Distinctive roles of Rac1 and Rab29 in LRRK2 mediated membrane trafficking and neurite outgrowth
Min Feng,Xin Hu,Na Li,Fan Hu,Fei Chang,Hongfei Xu,✉,Yongjian Liu,✉
1Department of Physiology;2Analytical and Testing Center,School of Basic Medical Science,Nanjing Medical University,Nanjing,Jiangsu 211166,China.
Introduction
Parkinson's disease(PD)associated leucine-rich repeat kinase 2(LRRK2)mutants have been widely studied for their pathogenic roles in a variety of neurodegeneration related subcellular processes such as altered membrane trafficking and neurite shortening[1-9].As a large-complex scaffold protein,LRRK2 contains multiple functional domains including Ras-like small GTPase domain(Roc),C-terminal of Roc domain(COR)and MAPKKK-like kinase domain in which several pathogenic mutations are harbored[10-13].However,the function of these domains in PD pathogenesis as well as their regulators and downstream effectors are largely unknown.Both wild type and PD-linked mutant LRRK2 have been shown to be involved in multiple intracellular membrane trafficking pathways through binding to multiple cytosolic machineries.For example,LRRK2 has been associated with various vesicular trafficking events through interactions with cytoskeletal components including tubulin-associated tau and actin[14-15].Other interactions with endocytic regulators such as clathrin,adaptor proteins(AP-1,AP-2),and endophilinA also indicated LRRK2's involvement in early endosomal trafficking[16-17].Interestingly,LRRK2 has been shown to interact with several small GTPases including Rac1,Rab29(Rab7L1),Rab7,and Rab5 to regulate endocytic vesicular trafficking events[18-21].However,the detailed mechanisms by which these GTPases act as LRRK2 effectors in membrane trafficking remain unexplored.
As one of the classical Rho GTPases,Rac1 is involved in a variety of cellular events including vesicular transport,microtubule dynamics and actin cytoskeleton remodeling[11,19,22-25].specifically,Rac1is critical for axonal outgrowth,maintenance of dendritic spine,and neurite morphology[22-24,26].Rac1 was reported as a substrate of LRRK2 kinase as the pathological mutantLRRK2G2019Sattenuated activation of Rac1,causing disassembly of actin filaments,and leading to neurite shortening.Rab29 was originally identified as PD gene at PARK16 locus[27-28]and its biological function may be linked to TGN mediated retrograde trafficking[29-30].Recent studies have suggested that Rab29 interacted with LRRK2 and participated in LRRK2 mediated retrograde trafficking pathway[20,31].Interestingly,Rab29 was also involved in neurite shortening induced by LRRK2 mutant[20],suggesting its rather complicated involvement in subcellular processes in neuron.With both small GTPases interacting with LRRK2 and acting as the downstream effectors,how their interactions are coordinated with different domains of LRRK2 and whether they share similar LRRK2 signaling pathways become an outstanding issue to be addressed.
Here,we report that Rac1 and Rab29 preferentially interacted with LRRK2 with different affinity and domain binding selectivity.Functionally,Rab29,but not Rac1,participated in retrograde trafficking of CIM6PR,a classical cargo protein used for studying endosome-to-TGN trafficking.On the other hand,both Rac1 and Rab29 could rescue neurite shortening in differentiated SH-SY5Y cells induced by PD mutant LRRK2G2019S.Our study provided clear evidence that these two small GTPases identified as the substrate of LRRK2 are involved in distinct mechanisms underlying LRRK2 mediated membrane trafficking and neurite outgrowth.
Materials and methods
Cell culture and transfection
HeLa Swiss and COS7 cells were cultured in DMEM(Invitrogen)supplemented with 10%cosmic calf serum(CCS,HyClone)and 1%penicillin/streptomycin at 37°C and 5%CO2.SH-SY5Y cells were maintained in DMEM supplemented with 10%fetal bovine serum(FBS,HyClone)and 1%penicillin/streptomycin at 37°C in 5%CO2.Transient transfection and expression were performed by transfecting the plasmids using Lipofectamine2000(Invitrogen),according to the manufacturer's instructions followed by harvesting transfected cells for analyzing 24-48 hours later.For neuritie analysis,SH-SY5Y cells were plated on poly-L-lysine/Marigel double coated glass coverslips followed by differentiation with 10 μmol/L retinoic acid(RA)treatment for 72 hours.The differentiated SHSY5Y cells were then transfected with appropriate plasmids using Lipofectamine 2000.
Three cell lines were used for the following specific experimental purposes.In CO-IP assays,we used COS7 to achieve high protein expression to detect specific protein-protein interaction in cell based in vivo system.HeLa Swiss cells were used due to their better morphology and clear subcellular distribution of Golgi complex for analyzing retrograde trafficking.Furthermore,RA induced differentiated SH-SY5Y cells were used as a cell culture model to investigate whether Rac1 and Rab29 influence the outgrowth of neuron.
Antibodies
The following antibodies were used in the study:rabbit polyclonal antibodies against CI-M6PR from Abcam(Abcam,HK),mAb against Myc-tag(9E10)from Santa Cruz Biotechnology(Santa Cruz,CA),mAb against GFP from the Clontech(Palo Alto,CA,USA),mAb and rabbit polyclonal antibodies against HA from Abcam,mAb and rabbit polyclonal antibodies against Flag from Abcam,HRP-conjugated secondary antibodies from Pierce(Rockford,IL),secondary donkey anti-rabbit IgG Alexa Fluor488,goat antimouse IgG Alexa Fluor568,and goat anti-rat IgG Alexa Fluor647 from Life Technologies(Life Technologies,USA).
