Purification and Crystallographic Analysis ofEscherichia coli GhoS
, ,
(Department of Physiology and Biophysics, School of Life Sciences, Fudan University, Shanghai 200438, China)
Toxin-antitoxin systems(referred to as TAs hereafter) broadly exist in the genomes of eubacteria and archaea; besides chromosome, the TA loci have also been found at low-copy plasmids[1]. TAs play many physiologically important roles, such as phage inhibition[2], global gene regulation[3]and antibiotics tolerance[4]. Normally, TAs are encoded by small bicistronic genes; toxins are stable and can interfere with the basic cellular metabolism, antitoxins can neutralize the toxicities of the toxins but are very labile. When suffering from some environmental stresses, such as the lack of nutrition and oxygen, DNA damage, antibiotics or phage infection[5-7], TAs can trigger the death of the bacteria. The death process begins with the degradation of antitoxin, leading to the activation of toxin; then, the activated toxin will execute its interferase activity.
Unlike Toxins, which are all proteins, antitoxins could be either proteins or RNAs. According to the nature of the antitoxins and their toxin inhibiting mechanisms, TAs can be classified into five different types. For type Ⅰ TAs, the antitoxins are antisense RNAs, which pair with the SD sequences of the toxin mRNAs and prevent their translation[8-9]. Both toxin and antitoxin are proteins for the type Ⅱ TAs, the antitoxins directly bind with the toxins and neutralize their toxicities[10-11]. Similar to type Ⅰ TAs, the type Ⅲ antitoxins are also RNAs; however, instead of the toxin mRNAs, type Ⅲ antitoxins bind with the toxin proteins[12-13]. Like the type Ⅱ TAs, the antitoxins of type Ⅳ and Ⅴ TAs are also proteins. However, instead of direct toxin-antitoxin interactions, type Ⅳ antitoxins interfere with the binding of the toxins to their targets, which are usually protein filaments[14]. Different form the type Ⅱ and type Ⅳ antitoxins, type Ⅴ antitoxins cleave the mRNAs of the toxins[15].
Owing to their functional importance, TAs of many species, such asBacillussubtilis,Mycobacteriumtuberculosis, andEscherichiacoli(E.coli) have been extensively studied in the past; numerous TA pairs have been identified, most of them belong to the type Ⅱ family. Very interesting, there is only one type Ⅴ TA pair, theE.coliGhoST pair[15], discovered to date. GhoT is the toxin protein and possesses various cellular activities. When produced at limited level, GhoT increases the persistence of the cells. Therefore, the cells can survive under environmental stress and exhibit tolerance to adverse treatments, such as antibiotic, hydrogen peroxide, DNA damage, and more. When overexpressed, GhoT can damage the cell membrane and lead to the ghost cell formation. Unlike the normal cells, centers of the ghost cells are transparent and the cell poles become dense[16]. In addition, GhoT can also increase early biofilm formation. The antitoxin protein GhoS is an endoribonuclease that recognizes and cleaves GhoT mRNA at specific sites. Different from other antitoxins, GhoS is not sensitive to the environmental stress and it does not bind to DNA to regulate its own transcription[15]. The cellular functions of GhoST are regulated by MqsAR[17], one member of the type Ⅱ TAs inE.coli. MqsR(the toxin protein of the MqsAR pair) recognizes the target mRNA and cleaves at the 5’-GCU-3’ site; while it is present in the mRNA of GhoS, the GCU motif is absent in GhoT mRNA. Under adverse treatments, the antitoxin MqsA is degraded; the liberated MqsR cleaves the GhoS mRNA and triggers the GhoT translation(Fig.1).
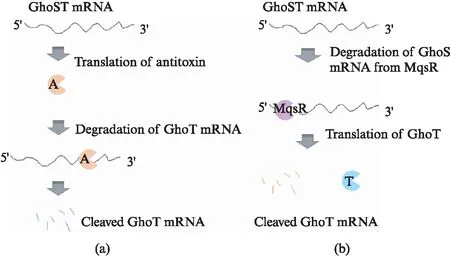
Fig.1 Regulation of GhoST system: (a) under normal conditions or (b) when treated with environmental stressA: antitoxin GhoS, T: toxin GhoT.
