus intraradices),and EM:Pisolithus albus(Cooke&Massee)Priest]in salt tolerance inA.auriculiformisseedlings were studied under greenhouse conditions(Diouf et al.2005).That study showed that dual inoculation ofR.intraradicesand nodulating bacteria imparted better salinity tolerance to seedlings than did other tested combinations.To the best of our knowledge,no studies have assessed the role of PSB and various AM fungi onA.auriculiformisseedling growth and quality.Hence,the objective of the work was to compare the effectiveness of various AM fungi and PSB inoculation individually or in combinations,onA.auriculiformisseedling growth,uptake of nutrients and seedling quality in a nutrient de ficient Al fisol soil in a tropical nursery.
Materials and methods
In the nursery we tested eight treatments including a control with two AM fungi and the PSB inoculated individually and in various combinations.The treatments involved three individual inoculations(AM fungi–1,AM fungi–2,PSB),three dual inoculations(AM fungi–1+AM fungi–2,AM fungi–1+PSB,AM fungi–2+PSB)and one triple inoculation(AM fungi–1+AM fungi–2+PSB)of the microbes and an uninoculated control.There were 10 replicates per treatment.
Study site and materials
The study was conducted in the Department of Botany plant nursery at the Bharathiar University(11.039°N and 76.876°E,498 m,a.s.l),Coimbatore,India.Seeds ofA.auriculiformiswere procured from the Institute of Forest Tree Genetics and Plant Breeding(IFGTB),Coimbatore,India.The seeds were scari fied in hot water(70°C)for 3 min.and soaked overnight in water to ensure rapid germination.The soaked seeds were germinated in trays containing sterile sand.Fifteen-day-old uniformly healthy seedlings were individually transplanted into 12×30 cm black polythene bags containing 3.0 kg of non-sterile Al fisol field soil collected from the Bharathiar University campus,Coimbatore.The field soil was freed of organic debris,crushed and sieved through a 2-mm sieve prior to filling.The physicochemical characteristics of the soil as assessed according to Jackson(1971)were:pH(H2O)7.8,7.22 mg/kg of total nitrogen(N),0.85 mg/kg of total P,0.29 mg/kg of available P,and 15.21 mg/kg of exchangeable potassium(K).The native soil contained 7.63 infective propagules of AM fungi per gram of soil as assessed according to the most probable number technique(Porter 1979).The morphological characterization of the AM fungal spores and their comparison with original descriptions on Schu¨ssler’s web page(http://www.amf-phylogeny.com/)indicated that the indigenous AM flora consisted ofFunneliformis mosseae(T.H.Nicolson&Gerd.)C.Walker&A.Schuessler,Glomus sinuosum(Gerd.&B.K.Bakshi)R.T.Almeida&N.C.Schenck,Rhizoglomus aggregatum(N.C.Schenck&G.S.Sm.)Sieverd.,G.A.Silva&Oehl,andScutellospora heterogama(T.H.Nicolson&Gerd.)C.Walker&F.E.Sanders.The bags containing seedlings were ordered in a completely randomized block design and maintained in a shade house(temperature:26 ± 2°C,relative humidity:76±5%)under natural light and watered when needed.No fertilizers were added.
Microbial inocula
The AM fungal speciesAcaulospora scrobiculataTrappe andScutellospora calospora(Nicol.&Gerd.)Walker&Sanders used in the study were originally isolated from the semi-arid grassland soils of the Western Ghats,southern India(Muthukumar and Udaiyan 2002).The AM fungal inocula were the culture soil consisting of extraradical hyphae,mycorrhizal roots,and spores.The AM fungal inocula were prepared in open pot cultures with mycorrhized maize plants(Zea maysL.)grown for 24 weeks.The AM fungal inoculum contained 246 and 180 propagules g-1of inoculum as assessed according to Porter(1979).At transplantation,4.0 g ofA.scrobiculataor 5.5 g ofS.calosporaor 4.75 g of consortia(2 g ofA.scrobiculata+2.75 g ofS.calospora)containing 984,990,and 987 fungal propagules,respectively,were placed in the planting hole.A similar quantity of sterilized inoculum(autoclaved at 121°C for 90 min.)was added to treatments not involving AM fungi.The co-occurring soil microorganisms in AM fungal inoculum were made similar across treatments by adding 25 ml of the microbial commixture to each bag prepared as per Muthukumar et al.(2001).
The culture ofPaenibacillus polymyxa(Prazmowski)Ash et al.,isolated and maintained in Pikovskaya’s medium was used(Muthukumar and Udaiyan 2010).For inoculum preparation,the PSB was inoculated in Pikovskaya’s broth and cultured for 36 h at 30°C in a rotary shaker.The bacterial cells were collected by centrifugation at 7000 g for 15 min.The supernatant was removed and the pellet was washed using sterile distilled water and centrifugation(7000 g for 15 min).The bacterial cells were then redispersed in a sterile saline solution and the bacterial cell number was altered to 5×107CFU/mL(Van et al.2000).At the time of transplanting the seedling,5 mL of the PSB inoculum was added.
Harvest and measurements
Sixty days after transplantation(DAT),the plants were removed from the polythene bags and the root system was freed from the soil with water.Nodule numbers were scored visually and the shoot and taproot lengths were measured.Stem girth was measured with screw gauze above the root collar.Cladode area was measured using a LI-3000 portable leaf area meter(Li-Cor,USA).A weighed portion of the root of each plant was removed for estimating AM fungal colonization.The dry weights of shoot,root,and nodules were determined separately after drying them for 48 h at 60°C in a hot air oven.The root by shoot(R/S)ratio was estimated from their respective dry weights.
AM fungal colonization
To estimate AM colonization,1-cm fragments were cleared in 2.5%KOH at 90°C in a water bath for 60 min.The cleared roots were then acidi fied(5 N HCl)and stained with 0.05%trypan blue lactoglycerol.Twenty root bits were observed per root system under an Olympus BX5 compound microscope.The magni fied intersection method of McGonigle et al.(1990)was used to determine the magnitude of AM fungal colonization in the processed roots.
Plant nutrients
We quanti fied the nutrient contents of plant shoot and root tissues.N content in plant tissues was measured after micro-Kjeldahl digestion using concentrated digest and selenium as a catalyst using a Technicon Auto Analyser(M/s Gedko International,UK).P content in plant tissues was quanti fied by use of the molybdenum blue method after wet ashing the samples in a HNO3– H2SO4–HClO4mixture(Jackson 1971).K content in plant tissues was estimated by the flame photometric method of Davis(1962).
Mycorrhizal dependency,nutrient uptake and seedling quality
The relative field mycorrhizal dependency(RFMD)for treatments involving AM inoculation was calculated using the dry weights of AM inoculated and uninoculated plants following Plenchette et al.(1983).The ef ficiency of nutrient uptake(ENuU),was estimated following Gray and Schlesinger(1983)and the seedling quality was categorized following Dickson et al.(1960).
Statistical analyses
One-way analysis of variance(ANOVA)was used to assess the in fluence of bioinoculation onA.auriculiformisseedling growth,nodulation,AM colonization and nutrient uptake.Means were compared with Duncan’s multiple range test(DMRT)at a signi ficance threshold ofp<0.05.Relationships between variables were quanti fied using Pearson’s correlation.AM fungal colonization values were normalized using arcsine transformation prior to statistical analysis.