Plasmids,oligonucleotides and reagents
The cDNA fragments in the plasmids of pcDNA3.1-3HA-Roc,pcDNA3.1-3HA-COR,pcDNA3.1-3HA-kinase and pcDNA3.1-3Flag-Rab29 were generated by proof-reading PCR and then inserted into corresponding vectors.The pcDNA3.1-3HA-LRRK2 and its variants were described previously[32].The pEGFP-C1-Rac1 was a gift from Dr.Du Jun at NJMU.The following regents were purchased from Sigma-Aldrich:cycloheximide(CHX),NP-40 and saponin.
Western blot analysis
Proteins were separated by electrophoresis through discontinuous 10%SDS-polyacrylamide gels before electrotransfer to nitrocellulose(Tanon,Shanghai).The filters were then blocked in PBS containing 0.1%Tween-20(TBS)and 5%nonfat dry milk,incubated in TBS with 1%nonfat dry milk and primary antibody at dilutions from 1:1,000 to 1:10,000 for 60 minutes at room temperature,washed three times in TBS,and incubated in appropriate secondary antibody conjugated to peroxidase for an additional 60 minutes followed by washing in TBS and visualization by enhanced chemiluminescence with the Tanon 5200 gel imaging system(Tanon,Shanghai).
Immunoprecipitation and protein half-life analysis
Cell transformants were washed with phosphate buffered saline(PBS)and lysed in NP-40 lysis buffer(0.5%NP-40,150 mmol/L NaCl,50 mmol/LTris-HCl,pH 7.0 and 5 mmol/L EDTA)supplemented with 1 mM PMSF and 0.1 m Mleupeptin for 10 minutes on ice.Cell lysate was centrifuged at 1,600 g and the supernatants were then precleared by incubation for 60 minutes at 4°C with 30 μL protein A/G agarose beads(Thermo scientific Pierce)and centrifugation at 8,000 g for 5 minutes.The precleared lysates were incubated for 2 hours at 4°C with 30 μL protein A/G agarose beads bound to polyclonal antibody to tagged protein.After immunoprecipitation,the beads were washed 4 times with wash buffer(0.5%NP-40,150 mmol/L NaCl,50 mmol/L Tris-HCl,pH 7.0 and 5 mmol/L EDTA)and then processed for SDS-PAGE analysis.
For half-life detection,HeLa Swiss cells were transiently transfected with 3HA-LRRK2,3Flag-Rab29 or EGFP-Rac1.After 24 hours,cells were treated with 100 μg/mL CHX and samples were collected at the time interval of 0,6,or 12 hours.Expression levels of endogenous CI-M6PR were then analyzed by immunoblotting.Prestained protein standard marker(Thermo scientific)(Cat.26616)was used for side-labeling Western blot.
Immuno fluorescence and confocal microscopy
Immunofluorescent staining was performed as previously described[33].Brie fly,cells were plated onto glass coverslips coated with poly-D-lysine and Matrigel(Collaborative Research),and fixed with 4%paraformaldehyde in 0.1 mol/L phosphate buffer,pH 7.2.After permeabilization and blocking in blocking buffer(BB,2%BSA,1% fish skin gelatin and 0.02%saponin in PBS),the cells were incubated with primary antibody in BB for 1 hour at room temperature,washed and further incubated with the appropriate secondary antibody for 1 hour followed by washing.For confocal laser microscopy,staining was visualized with a confocal laser microscope(LSM710,Zeiss)and the images processed using the NIH Image program and ZEN program.
Statistical analysis
Statistical analysis was performed using the Graph-Pad Prism software(version 5.0,GraphPad Software).For quantitative neurite length analysis were determined by unpaired t-test as indicated,and the expression level of endogenous in half-life studies were quantified by densitometry of the bands between two treatment groups and statistical significance were performed by one-way ANOVA followed by Tukey's post hoctest,and denoted*if P<0.05,**if P<0.01 and***if P<0.001.Results are expressed as mean±SEM if not indicated otherwise.
Results
LRRK2 preferentially interacted with Rac1 and Rab29
Previous studies showed that LRRK2 interacted with many small GTPases,such as Rab29,Rab10 and Rac1[2,11,19,22].To determine whether Rac1 and Rab29 interacted with LRRK2 differently,we conducted coimmunoprecipitation assay.As shown in Fig.1,with 3HA-LRRK2 co-transfected with EGFP-Rac1 or EGFP-Rab29 in COS7 cells,LRRK2 showed specific co-precipitation with either Rac1 or Rab29(Fig.1A).Interestingly,Rab29 displayed higher affinity than Rac1 for interacting with the full length of LRRK2(Fig.1A).To further determine the regions of LRRK2 responsible for their binding,we constructed various deletion mutation of LRRK2 fragments(Fig.1B).Consistent with previous study,Rac1 had strong binding to the COR or kinase domains of LRRK2(Fig.1C).On the other hand,Rab29 showed robust interaction with COR domain and rather weak binding with Roc or kinase domain of LRRK2(Fig.1D).Statistical analysis indicated that the binding intensity of Rab29 with COR domain were 2~3folds stronger than that with Roc or kinase domain of LRRK2(Fig.1D).Therefore,combined data from full length LRRK2 and domain binding analysis strongly suggested that Rac1 and Rab29 interacted with LRRK2 preferentially,which further suggested that Rac1 and Rab29 might participate in distinct pathways in LRRK2 signaling and functions.