GhoS cleaves the GhoT mRNA at several different locations; product analysis revealed one putative targeting sequence, corresponding to 5’-UNNU(A/C)N(A/G)(A/U)A(A/U)-3’. GhoS can exist as either monomer or dimer and both forms are functional. However, the basis for dimerization and substrate recognition of GhoS is not clear. Here, we report the expression and crystallographic studies of GhoS. The crystal diffracted to 1.5 Å resolution and belongs to the P21space group. Optimization of Se-GhoS crystals are in progress; determination of these GhoS structures will provide better insights into the structure-and-function relationship of GhoS protein.
1 Materials and methods
1.1 DNA construction
The pET28a plasmid containing the codon-optimized cDNA of the full-length GhoS F14A mutant was purchased from GENERAY Biotechnology Co. Ltd., Shanghai, China(http:∥www.generay.com.cn); the F14A mutation was introduced to favor the dimerization of GhoS protein. The target cDNA was amplified by polymerase chain reaction(PCR) using two primers, GhoS_F and GhoS_R. The detailed sequences of cDNA and the primers are listed in Tab.1.
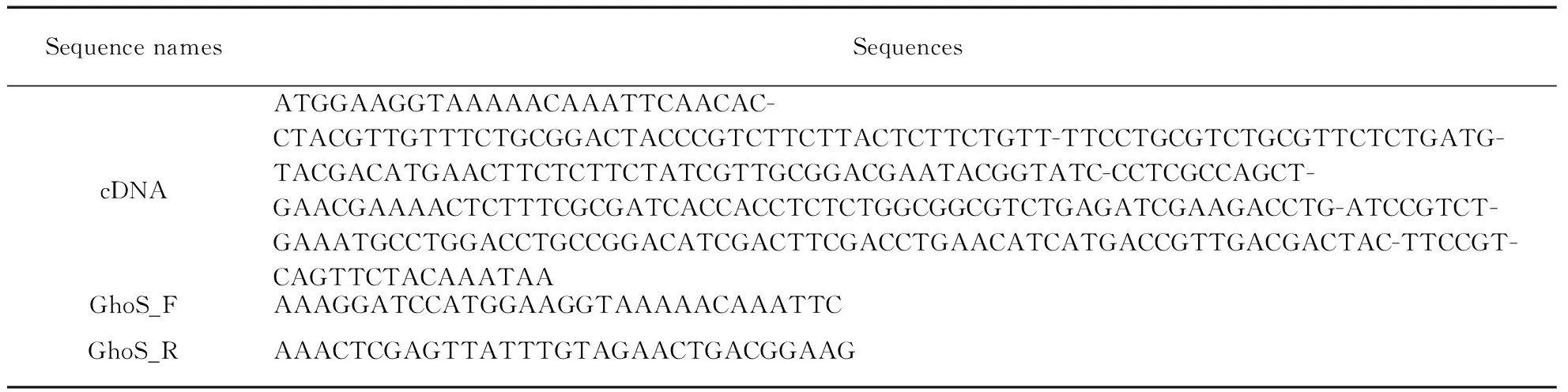
Tab.1 Optimized cDNA sequence and primers for GhoS F14A mutant construction(from 5’ to 3’)
The amplicon was resolved on agar gel and the target DNA was extracted using the commercial gel extraction kit(Qiagen). The target DNA was double-cleaved withBamHⅠ andXhoⅠ, and inserted into theBamHⅠ/XhoⅠ site of the pET28-SUMO vector. The recombinant vector(coding the His-Sumo-GhoS) was then transferred into theEscherichiacoliBL21(DE3) competent cell. The plasmid DNA was extracted according to standard Miniprep protocols and the sequence of the plasmid was confirmed by DNA sequencing. All the recombinant strains are protected by 30% glycerol and stored at -80℃ freezer till use.