Results
Plant growth
Inoculation significantly enhanced the growth ofA.auriculiformisseedlings(Table 1).The extent of enhancement varied according to whether inoculants were applied individually(single inoculation),in combinations with one other inoculant(dual inoculation),or in combination with two other inoculants(triple inoculation).A.auriculiformisseedlings inoculated singly or using combinations of AM and PSB were 14–29%and 36–59%taller,respectively,than controls.Single or dual inoculations yielded seedlings 83%taller than controls;triple inoculation yielded seedlings 42–60%taller than singly inoculated seedlings and 15–35%taller than dually inoculated seedlings.Similar to seedling height,taproot lengths ofA.auriculiformisseedlings inoculated withA.scrobiculata,S.calospora,and PSB either individually or in combinations,were 11%to 72%longer than those of controls(Table 1).Seedlings inoculated with two AM fungi and PSB(triple inoculation)had taproots that were 33–55%and 3–28%longer than the individual or dual inoculated seedlings,respectively.A.auriculiformisseedlings treated with AM fungi and PSB had 22–95%more cladodes with 12–99%higher surface area than control seedlings.Triple inoculated seedlings had 41–60%more cladodes with 54–81%more cladode areas than seedlings inoculated with any of the three bioinoculants individually.This increase in cladode number and area was,however,slightly less(1–27%and 4–37%,respectively)than recorded for dually inoculated seedlings.The stem thickness ofA.auriculiformisseedlings inoculated with bioinoculants was 12–118%higher than in control seedlings.Stem girth of multiple bioinoculant treated seedlings was 54–95%greater than recorded for individually inoculated and 3–39%greater than for dually inoculated seedlings(Table 1).Inoculation ofA.scrobiculata,S.calospora,and PSB either alone or in various combinations increased shoot dry weights ofA.auriculiformisseedlings by 35–135%and root dry weights by 38–316%over control seedlings.Simultaneous inoculation of all three microbes maximized seedling shoot biomass,which was 28–74%greater than in individually inoculated and 2–20%greater than in dually inoculated seedlings.Similarly,seedlings inoculated with all three bioinoculants simultaneously accumulated more root mass compared to seedlings that were inoculated with these bioinoculants either individually(104–202%)or dually(3–70%).
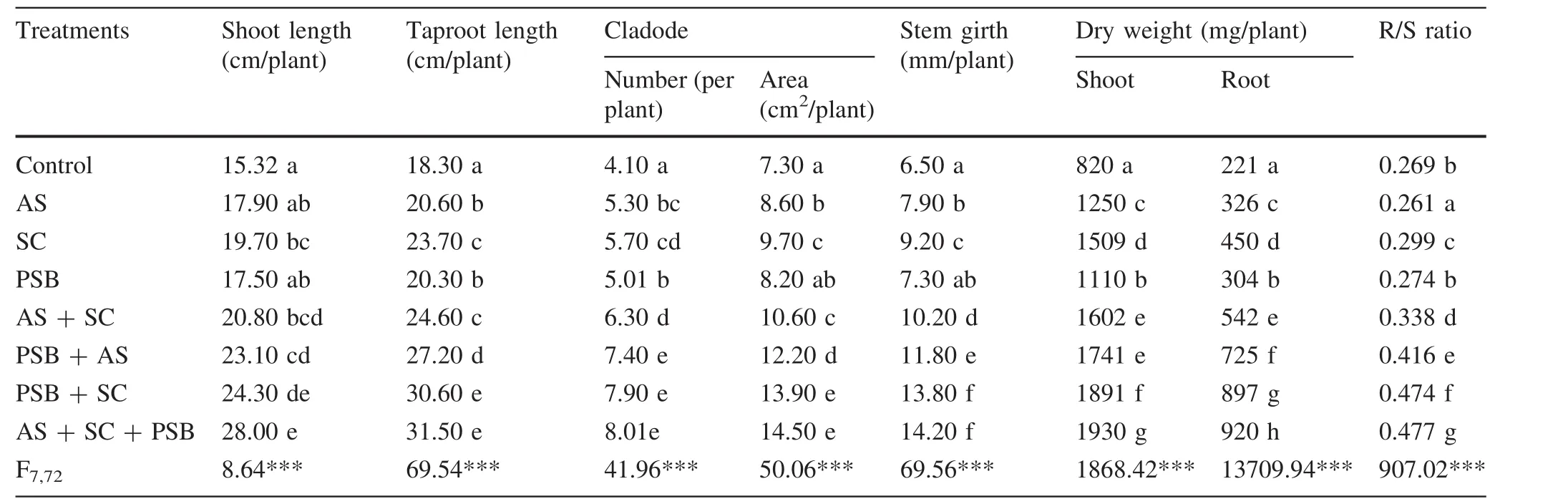
Table 1 In fluence of bioinoculant inoculation on growth of Acacia auriculiformis at 60 days after transplantation
Bioinoculant inoculation increased the R/S ratios ofA.auriculiformisseedlings by 2–77%over control seedlings except for those inoculated withA.scrobiculata(Table 1).Seedlings inoculated withA.scrobiculatahad R/S ratios 3%lower than recorded for controls.Maximum R/S ratio was recorded for seedlings inoculated with multiple bioinoculants and means were 77%greater than for control seedlings.