Rab29 but not Rac1 acts downstream of LRRK2 signaling in regulating retrograde trafficking of CIM6PR
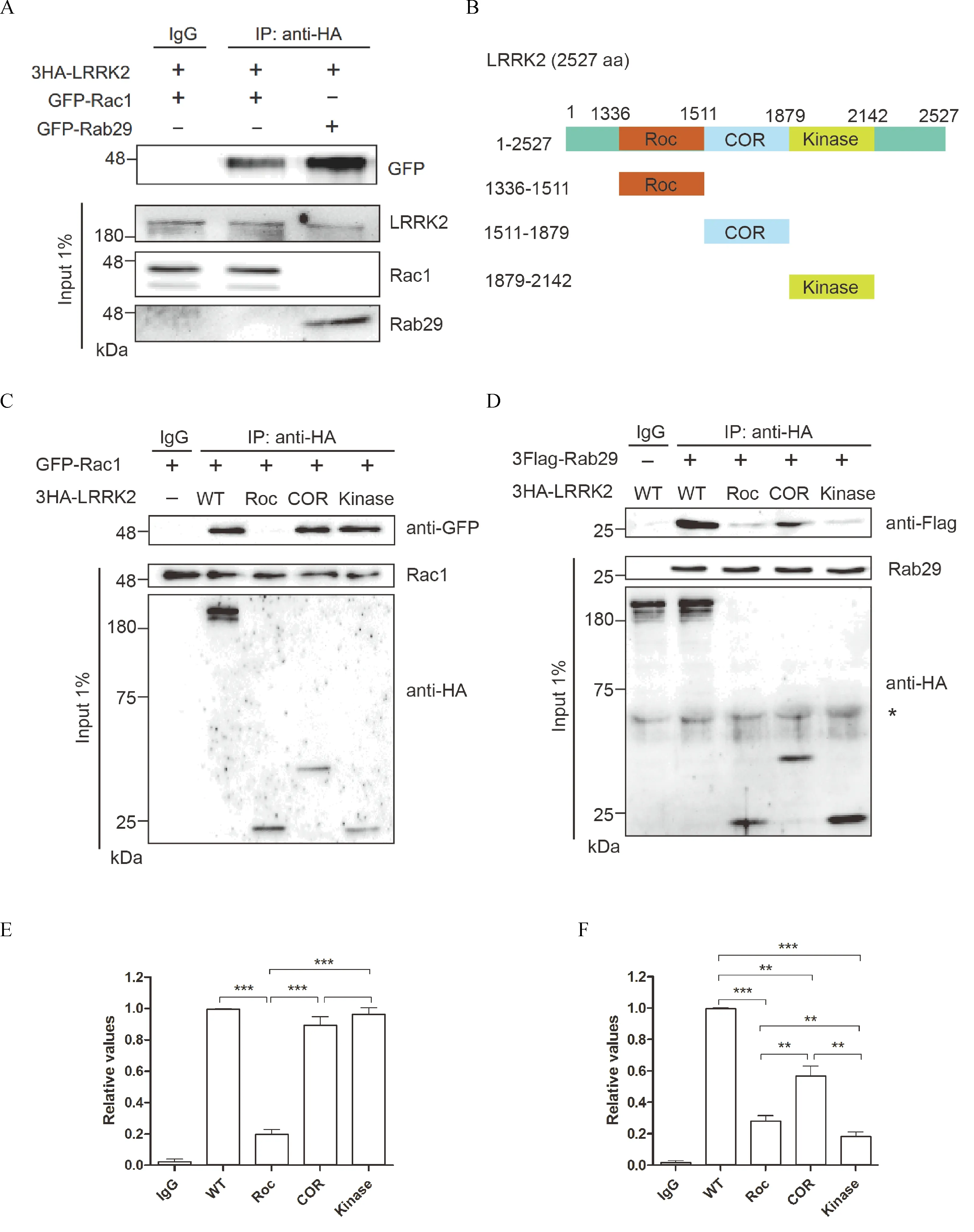
Fig.1 LRRK2 preferentially interacted with Rac1and Rab29.A:Co-Immunoprecipitation of EGFP-Rab29 or EGFP-Rac1 with 3HALRRK2WToverexpressed in COS7 cells.The data indicated that Rac1 and Rab29 specifically interacted with LRRK2.B:Schematic representation of LRRK2 constructs used.C&D:Co-Immunoprecipitation of GFP-Rac1(C)or 3Flag-Rab29(D)with different fragments of 3HA-LRRK2(full length,Roc,COR or Kinase domains)overexpressed in COS7 cells.The data indicated that Rac1 specifically bound to COR and kinase domain while Rab29 mainly interacted with COR domain of LRRK2.E&F:Quantification analysis of the relative Rab29 levels(normalized by the WT)was presented in C and D,Data presented were mean±SEM from two independent experiments.*P<0.05,**P<0.01,***P<0.001,one-way ANOVA followed by Tukey's post hoc test.