1.2 Protein expression and purification
The frozen recombinant strains were revived in Lysogeny broth(LB) medium supplemented with 50μg/mL kanamycin at 37℃ overnight. Every 20mL revived bacterium suspension was inoculated into 1L LB medium supplemented with kanamycin(50μg/mL) and cultured at 37℃ with continuous shaking(225r/min). The protein expression was induced by addition of isopropylβ-D-1-thiogalactopyranoside(IPTG, final concentration is 0.2mmol/L), when OD260reached 0.6—0.8. The induced cultures were then grown at 18℃ for an additional 16—18 h.
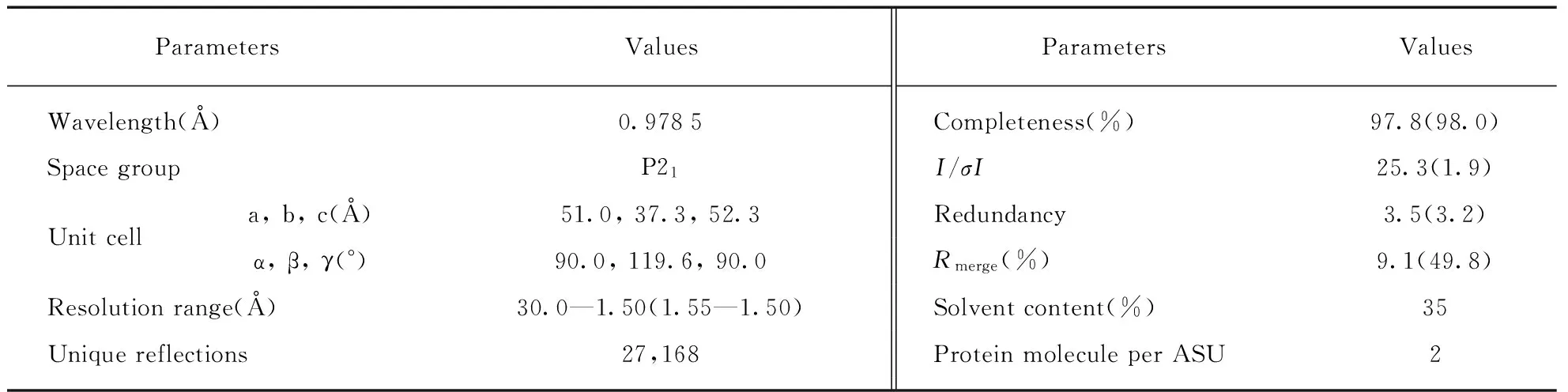
Tab.2 Data collection and processing statistics*
* Values in parentheses are for the highest resolution shell.
The cell pellet was harvested by centrifugation(4 500 r/min, 20min), resuspended in lysis buffer(Buffer A, 20 mmol/L Tris-HCl pH 8.0, 500 mmol/L NaCl, 25 mmol/L imidazole), and lysed under high pressure via JN-02C cell crusher. The homogenate was clarified by centrifugation(17000r/min) at 4℃ for 1h and the supernatant was loaded onto a HisTrapTMcolumn(GE Healthcare) equilibrated with buffer A. The His-Sumo-GhoS fusion protein was eluted from the column using elution buffer(Buffer B, 20 mmol/L Tris-HCl pH 8.0, 500 mmol/L NaCl, 500 mmol/L imidazole) with a stage-wise gradient. The fraction containing the desired proteins were pooled, digested with Ulp1 protease, and dialyzed against Buffer A at 4℃ for 3h. The sample was again loaded onto the HisTrapTMcolumn, the flow through containing the GhoS protein was collected and further purified by gel filtration chromatography using a Hiload 16/60 Superdex G75 column(GE Healthcare) equilibrated with Buffer C(10mmol/L Tris-HCl pH 8.0, 100 mmol/L NaCl, 2mmol/L DTT).