Plant nutrient content
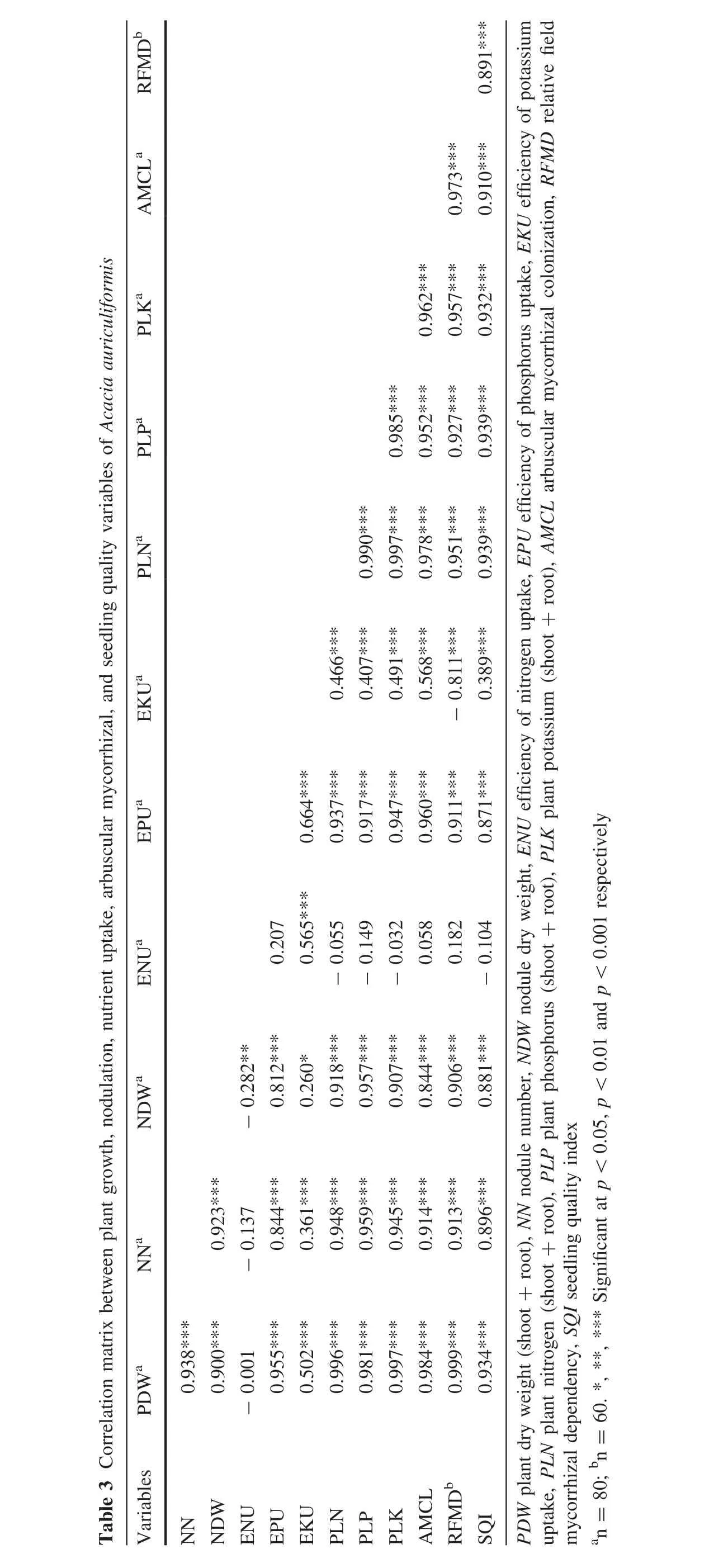
A.auriculiformisseedlings inoculated withA.scrobiculataandS.calosporaeither individually or in combinations had 9–65,11–162 and 6–83%more N,P,and K,respectively,than recorded in control shoots(Table 2).The root N,P,and K contents of these seedlings were 20–108,51–366 and 38–138%greater,respectively,than in uninoculated seedlings.Generally,A.auriculiformisseedlings inoculated with PSB had the lowest nutrient concentrations in their roots and shoots,whereas seedlings simultaneously inoculated withA.scrobiculata,S.calospora,and PSB had greater nutrient concentrations in their roots and shoots.The seedlings simultaneously inoculated with all bioinoculants had 2–51,8–177,1–20%more N,P,and K,respectively,in their shoots and 1–74,14–208,2–11%more N,P,and K,respectively,in their roots than did individual and dual inoculated seedlings.Tissue nutrient contents were positively related to seedling growth,nodulation and AM colonization(Table 3).
Nutrient uptake
Ef ficiency of nutrient uptake for all tested nutrients was greaterforseedlingstreated with variousmicrobial bioinoculants(Table 2).The uptake ef ficiencies of N,P,and K were respectively 5–21,16–110 and 8–38%greater than recorded for uninoculated seedlings.Maximum N uptake ef ficiency was recorded for seedlings inoculated withS.calosporaalone.In contrast,maximum K uptake ef ficiency was recorded for seedlings coinoculated with both AM fungi,while P uptake ef ficiency was greater for seedlings inoculated with the two AM fungi in combination with PSB.The ef ficiency of nutrient uptake(except N)was positively related to plant growth,nutrient content nodulation and mycorrhizal variables(Table 3).
Nodulation
AllA.auriculiformisseedlings nodulated naturally and nodulation was significantly enhanced by bioinoculant inoculation(F7,72=159.645;p<0.001;Fig.1a).Inoculated seedlings had 33–400%more nodules compared to controls.Seedlings inoculated with multiple bioinoculants had 122–277%and 28–87%more nodules than seedlings that received one or two inoculants.Likewise,nodule dry weight of inoculated seedlings was 40–300%greater than recorded for controls (F7,72=595.747;p<0.001;Fig.1b).Multiple inoculations increased nodular biomass by 281–336%and 24–177%compared to single and dual inoculated seedlings.Taproot length was significantly and positively correlated(p<0.001,n=80)to nodule number(r=0.912)and nodule dry weight(r=0.816).Similarly,plant growth was also significantly and positively correlated to nodulation and nodule dry weight(Table 3).
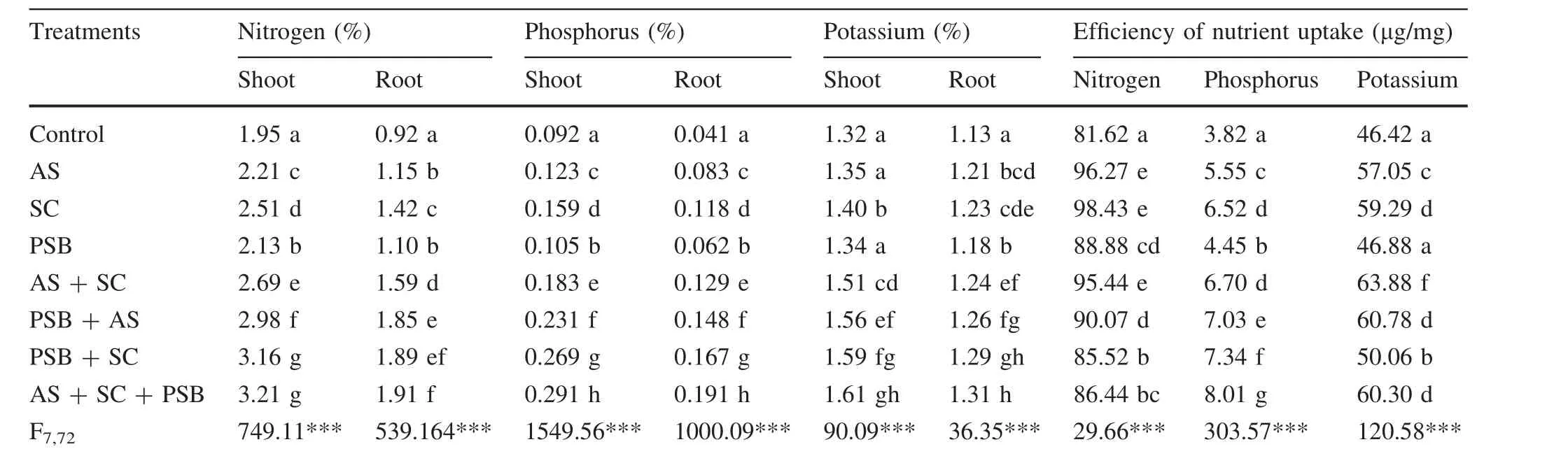
Table 2 In fluence of bioinoculant inoculation on nutrient contents and ef ficiency of nutrient uptake of Acacia auriculiformis as assessed after 60 days after transplantation
?