LRRK2 has been suggested an involvement in endosome-to-TGN transport mediated by the retromer complex as LRRK2G2019Scould induce mistargeting of CI-M6PR,a known cargo protein of the retrograde trafficking pathway,to lysosomes[30].To first investigate whether Rab29 and Rac1 function similarly in retrograde trafficking,we examined whether the localization of CI-M6PR could be altered by overexpressed GTPases mutants.As shown in Fig.2B,overexpression of Rab29WTseemed not interfere with the normal perinuclear localization of CI-M6PR at TGN compartment in HeLa cells.However,overexpression of dominant negative(DN)or constitutive active(CA)mutant of Rab29(Rab29T21Nand Rab29Q67L)led to mistargeting of CI-M6PR with rather diffused subcellular distribution.On the other hand,both Rac1WTand its mutant forms did not alter the perinuclear localization of CI-M6PR(Fig.2C).These results suggested that Rab29 and Rac1 participated in different pathways in LRRK2 mediated subcellular activity.
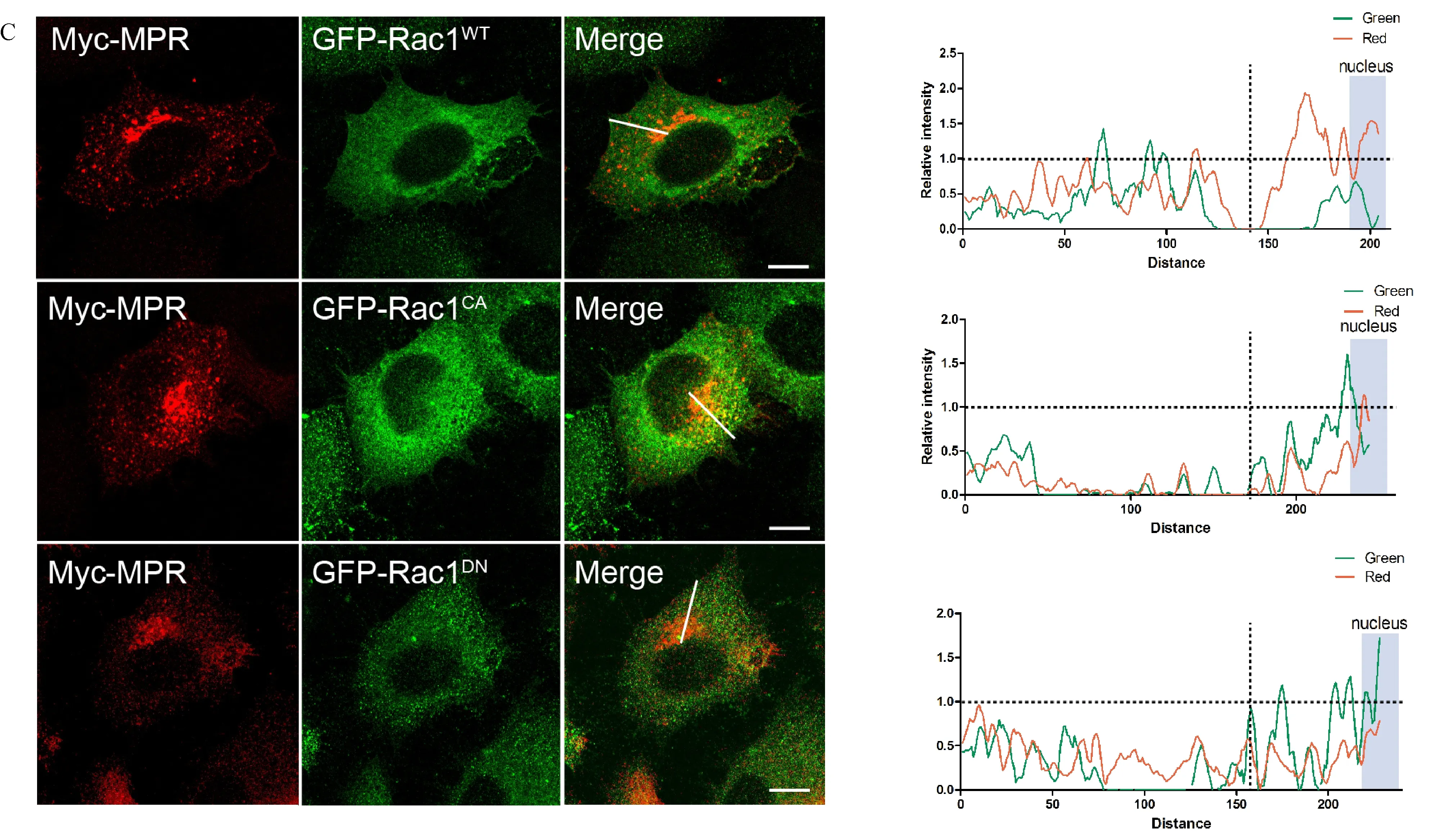
Fig.2 Rab29 but not Rac1 acts downstream of LRRK2 signaling in regulating retrograde trafficking of CI-M6PR.A:Double immunostaining of HeLa Swiss cells transiently expressing Myc-CI-M6PR with wild type,G2019S or K1906M of 3HA-LRRK2,respectively.The data showed that LRRK2G2019Saltered the perinuclear localization of CI-M6PR with diffused staining pattern.B:Double immunostaining of HeLa Swiss cells transiently expressing Myc-CI-M6PR and wild type or mutant 3Flag-Rab29,respectively.The data showed that Rab29 mutations(CA and DN)altered the perinuclear localization of CI-M6PR.C:Double immunostaining of HeLa Swiss cells overexpressing Myc-CI-M6PR and wild type or mutant EGFP-Rac1,respectively.The data showed that Rac1 did not alter the perinuclear localization of CI-M6PR.Fluorescence intensity pro files of stained proteins were shown in the green and red channels of the regions indicated by the white lines.The CIM6PR fluorescence intensity near the nucleus represents the quantitative perinuclear distribution.Scale bar=10 μm.