1.3 Crystallization and data collection
The initial crystallization conditions were identified using the Gryphon crystallization robot system(Arts Robbin Instrument) and the commercial crystallization kits(Hampton Research and Emerald) at 18℃. During the initial screening, the sitting-drop vapor diffusion method with the 3-drop Intelli-Plates was utilized; the droplet contains 0.2 μL protein sample and 0.2μL well solution. The optimization procedure was performed at room temperature using the hanging-drop vapor diffusion method.
The crystals were cryoprotected using the mother liquor supplemented with 25% glycerol and flash-frozen in liquid nitrogen. The diffraction data were collected on beamline BL19U at the Shanghai Synchrotron Radiation Facility(SSRF) at cryogenic temperatures, maintained with a cryogenic system. One single crystal was utilized for the data collection; data processing was carried out using the HKL3000 programs[18].The data collection and processing statistics are summarized in Tab.2.
2 Results and discussion
The full length GhoS contains 98 amino acids, and together with two residues(Ser and Gly) left from the fusion tag, the target protein has a theoretic molecular weight of 11.5kDa. When purified on the Hiload 16/60 Superdex G75 column(Fig.2(a)), two protein peaks were observed. As revealed by the SDS-Page gel analysis(Fig.2(b)), both peaks are the GhoS F14A mutant proteins, which are more than 95% in purity. The two peaks were eluted at the volume of 65.5 mL and 77.4 mL, the apparent molecular weights are 25 kDa and 13 kDa, respectively; the molecular weights are calculated using the following formula:Kov=(Ve-Vo)/(Vt-Vo)(whereVe= elution volume of the protein,Vo= column void volume,Vt= total bed volume), which was calibrated using the standard proteins purchased from GE healthcare company. These observations suggested that the F14A mutant of GhoS exists as both dimer and monomer; the ration between the dimer and monomer is around 3∶7, which is similar to the value previously reported[15].

Fig.2 GhoS F14A mutant purification and analysis(a) Profile of the size exclusion chromatography. (b) SDS-Page gel analysis of the purified protein. M: protein marker. The number on top of the SDS-Page gel corresponds to the fraction number in the panel (a).
Both monomeric and dimeric proteins were concentrated and screened against 12 crystallization kits purchased from the commercial company. Might due to the very low concentration, the dimeric GhoS F14A protein failed to generate any crystal during the screening process. Fortunately, the monomeric protein with much higher concentration(36mg/mL) was able to give crystals under several similar conditions; the sizes of all the initial crystals are very small(less than 0.05mm×0.04mm×0.02mm).
After several round of optimization, some crystals(Fig.3) with size around 0.2mm×0.15mm×0.05mm were obtained under the crystallization condition composed of 100 mmol/L Hepes/NaOH pH 7.0, 30% PEG400, 200 mmol/L MgCl2. The droplet contains 0.5μL protein sample and 1.0μL well solution. The crystal appeared one week after the drop was setup and reached their full sizes after one additional week.
The crystals were cryoprotected and used for data collection on beamline BL19U at the Shanghai Synchrotron Radiation Facility. The best diffraction data was collected from one single crystal at temperature of 100 K. Totally, 360 diffraction images(oscillation angle 1.0°) were collected(Fig.4 shows one of them) and the data was processed to 1.5 Å resolution(Tab.2). The crystal belongs to the monoclinic P21space group with unit cell parameters:a=51.0,b=37.3,c=52.3,a=90.0°,β=119.6°,γ=90.0°. As indicated by the Matthews coefficient value(1.88), there are 2 GhoS molecules contained in the asymmetric unit, the corresponding solvent content is 35%.

One GhoS NMR structure(PDB_ID: 2LLZ) has been previously reported[15]; however, this structure has failed to serve as the searching model to solve the crystal structure with molecular replacement method, suggesting that conformations of the NMR and crystal structures are significantly different from each other. Crystallization and optimization of the Se-Met substituted GhoS proteins are in progress, the diffraction data of the Se-GhoS crystal will facilitate the structure determination and provide better insights into the structure-and-function relationship of GhoS.
References:
[1] MAGNUSON R D. Hypothetical functions of toxin-antitoxin systems [J].JBacteriol, 2007,189(17): 6089-6092.