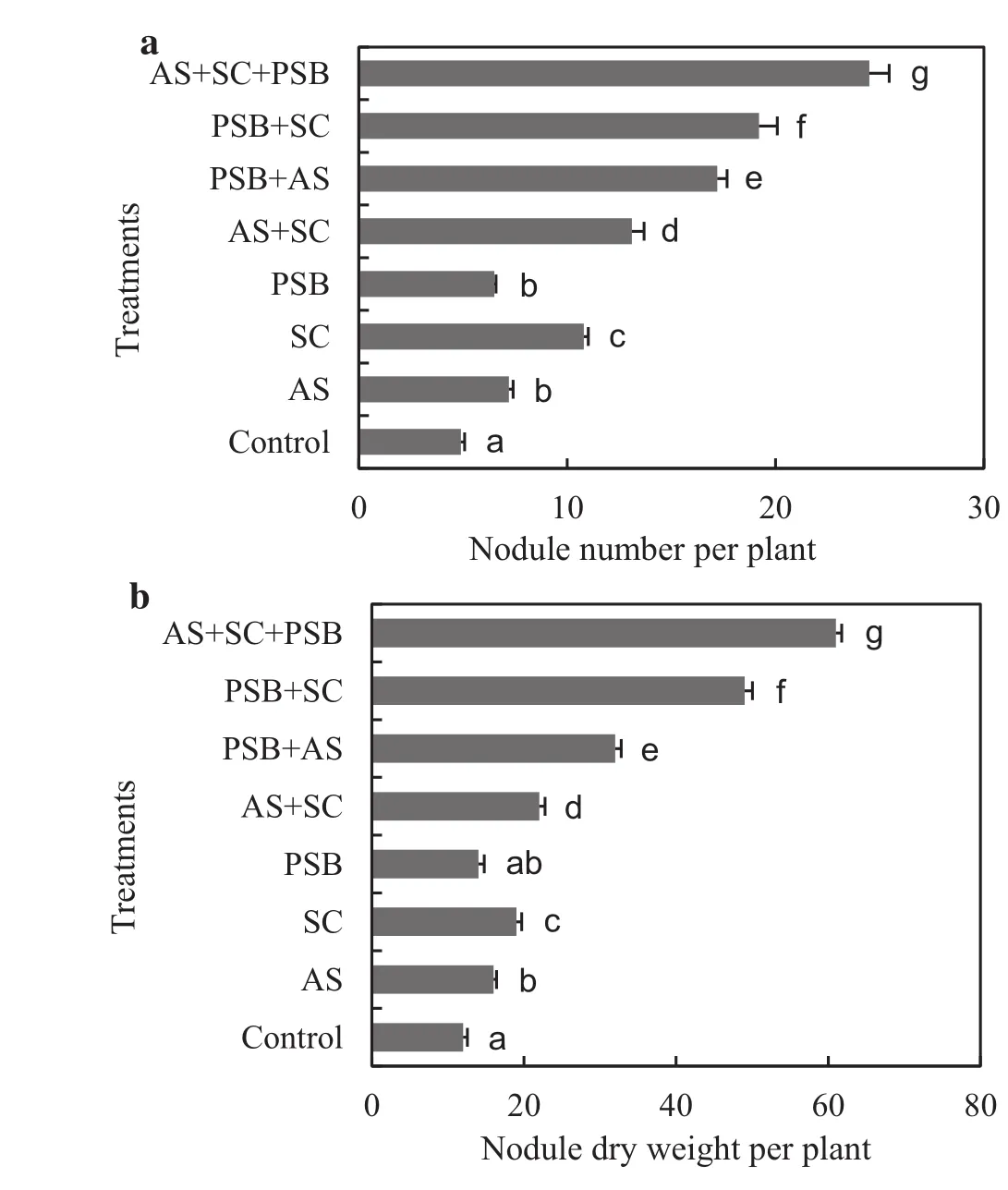
Fig.1 Nodule numbers(a)and nodule dry weights(b)(mean±1 S.E.)of Acacia auriculiformis inoculated with bioinoculants and assessed after 60 days after transplantation.AS,Acaulospora scrobiculata;SC,Scutellospora calospora;PSB,phosphate solubilizing bacteria.Signi ficant differences(p<0.05)according to DMRT are denoted by different letters
Mycorrhizal colonization
A.auriculiformisseedlings developed significantly lower levels of AM colonization when grown on soil containing indigenous AM fungi(F7,72=1458.094,p<0.001,Fig.2).But inoculation stimulated AM fungal colonization and inoculated seedlings had significantly higher colonization levels compared to controls.Inoculation ofA.scrobiculataandS.calosporaeither individually or in combination increased AM fungal colonization of seedlings by two to four times.Inoculation with PSB increased the colonization of native AM fungi by 119%and its inoculation in combination withA.scrobiculataandS.calosporaincreased AM colonization by 158 and 46%compared to individual inoculation of these fungi.Simultaneous inoculations ofA.scrobiculataandS.calosporatogether with PSB increased AM colonization in roots by seven to eight fold compared to uninoculated controls.Mycorrhizal colonization of seedlings was positively correlated to plant growth,nutrient,and nodulation variables(Table 3).
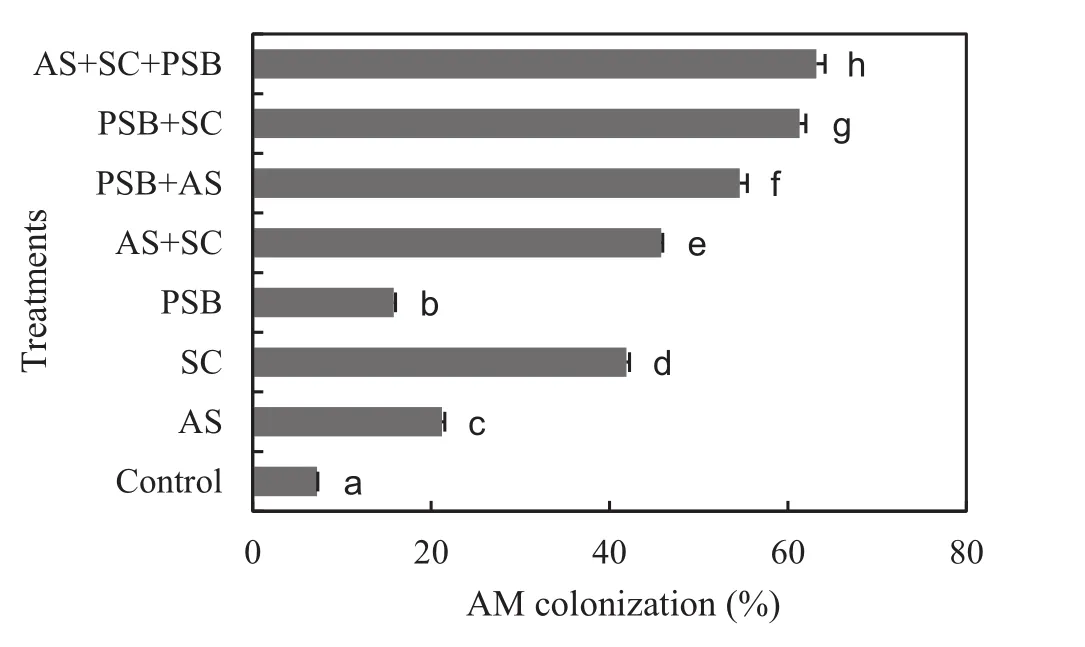
Fig.2 Arbuscular mycorrhizal(AM)colonization(mean±1 S.E.)of Acacia auriculiformis inoculated with bioinoculants and assessed after 60 days after transplantation.AS,Acaulospora scrobiculata;SC,Scutellospora calospora;PSB,phosphate solubilizing bacteria.Signi ficant differences(p<0.05)according to DMRT are denoted by different letters
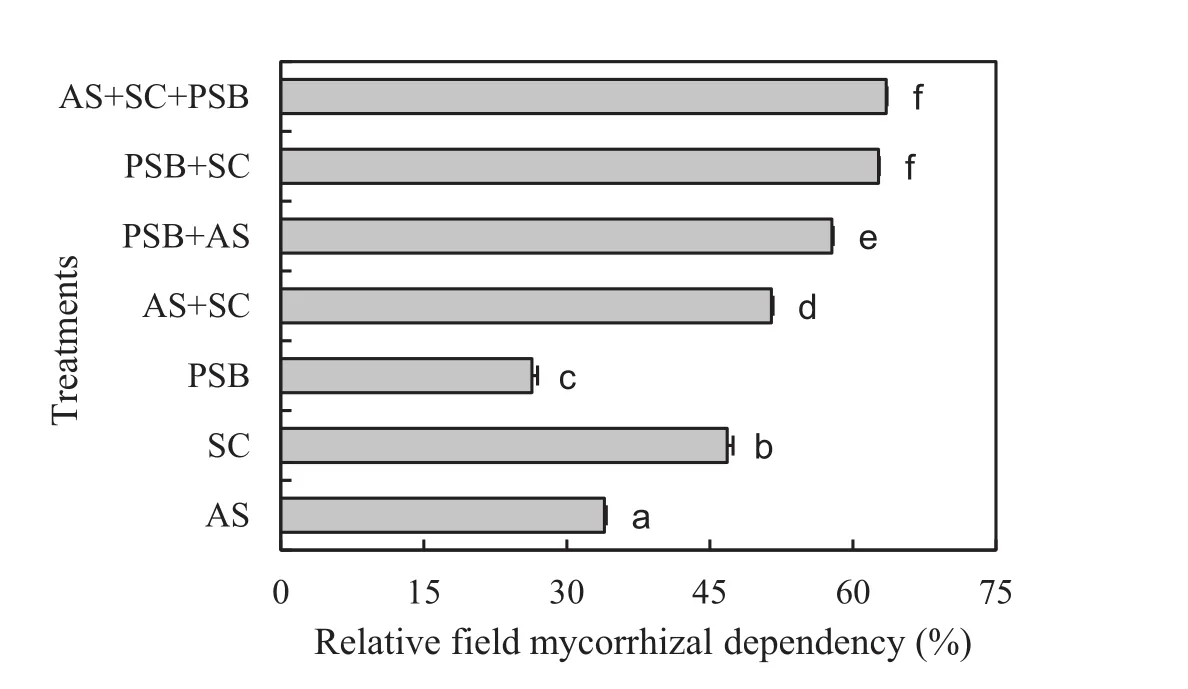
Fig.3 Relative field mycorrhizal dependency(mean±1 S.E.)of Acacia auriculiformis inoculated with bioinoculants.AS,Acaulospora scrobiculata;SC,Scutellospora calospora;PSB,phosphate solubilizing bacteria.Signi ficant differences(p<0.05)according to DMRT are denoted by different letters
Relative field mycorrhizal dependency
Inoculation positively affected the relative field mycorrhizal dependency ofA.auriculiformisseedlings(F6,63=1746.19,p<0.001,Fig.3).The relative field mycorrhizal dependency of seedlings treated with bothA.scrobiculataandS.calosporawas 11–55%greater than for seedlings inoculated with eitherA.scrobiculataorS.calospora.Similarly,seedlings coinoculated with PSB andA.scrobiculataorS.calosporaor both increased the relative field mycorrhizal dependency by 122–138%.Triple inoculation of all the microbes increased the relative field mycorrhizal dependency by 2–142%over individual and dual inoculations,respectively.The relative field mycorrhizal dependency was correlated with all plant growth,nutrient(except speci fic N uptake),nodulation,and AM variables(Table 3).
Seedling quality index
Seedling quality indices were higher for seedlings treated with bioinoculants either alone or in various combinations than in control seedlings(F5,54=1578.40,p<0.001,Fig.4).Seedlings inoculated with all three microbes orS.calosporaand PSB had the highest seedling quality index,which was 2–184%higher than seedlings receiving other inoculations and 271–280%higher than for control.The seedling quality index correlated positively with all plant growth,nutrient,AM and nodulation variables(Table 3).