Rab29 mutants but not Rac1 accelerated CI-M6PR degradation
Since altered retrograde trafficking may result in lysosomal targeting of membrane proteins,we next examined whether aberrant distribution of CI-M6PR had impacts on its stability in cells overexpressing mutant Rab29 and LRRK2G2019Scells through protein half-life assay.HeLa cells that were transiently expressing LRRK2WTor LRRK2G2019Sfor 24 hours were treated with CHX at 0,6,12 hours to detect endogenous CIM6PR protein levels.As shown in Fig.3A,compared with cells overexpressing LRRK2WT,overexpression of LRRK2G2019Scaused a distinctly decreased half-life of CIM6PR,strongly suggesting its decreased stability.Furthermore,in cells overexpressing Rab29Q67Lor Rab29T21N,CI-M6PR also displayed accelerated degradation in contrast with that with Rab29WT(Fig.3B).However,in cells overexpressing Rac1 variants(Rac1WT,Rac1CAor Rac1DN),there was no obvious difference of protein levels among three groups(Fig.3C).These combined data based on the subcellular localization and protein stability studies indicated that two downstream GTPases of LRRK2 might be involved in different intracellular pathways,with Rab29 selectively at the retrograde trafficking altered by LRRK2.
Rab29 but not Rac1 is a downstream effector of LRRK2 involved inretrograde trafficking of CIM6PR
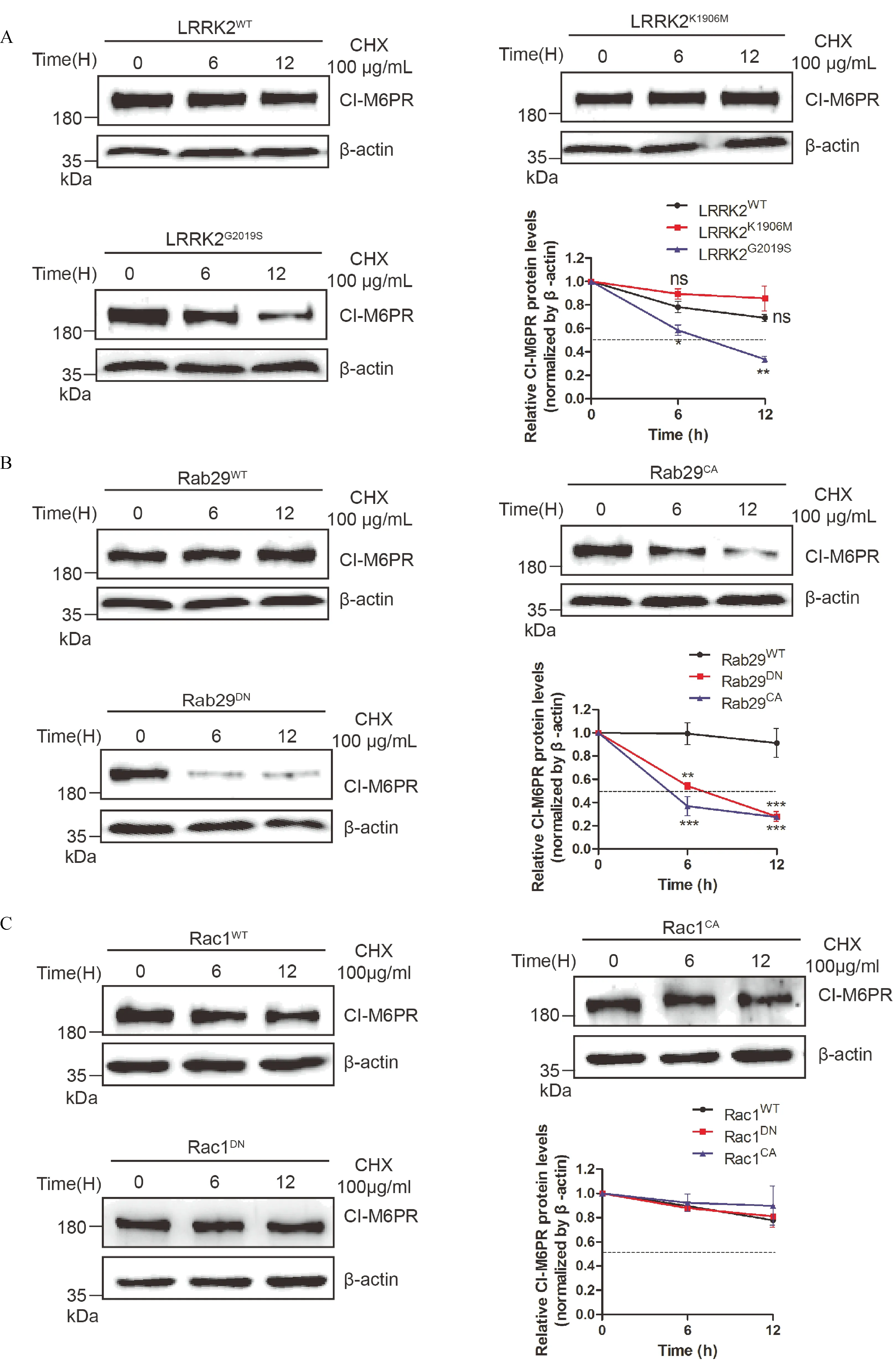
Fig.3 Overexpressed Rab29 mutants but not Rac1 accelerated CI-M6PR degradation.A-C:CI-M6PR was co-transfected in HeLa Swiss cells with indicated plasmids and the cells were treated with 100 μg/mL CHX and collected samples at the indicate time points(0,6 and 12 hours)for detecting the levels of the overexpressed proteins.The data suggested that LRRK2G2019Sreduced the half-life of CI-M6PR compared with that in wild type or LRRK2K1906Mgroup(A).Furthermore,overexpressed mutant Rab29s but not Rac1 reduced the half-life of CI-M6PR compared with that in wild type group(B&C).The arbitrary densitometry value in A,B and C was measured using imaging analysis software Image J.Data(mean±SEM)were from the indicated number of independent experiments and comparisons were analyzed usingone-way ANOVA followed by Tukey's post hoc test.*P<0.05,**P <0.01 and***P<0.001.