[2] PECOTA D C, WOOD T K. Exclusion of T4 phage by the hok/sok killer locus from plasmid R1 [J].JBacteriol, 1996,178(7): 2044-2050.
[3] WANG X, WOOD T K. Toxin-antitoxin systems influence biofilm and persister cell formation and the general stress response [J].ApplEnvironMicrobiol, 2011,77(16): 5577-5583.
[4] LEWIS K. Persister cells [J].AnnuRevMicrobiol, 2010,64: 357-372.
[5] ENGELBERG-KULKA H, AMITAI S, KOLODKIN-GAL I, et al. Bacterial programmed cell death and multicellular behavior in bacteria [J].PLoSGenet, 2006,2(10): e135.
[6] HAZAN R, ENGELBERG-KULKA H.EscherichiacolimazEF-mediated cell death as a defense mechanism that inhibits the spread of phage P1 [J].MolGenetGenomics, 2004,272(2): 227-234.
[7] KOBAYASHI I. Genetic addiction-a principle in symbiosis of genes in a genome [M]∥Phillips G, Funnell B, eds. Plasmid Biology. Washington, DC, USA : ASM Press, 2004 : 105-144.
[8] FOZO E M, HEMM M R, STORZ G. Small toxic proteins and the antisense RNAs that repress them [J].MicrobiolMolBiolRev, 2008,72(4): 579-589.
[9] FOZO E M, MAKAROVA K S, SHABALINA S A, et al. Abundance of type Ⅰ toxin-antitoxin systems in bacteria: Searches for new candidates and discovery of novel families [J].NucleicAcidsRes, 2010,38(11): 3743-3759.
[10] GERDES K, CHRISTENSEN S K, LOBNER-OLESEN A. Prokaryotic toxin-antitoxin stress response loci [J].NatRevMicrobiol, 2005,3(5): 371-382.
[11] LEPLAE R, GEERAERTS D, HALLEZ R, et al. Diversity of bacterial type Ⅱ toxin-antitoxin systems: A comprehensive search and functional analysis of novel families [J].NucleicAcidsRes, 2011,39(13): 5513-5525.
[12] FINERAN P C, BLOWER T R, FOULDS I J, et al. The phage abortive infection system, ToxIN, functions as a protein-RNA toxin-antitoxin pair [J].ProcNatlAcadSciUSA, 2009,106(3): 894-899.
[13] BLOWER T R, SHORT F L, RAO F, et al. Identification and classification of bacterial type Ⅲ toxin-antitoxin systems encoded in chromosomal and plasmid genomes [J].NucleicAcidsRes, 2012,40(13): 6158-6173.
[14] MASUDA H, TAN Q, AWANO N, et al. YeeU enhances the bundling of cytoskeletal polymers of MreB and FtsZ, antagonizing the CbtA(YeeV) toxicity inEscherichiacoli[J].MolMicrobiol, 2012,84(5): 979-989.
[15] WANG X, LORD D M, CHENG H Y, et al. A new type Ⅴ toxin-antitoxin system where mRNA for toxin GhoT is cleaved by antitoxin GhoS [J].NatChemBiol, 2012,8(10): 855-861.
[16] FARIDANI O R, NIKRAVESH A, PANDEY D P, et al. Competitive inhibition of natural antisense Sok-RNA interactions activates Hok-mediated cell killing inEscherichiacoli[J].NucleicAcidsRes, 2006,34(20): 5915-5922.
[17] WEAVER K E, WEAVER D M, WELLS C L, et al.Enterococcusfaecalisplasmid pAD1-encoded Fst toxin affects membrane permeability and alters cellular responses to lantibiotics [J].JBacteriol, 2003,185(7): 2169-2177.
[18] MINOR W, CYMBOROWSKI M, OTWINOWSKI Z, et al. HKL-3000: The integration of data reduction and structure solution-from diffraction images to an initial model in minutes [J].ActaCrystallogrDBiolCrystallogr, 2006,62(Pt 8): 859-866.