Discussion
Our results show that inoculation can improve growth and quality ofA.auriculiformisseedlings under experimental conditions.This supports the hypothesis that tree legumes are generally dependent on microsymbionts and other soil microorganisms to thrive in stressed soils(Bashan et al.2009;Khan et al.2014).Most previous studies investigating the interactive in fluence of AM fungi and PSB on plant growth involved herbaceous crop plants,and information for woody perennial species is very limited(Muthukumar and Udaiyan 2010).
Height is an important criterion for determining the eligibility of tree seedlings for transplantation to field sites.Plantable size forA.auriculiformisseedlings is 15–30 cm(Ratha Krishnan et al.2014)and in the present study,all the seedlings inoculated with dual and triple combinations of bioinoculants attained a size>20 cm within nearly 60 days after inoculation.Inoculation also increased taproot lengths,which supports findings of other studies where inoculation ofAM orPSB enhanced rootlength(Muthukumar and Udaiyan 2006;Prasad et al.2012).Yao et al.(2009)showed that AM fungal inoculation did not affect taproot length inCitrus trifoliata,Rutaceae,a nonlegume.Taproots help plants to anchor deeply and stabilize on sites subject to strong winds.Long taproots also help plants to exploit moisture in deeper soil horizons and thereby resist drought(Gregory 2006).A recent metaanalysis on the responsiveness to mycorrhizal colonization of plants with taproots versus those with fibrous root systemsrevealed thatplantswith taprootsweremore responsive to mycorrhizal colonization than those with fibrous root system(Yang et al.2015).The signi ficant increase in cladode number and area of inoculated seedlings supports reports concluding that inoculation with AM fungi or PSB increased leaf area and numbers(Rajan et al.2000;Ghosh and Verma 2006;Yooyongwech et al.2013).Both AM fungi and PSB are shown to either produce or modulate plant phytohormone content,which is important in the development of leaves(Rodr´ıguez and Fraga 1999;Smith and Read 2008).Inoculation with AM fungi is generally reported to increase the ef ficiency of root systems such that plants tend to invest less biomass in root production and have low R/S ratios(Smith and Smith 2012).Contrary to this general observation,AM fungal inoculation increased the R/S ratios ofA.auriculiformisseedlings in this study.Nevertheless,the increase in R/S ratios of inoculated seedlings suggests that inoculation increased the carbon allocation for root growth.Altered root production in response to microbial inoculation can probably be attributed to modi fied nutrient availability or to changes in phytohormone levels in the seedlings.As microbial inoculation increased P content ofA.auriculiformisseedlings it might have induced proliferation of the root system.Changes in root architecture induced by application of P have been reported for many plant species(Lo´pez-Bucio et al.2003).Phytohormone-mediated differences in root development have also been well documented(Perez–Perez 2007).