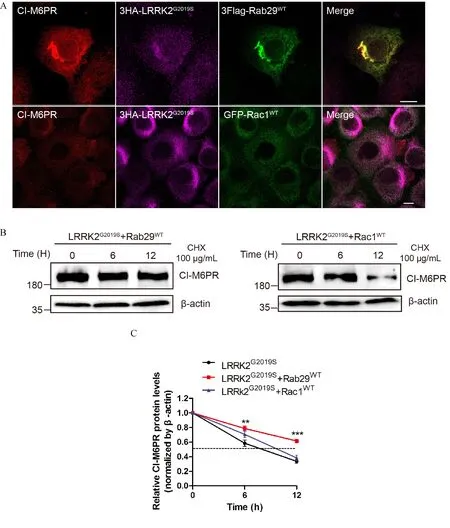
Fig.4 Rab29 but not Rac1 is a downstream effector of LRRK2 involved in retrograde trafficking of CI-M6PR.A:HeLa Swiss cells overexpressing Myc-CI-M6PR,3HA-LRRK2G2019Sand 3Flag-Rab29WTor EGFP-Rac1WTwere immunostained,which indicated that Rab29 but not Rac1 could rescue the altered subcellular distribution of CI-M6PR induced by 3HA-LRRK2G2019S.B:Co-overexpression of LRRK2G2019Sand Rab29WTor Rac1WTHeLa in Swiss cells was used to determine the half-life of CI-M6PR.The data showed that Rab29WT could rescue the reduced half-life of CI-M6PR induced by LRRK2G2019S.C:Quantitative analysis of the relative CI-M6PR levels(normalized by β-actin,time point 0 hour)in(B).Data(mean±SEM)were from the indicated number of independent experiments and comparisons were made using one-way ANOVA followed by Tukey's post hoc test.*P<0.05,**P<0.01 and***P<0.001.
Recent studies suggested that both Rab29 and LRRK2 participated in modulating the retrograde trafficking of CI-M6PR[29-30,34].We thus further explore whether LRRK2 mediated the retrograde trafficking of CI-M6PR via regulating Rab29 and Rac1.We overexpressed CI-M6PR along with LRRK2G2019Sand Rab29WTor Rac1WTin HeLa cells to investigate functional relationship between LRRK2 and two GTPases.As shown in the top panel of Fig.4A,immuno fluorescent staining revealed that overexpressed Rab29WTrestored the perinuclear distribution of CI-M6PR which was altered by LRRK2G2019S(see in Fig.2A).However,in cells co-overexpressing Rac1WTand LRRK2G2019Sdid not rescue the altered subcellular distribution ofCI-M6PR(Fig.4A,low panel).To further characterize the functional interactions between the two small GTPases and LRRK2,we performed a series of half-life assay to measure the stability of endogenous CI-M6PR.We further showed that overexpression of Rab29 suppressed the accelerated degradation of CIM6PR induced by LRRK2G2019S(Fig.3A,Fig.4B left panel).On the other hand,co-expressing Rac1WTwith LRRK2G2019Sshowed significantly decreased half-life,consistentwiththatincellsoverexpressing LRRK2G2019Salone(Fig.3A,Fig.4B right panel).These results suggested that Rab29 and Rac1 as downstream effectors of LRRK2 play different roles in retrograde trafficking of CI-M6PR.
Both Rab29 and Rac1 rescue neurite shortening induced by LRRK2G2019Sin differentiated SH-SY5Y cells
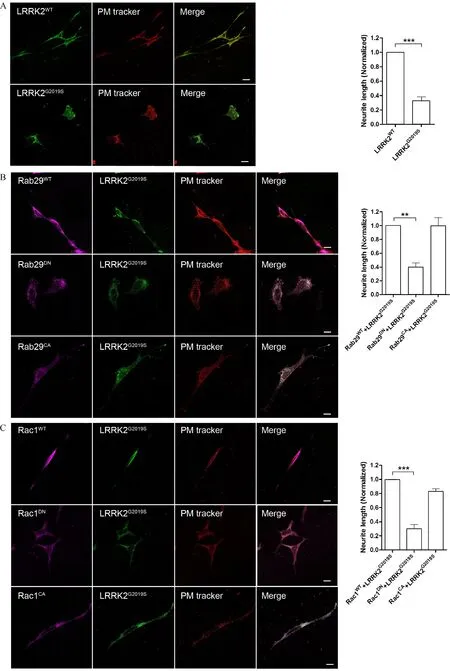
Fig.5 Both Rab29 and Rac1 rescued neurite shortening induced by LRRK2G2019Sin differentiated SH-SY5Y cells.A:Overexpressed 3HA-LRRK2G2019Sin differentiated SH-SY5Y cells reduced neurite length compared with that for wild type protein.Na+-K+ATPase was used to track plasma membrane of the cells.B:Co-overexpression LRRK2G2019Swith Rab29WTor Rab29CAbut not Rab29DNcould significantly rescue neurite retraction induced by LRRK2G2019S.C:Co-overexpression LRRK2G2019Swith Rac1WTor Rac1CAbut not Rac1DNcould significantly rescue neurite retraction induced by LRRK2G2019S.These results suggested that Rab29 and Rac1 can act as downstream of LRRK2 mediated neurite outgrowth.Quantification analysis of the relative neurite length(normalized by the WT)was presented in A,B,and C.Data presented were mean±SEM from two independent experiments.*P<0.01 and***P<0.001,unpaired t-test.
Previous studies have shown that the pathogenic mutant LRRK2G2019Scould induce striking phenotypes of Parkinson's disease such as neurite shortening.As shown in Fig.5A,consistent with previous study,we showed that overexpressed LRRK2G2019Sinduced significantly neurite shortening compared with that expressing LRRK2WT(Fig.5A).Statistical analysis indicated that the neurite length was about 70%shorter in LRRK2G2019Scells in comparison to that in LRRK2WT(Fig.5B).To test whether the function of Rab29 in membrane trafficking and Rac1 in cytoskeleton have relevance to neurite progress and retraction,we used differentiated SH-SY5Y cells to co-transfect LRRK2G2019Swith variants of Rab29 or Rac1.As shown in Fig.5B,Rab29WTand Rab29CAbut not Rab29DNcould rescue neurite shortening caused by LRRK2G2019Sin differentiated SH-SY5Y cells.Furthermore,Rac1 had the same effect as Rab29 in rescuing LRRK2 mediated neurite shorthening(Fig.5C and D).Thus,our results indicated that both Rab29 and Rac1 participated in LRRK2G2019Smediated signaling process in neurite growth in differentiated SH-SY5Y cells.
Discussion
We have shown in this paper that,as LRRK2 interacting proteins,Rac1 and Rab29 preferentially bound to LRRK2 subdomains.specifically,Rac1 selectively bound to COR and kinase domain while Rab29 mainly to COR domain.Notably,our data on binding domain analysis between LRRK2 and Rab29 is the first to reveal such binding detail at the molecular level.More importantly,Rac1 and Rab29 participated in different subcellular pathways of LRRK2 signaling.Using a specific marker protein CI-M6PR,we showed that Rab29,but not Rac1,was involved in retrograde trafficking.On the other hand,overexpression of both wild type Rac1 and Rab29 could rescue the LRRK2 mediated neurite shortening in SH-SY5Y cells.Our study suggested that Rac1 and Rab29,as the downstream effectors of LRRK2 signaling pathway,are involved in a distinct function in LRRK2 mediated membrane trafficking and neurite outgrowth.
As a scaffold protein,LRRK2 may execute its biological function via recruiting downstream effectors.Recent identification of several small GTPases whose activation could be regulated through binding to and further altered by LRRK2 kinase activity[2,31].Our data strongly suggested that Rab29 has higher affinity in LRRK2 binding than that for Rac1,presumably through COR domain(Fig.1),which may play an important role in intramolecular regulation between the endogenous GTPase Roc and kinase of LRRK2[35].Consistently,the interaction between Rab29 and LRRK2 are related to their involvement in retrograde trafficking pathway as Rab29 has been localized clearly on TGN in literature as well as our own work[30](data not shown).Both Rab29 mutants(Rab29CAand Rab29DN)had the same phenotype as LRRK2G2019Son the induction of altered subcellular distribution and reduced half-life of CI-M6PR,suggesting their functional relevance in retrograde trafficking.Furthermore,we have not identified any candidate phosphorylation residue in Rab29 as LRRK2 kinase substrate that may alter its function(data not shown).Thus,LRRK2-Rab29 signaling may be different from that for the LRRK2-Rac1 pathway in which Rac1 was inactivated by LRRK2 mediated phosphorylation[2,11].These results further support that LRRK2 regulates the Rab mediated different intracellular pathways through specific functional domains and different molecular mechanisms.