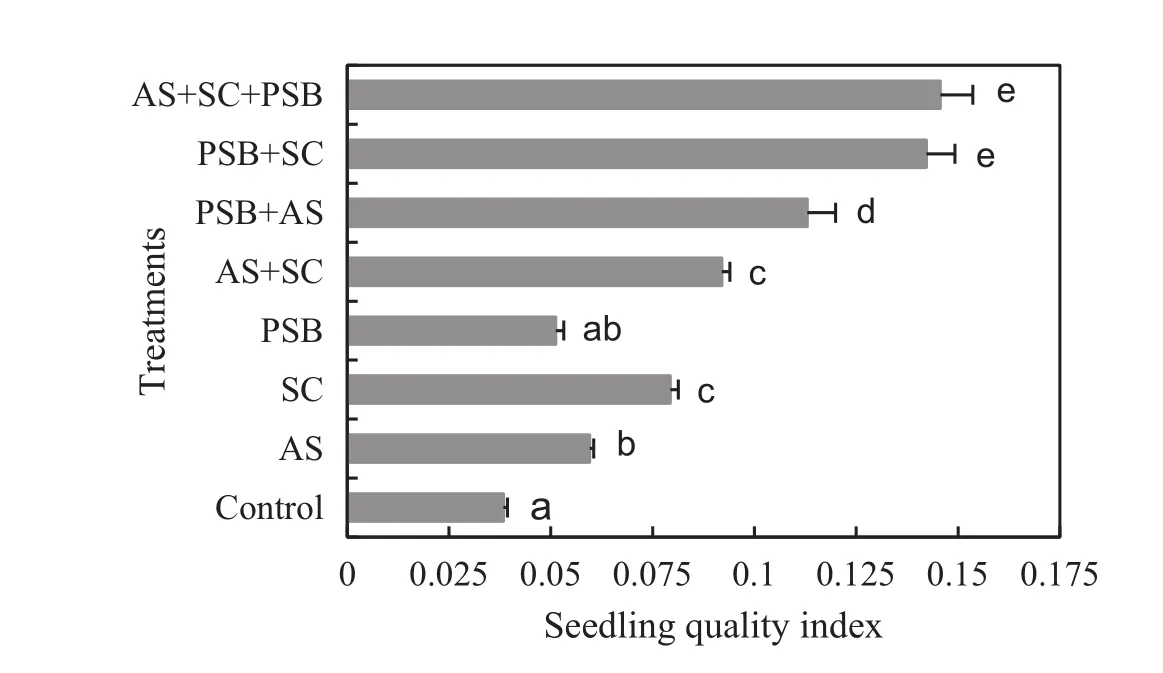
Fig.4 Seedling quality index(mean±1 S.E.)of Acacia auriculiformis inoculated with various bioinoculants.AS,Acaulospora scrobiculata;SC,Scutellospora calospora,PSB,phosphate solubilizing bacteria.Signi ficant differences(p<0.05)according to DMRT are denoted by different letters
In our study,nodulation occurred on all seedlings in spite of the absence of nodulating bacteria from our inoculations.This suggests that the soil contained indigenous nodulating bacteria that were capable of nodulating the root systems ofA.auriculiformis.Diouf et al.(2005)also reported nodulation inA.auriculiformisandA.mangiumby indigenous bacterial strains raised in an unsterile Senegal field soil.Individual or coinoculation of AM fungi and PSB increased nodulation by native nodulating bacterialstrains.Generally,the high energy demanding processes of nodule formation and nitrogen fixation by the bacterial symbiont requires an optimum level of P in the host plant.This was con firmed in this study where plant P was significantly related to nodule number and nodule dry weight.However,a signi ficant relation between nodule numbers and nodule dry weight in our study contradicts the results of Diouf et al.(2005)that showed no correlation between these variables.Further,the signi ficant correlation(p<0.001;n=8)of nodule abundance(r=0.987)and nodular mass(r=0.944)to proportional allocation of biomass to root(calculated as a proportion of the whole plant)suggests that the increased carbon availability in the roots for the nodulating bacteria could have positively in fluenced nodulation and nodular biomass.The positive correlation between nodulation and taproot length ofA.auriculiformisseedlings suggests the involvement of common factors like plant hormones(especially auxins)in both root formation and nodulation(Bensmihen 2015).
All the uninoculated control seedlings were colonized by AM fungi,suggesting that indigenous AM fungi were capable of establishing mycorrhization.The colonization levels were,however,low(<10%)in these seedlings due to limited abundance of AM propagules in the studied soil.Arbuscularmycorrhizalinoculationremovedthisconstraint,and seedlings inoculated with AM fungi either alone or in combinations showed higher AM colonization levels than did control seedlings.Dual inoculation ofA.scrobiculataandS.calosporaresulted in more extensive colonization than did inoculation using either of these fungi individually.This supports the results of Giri et al.(2004)whereA.auriculiformisseedlingsinoculatedwithbothR.fasciculatusandG.macrocarpumdeveloped more colonization than did seedlings inoculated with eitherR.fasciculatusorG.macrocarpum.Seedlings ofA.auriculiformisinoculated withS.calosporahad more root length colonized than did seedlings inoculated withA.scrobiculata.This contradicts the view that isolates of the Gigasporaceae are poorer root colonizers than are isolates of the Acaulosporaceae.The variation in root colonizing ability between the two AM fungi could be attributed to the inherent colonizing behaviour of these fungal isolates(Hart and Reader 2002).
Inoculation with PSB not only stimulated root colonization by the indigenous AM fungi,but also by the inoculated AM fungi.This was evidenced by the higher colonization levels in seedlings coinoculated with AM fungi and PSB compared to those inoculated with AM fungi alone.It is well established that some plant growth promoting rhizobacteria includingPaenibacilluscan stimulate mycorrhizal formation in different plant species(Hildebrandt et al.2006;Arthurson et al.2011)and are considered mycorrhizal helper bacteria(Frey-Klett et al.2007).Frey-Klett et al.(2007)reported that in addition to stimulating the process of mycorrhizal formation,Paenibacillusalso act as mycorrhizal helper bacteria by positively interacting with the functioning of the mycorrhizal fungi.This was evidenced in our study by the increased nutrient content of dual and triple inoculatedA.auriculiformisseedlings involving PSB.Though mycorrhizal helper bacteria lack plant speci ficity,they may be fungal speci fic(Garbaye 1994).In the present study,Paenibacillusstimulated colonization ofS.calosporainoculated seedlings more than it did seedlings inoculated withA.scrobiculata.
The increased NPK contents of shoots and roots ofA.auriculiformisseedlings inoculated in the current study could be attributed to various factors.First,an elaboration of the root surface area in response to AM fungal colonization could have resulted in greater flow of nutrients to roots(Smith and Smith 2012).This was suggested by the highly signi ficant positive relationship between root length colonized by AM fungi and plant tissue nutrient contents.Second,inoculation of the PSBPaenibacillusmight have improved the solubility and the subsequent availability of P in the soil,facilitating its uptake either directly or indirectly by roots(Arthurson et al.2011).Third,increased activity of the nodulating bacteria could have contributed to the increased levels of N in plant tissues.This was evidenced by the positive correlation between seedling N content and nodulation variables.Nevertheless,the lack of correlation between the ef ficiency of N uptake and seedling N content implies thatA.auriculiformisin the present experimental conditions obtained most of its N from its symbiosis with the nodulating bacterium rather than from its direct uptake from the soil.And finally,increased root proliferation subsequently resulting in increased root surface area as discussed earlier could have also contributed to the increased nutrient content of inoculated seedlings.Interestingly,nutrient content of seedlings inoculated withS.calosporawas always higher than in seedlings inoculated withA.scrobiculata.This variation could be attributed to differences in the amount of extraradical hyphae produced by these two fungi.Although not examined in the present study,S.calosporatend to colonize soil more extensively than members ofAcaulosporaresulting in more host bene fits(Hart and Reader 2002).Moreover,the response of the host plant to AM symbiosis could vary significantly not only by fungal species but even among the isolates of a species(Lee et al.2013).The mechanisms for this functional diversity are largely unknown but evidence suggests that the differences in the exchange of nutrients and photosynthates between plants and AM fungus might be a contributing factor(Kiers et al.2011).Therefore,inoculation with multiple AM fungi appears to be more bene ficial than inoculation with only one isolate.
Our results showed that dual and triple inoculation withA.scrobiculata,S.calospora,and PSB increased the mycorrhizal dependency of seedlings compared to those receiving only single inoculations.This supports Giri et al.(2004),who showedthat thedependencyofA.auriculiformisseedlings on mycorrhizal fungi was greater when inoculated with bothR.fasciculatusandG.macrocarpuscompared to seedlings inoculated with eitherR.fasciculatusorG.macrocarpus.Though mycorrhizal dependency tends to differ with AMfungalspeciesandthedevelopmentalstageoftheplant,it generallyincreaseswiththenutrientdemandofthehostplant.Interestingly,the mycorrhizal dependency of seedlings inoculated withPaenibacillusandS.calosporawas greater than for seedlings inoculated withPaenibacillusandA.scrobiculata.PreleasedbyPSBoftenfailstoreachthesurfaceofplant rootsduetothelimiteddiffusionor fixationofthereleasedsoil P.Nevertheless,the released P is absorbed by the AM fungal mycelium and is transported to the plant roots,thereby synergistically improving the P uptake of the plants(Barea et al.2005).The capacity for this method ofP uptakeand transport varies with AM fungal species.In addition,the AM fungus and PSB can interact in the hyphosphere to promote the mineralization of phytate in the soil(Zhang et al.2014).Therefore,efficient nutrient acquisition and transfer byS.calosporaand its better compatibility withPaenibacillusmighthavecontributedtothehighmycorrhizaldependencyofA.auriculiformison this combination than on theA.scrobiculata-Paenibacilluscombination.
The enhancement of the quality of our seedlings by microbe inoculation supports the findings of studies where inoculation of microorganisms enhanced the quality of the forest tree seedlings raised in nurseries(Muthukumar and Udaiyan 2006,2010).We attribute the improved seedling quality to the positive in fluence of inoculation.
Conclusions
Inoculation positively affected seedling growth and quality ofA.auriculiformisin unsterile soil under nursery conditions.This con firms that AM fungi and PSB can play a pivotal role in growth and nutrient uptake by seedlings even innutrient-stressedsoils.Thegrowthofseedlingsinoculated with individual bioinoculants was less vigorous than the growth of seedlings inoculated with combinations of bioinoculants.This interactive effect among microbial inoculants could be exploited in nurseries involved in seedling production for raising plantations on degraded soils.
References
Arthurson V,Hjort K,Muleta D,Ja¨derlund L,Granhall U(2011)Effects onGlomus mosseaeroot colonization byPaenibacilluspolymyxaandPaenibacillus brasilensisstrains as related to soil P-availability in winter wheat.Appl Environ Soil Sci 2011:9.doi:10.1155/2011/298097
Barea JM,Pozo MJ,Azco´n R,Azco´n-Aguilar C(2005)Microbial cooperation in the rhizosphere.J Exp Bot 56(417):1761–1778
Bashan Y,Salazar B,Puente ME(2009)Responses of native legume desert trees used for reforestation in the Sonoran Desert to plant growth-promoting microorganisms in screen house.Biol Fertil Soils 45(6):655–662
Bensmihen S(2015)Hormonal control of lateral root and nodule development in legumes.Plants 4(3):523–547
Davis DJ(1962)Emission and absorption spectrochemical methods.In:Tracey MV,Peach K(eds)Modern methods of plant analysis.Springer,Berlin,pp 1–25
Dickson A,Leaf AL,Hosner JF(1960)Quality appraisal of white spruce and white pine seedling stock in forest nurseries.For Chron 36(1):10–13
Diouf D,Duponnois R,Ba AT,Neyra M,Lesueur D(2005)Symbiosis ofAcacia auriculiformisandAcacia mangiumwith mycorrhizal fungi andBradyrhizobiumspp.improves salt tolerance in greenhouse conditions. Funct Plant Biol 32(12):1143–1152
Dumroese RK,Jacobs DF,Davis AS(2009)InoculatingAcacia koawithBradyrhizobiumand applying fertilizer in the nursery:effects on nodule formation and seedling growth.HortSci.44(2):443–446
Duponnois R,Founoune H,Lesueur D,Thioulouse J,Neyra M(2000)Ectomycorrhization of sixAcacia auriculiformisprovenances from Australia,Papua New Guinea and Senegal in glasshouse conditions:effect on the plant growth and on the multiplication of plant parasitic nematodes.Aust J Exp Agric 40(3):443–450
Founoune HB,BaˆA,Plenchette C,El Jaafari S,Neyra M,Ducousso M(2000)Ectomycorrhization ofAcacia holosericeaA Cunn.ex G Don byPisolithusspp in Senegal:effect on plant growth and on the root-knot nematodeMeloidogyne javanica.Ann For Sci 57(4):345–350
Frey-Klett P,Garbaye J,Tarkka M(2007)The mycorrhiza helper bacteria revisited.New Phytol 176(1):22–36
Garbaye J(1994)Helper bacteria:a new dimension to the mycorrhizal symbiosis.New Phytol 128(2):197–210
Ghosh S,Verma NK(2006)Growth and mycorrhizal dependency ofAcacia mangiumWilld.inoculated with three vesicular arbuscular mycorrhizal fungi in lateritic soil.New For 31(1):75–81
Giri B,Kapoor R,Agarwal L,Mukerji KG(2004)Preinoculation with arbuscular mycorrhizae helpsAcacia auriculiformisgrow in degraded Indian wasteland soil.Commun Soil Sci Plant Anal 35(1–2):193–204
Girjashankar V(2011)Micropropagation of multipurpose medicinal treeAcacia auriculiformis.J Med Plants Res 5(3):462–466
Gray JT,Schlesinger WH(1983)Nutrient use by evergreen and deciduous shrubs in southern California.J Ecol 71(1):43–56
Gregory PJ(2006)Roots and the architecture of root systems.In:Gregory PJ(ed)Plant roots:growth,activity,and interaction with soils.Blackwell Publishing Ltd,Oxford,pp 18–44
Grif fin AR,Midgley SJ,Bush D,Cunningham PJ,Rinaudo AT(2011)Global uses of Australian acacias—recent trends and future prospects.Divers Distrib 17(5):837–847
Hart MM,Reader RJ(2002)Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi.New Phytol 153(2):335–344
Hildebrandt U,Ouziad F,Marner FJ,Bothe H(2006)The bacteriumPaenibacillus validusstimulates growth of the arbuscular mycorrhizal fungusGlomus intraradicesup to the formation of fertile spores.FEMS Microbiol Lett 254(2):258–267
Jackson ML(1971)Soil chemical analysis.Prentice Hall,New Delhi,p 498
Khan BM,Hossain MK,Mridha MAU(2014)ImprovingAcacia auriculiformisseedlings using microbial inoculant(Bene ficial Microorganisms).J For Res 25(2):359–364
Kiers ET,Duhamel M,Beesetty Y,Mensah JA,Franken O,Verbruggen E,Felbaum CR,Kowalchuk GA,Hart MM,Bago A,Palmer TM,West SA,Vandenkoornhuyse P,Jansa J,Bu¨cking H (2011)Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis.Science 333(6044):880–882
Kushalapa KA(1991)Performance ofAcacia auriculiformisin India.In:Turnbull,JW(ed.)Advances in tropical acacia research.Proceedings of an international workshop held in Bangkok,Thailand.Australian Centre forInternationalAgricultural Research,Canberra,pp 189–193
Lamani VK,Patil SK,Manjunath GO(2004)Growth ofAcacia auriculiformisas in fluenced by N,P and K fertilizer.Karnataka J Agric Sci 17(4):872–874
Lee E-H,Eo J-K,Ka K-H,Eom A-H(2013)Diversity of arbuscular mycorrhizal fungi and their roles in ecosystems.Mycobiology 41(3):121–125
Lo´pez-Bucio J,Cruz-Ramırez´A,Herrera-Estrella L(2003)The role of nutrient availability in regulating root architecture.Curr Opin Plant Biol 6(3):280–287
McGonigle TP,Miller MH,Evans DG,Fairchild GL,Swan JA(1990)A method which gives an objective measure of colonization of roots by vesicular–arbuscular mycorrhizal fungi.New Phytol 115(3):495–501
Muthukumar T,Udaiyan K(2002)Arbuscular mycorrhizal fungal composition in semi-arid soils of Western Ghats,southern India.Curr Sci 82(6):624–628
Muthukumar T,Udaiyan K(2006)Growth of nursery grown bamboo inoculated with arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria in two tropical soil types with and without fertilizer application.New For 81(3):469–485
Muthukumar T,Udaiyan K(2010)Growth response and nutrient utilization ofCasuarina equisetifoliaseedlings inoculated with bioinoculants under tropical nursery conditions.New For 40(1):101–118
Muthukumar T,Udaiyan K,Rajeshkannan V(2001)Response of neem(Azadirachta indicaA.Juss)to indigenous arbuscular mycorrhizal fungi,phosphate-solubilizing and symbiotic nitrogen- fixing bacteria under tropical nursery conditions.Biol Fertil Soils 34(6):417–426
Perez-Perez JM(2007)Hormone signalling and root development:an update on the latestArabidopsis thalianaresearch.Funct Plant Biol 34(3):163–171
Plenchette C,Fortin JA,Furlan V(1983)Growth responses of several plant species to mycorrhizae in a soil of moderate P-fertility I.Mycorrhizal dependency under field conditions.Plant Soil 70(2):199–209
Porter WM(1979)The most probable number method for enumerating infective propagules of vesicular–arbuscular mycorrhizal fungi in soil.Aust J Soil Res 17(3):515–519
Prasad K,Aggarwal A,Yadav K,Tanwar A(2012)Impact of different levels of superphosphate using arbuscular mycorrhizal fungi andPseudomonas fluorescensonChrysanthemum indicumL.J Soil Sci Plant Nutr 12(3):451–462
Rajan SK,Reddy BJD,Bagyaraj DJ(2000)Screening of arbuscular mycorrhizal fungi for their symbiotic ef ficiency withTectona grandis.Forest Ecol Manag 126(2):91–95
Ratha Krishnan P,Kalia RK,Tewari JC,Roy MM(2014)Plant nursery management:principles and practices.Central Arid Zone Research Institute,Jodhpur,p 40
Ravindranath NH,Murthy IK,Priya J,Upgupta S,Mehra S,Nalin S(2014)Forest area estimation and reporting:implications for conservation, management and REDD+. Curr Sci 106(9):1201–1206
Rodrı´guez H,Fraga R(1999)Phosphate solubilizing bacteria and theirrole in plantgrowth promotion.BiotechnolAdv 17(4–5):319–339
Shukla NK,Lal M,Singh RS,Khanduri AK(1990)Physical and mechanical properties ofAcacia auriculiformis,Fernandoa adenophyllaandMelia azaderach.J Timber Dev Assoc India 36(1):31–45
Smith SE,Read DJ(2008)Mycorrhizal symbiosis,3rd edn.Academic Press,San Diego,p 815
Smith SE,Smith FA(2012)Fresh perspectives on the roles of arbuscular mycorrhizal fungi in plant nutrition and growth.Mycologia 104(1):1–13
Uddin MB,Khan MASA,Mukul SA,Hossain MK(2008)Effects of inorganic fertilizers on biological nitrogen fixation and seedling growth of some agroforestry trees in Bangladesh.J For Res 19(4):303–306
Van VT,Berge O,Ke SN,Balandreau J,Heulin T(2000)Repeated bene ficial effects of rice inoculation with a strain ofBurkholderia vietnamiensison early and late yield components in low fertility sulphate acid soils of Vietnam. Plant Soil 218(1):273–284
World Bank(2011)Forest area(%of land area)[Data file].Retrieved August 14,2012 from http://data.worldbank.org/indicator
Yang L,Liu N,Ren H,Wang J(2009)Facilitation by two exoticAcacia:acacia auriculiformisandAcacia mangiumas nurse plants in South China.For Ecol Manag 257(8):1786–1793
Yang H,Zhang Q,Dai Y,Liu Q,Tang J,Bian X,Chen X(2015)Effects of arbuscular mycorrhizal fungi on plant growth depend on root system:a meta-analysis.Plant Soil 389(1):361–374
Yao Q,Wang LR,Zhu HH,Chen JZ(2009)Effect of arbuscular mycorrhizal fungal inoculation on root system architecture of trifoliate orange(Poncirus trifoliataL.Raf.)seedlings.Sci Hortic 121(4):458–461
Yooyongwech S,Phaukinsang N,Cha-Um S,Supaibulwatana K(2013)Arbuscular mycorrhiza improved growth performance inMacadamia tetraphyllaL.grown under water de ficit stress involves soluble sugar and proline accumulation.Plant Growth Regul.69(3):285–293
Zhang L,Fan J,Ding XD,He X,Zhang F,Feng G(2014)Hyphosphere interactions between an arbuscular mycorrhizal fungus and a phosphate solubilizing bacterium promote phytate mineralization in soil.Soil Biol Biochem 74:177–183
 Journal of Forestry Research2018年3期
Journal of Forestry Research2018年3期
- Journal of Forestry Research的其它文章
- Vacuum press drying studies on two fast-growing Indian wood species
- Water-repellent ef ficiency of thermally modi fied wood as affected by its permeability
- The nutritional composition and digestion of plants foraged by red deer(Cervus elaphus xanthopygus)in northeast China
- Predicting intensity of white-tailed deer herbivory in the Central Appalachian Mountains
- A taxonomic review of the genus Miaenia Pascoe,1864(Coleoptera:Cerambycidae)from South Korea with a new record of M.tonsa(Bates)
- A study on JA-and BTH-induced resistance of Rosa rugosa‘Plena’to powdery mildew(Sphaerotheca pannosa)