Although Rac1 and Rab29 showed different involvement in retrograde trafficking,they both rescued neurite shortening induced by LRRK2G2019Sin differentiated SH-SY5Y cells,suggesting their shared roles in neurite process.Since neuronal differentiation is a complex process involved in multiple cellular pathways,Rac1 and Rab29 could utilize different signaling processes to achieve similar consequence.Mechanistically,Rac1 may receive multiple upstream regulations.Our domain binding analysis data supported the role of LRRK2 kinase activity in Rac1 functional regulation,consistent with a previous report[2,36].Moreover,Rac1 processes its signal to the downstream effector p21-activated kinase(PAK)to promote survival and differentiation of neurons[2,37].Rac1 is also known for its role in stimulating the pro-survival PI3K/Akt pathway in leading to neuronal survival and differentiation[26,38].Thus,it remains to be studied how the inactivation of Rac1 by LRRK2 kinase contributes to the detailed molecular process of LRRK2-Rac1 signaling in neurite growth.On the other hand,Rab29 had previously been shown to localize primarily to the TGN and implicated in vesicular sorting.Thus,its function in the regulation of neurite outgrowth process might be due to sufficient supply of membranous materials required for neurite outgrowth and differentiation[39-41].Furthermore,whether LRRK2-Rab29 mediated retrograde trafficking in neuron are involved in vesicular trafficking of neurotrophin containing vesicles that are important not only in differentiation but anti-degeneration remains to be the important issue in PD research.In summary,our study has shown that Rac1 and Rab29,as the downstream effectors of LRRK2 signaling,interacted with different domains of LRRK2 and involved in distinct LRRK2 mediated subcellular pathways.
This work was supported by the National Nature Science Foundation of China(Grant No.31371436 and No.8157051134)and by the laboratory start-up grant from Nanjing Medical University to Y.Liu.The funders had no role in study design,data collection and analysis,decision to publish,or preparation of the manuscript.
[1]Sepulveda B,Mesias R,Li X,et al.Short-and long-term effects of LRRK2 on axon and dendrite growth.PLoS One,2013,8(4):e61986.
[2]Chan D,Citro A,Cordy JM,et al.Rac1 protein rescues neurite retraction caused by G2019S leucine-rich repeat kinase 2(LRRK2).J Biol Chem,2011,286(18):16140-16149.
[3]Plowey ED,Cherra SJ 3rd,Liu YJ,et al.Role of autophagy in G2019S-LRRK2-associated neurite shortening in differentiated SH-SY5Y cells.J Neurochem,2008,105(3):1048-1056.
[4]Berwick DC,Harvey K.LRRK2 signaling pathways:the key to unlocking neurodegeneration?Trends Cell Biol,2011,21(5):257-265.
[5]Berwick DC,Harvey K.LRRK2 functions as a Wnt signaling scaffold,bridging cytosolic proteins and membrane-localized LRP6.Hum Mol Genet,2012,21(22):4966-4979.
[6]Cookson MR.The role of leucine-rich repeat kinase 2(LRRK2)in Parkinson’s disease.Nat Rev Neurosci,2010,11(12):791-797.
[7]Dächsel JC,Farrer MJ.LRRK2 and Parkinson disease.Arch Neurol,2010,67(5):542-547.
[8]WinnerB,Melrose HL,Zhao C,et al.Adult neurogenesis and neurite outgrowth are impaired in LRRK2 G2019S mice.Neurobiol Dis,2010,41(3):706-716.
[9]Yang D,Li T,Liu Z,et al.LRRK2 kinase activity mediates toxic interactions between genetic mutation and oxidative stress in a Drosophila model:suppression by curcumin.Neurobiol Dis,2012,47(3):385-392.
[10]Anand VS,Braithwaite SP.LRRK2 in Parkinson’s disease:biochemical functions.FEBS J,2009,276(22):6428-6435.
[11]Boon JY,Dusonchet J,Trengrove C,et al.Interaction of LRRK2 with kinase and GTPase signaling cascades.Front Mol Neurosci,2014,7:64.
[12]Sancho RM,Law BMH,Harvey K.Mutations in the LRRK2 Roc-COR tandem domain link Parkinson’s disease to Wnt signalling pathways.Hum Mol Genet,2009,18(20):3955-3968.
[13]Dauer W,Ho CC.The biology and pathology of the familial Parkinson’s disease protein LRRK2.Mov Disord,2010,25(Suppl 1):S40-S43.
[14]Parisiadou L,Xie C,Cho HJ,et al.Phosphorylation of ezrin/radixin/moesin proteins by LRRK2 promotes the rearrangement of actin cytoskeleton in neuronal morphogenesis.J Neurosci,2009,29(44):13971-13980.
[15]Shin N,Jeong H,Kwon J,et al.LRRK2 regulates synaptic vesicle endocytosis.Exp Cell Res,2008,314(10):2055-2065.
[16]Soukup SF,Kuenen S,Vanhauwaert R,et al.A LRRK2-dependent endophilina phosphoswitch is critical for macroautophagy at presynaptic terminals.Neuron,2016,92(4):829-844.
[17]Milosevic I,Giovedi S,Lou X,et al.Recruitment of endophilin to clathrin-coated pit necks is required for efficient vesicle uncoating after fission.Neuron,2011,72(4):587-601.
[18]Auer M,Hausott B,Klimaschewski L.Rho GTPases as regulators of morphological neuroplasticity.Ann Anat,2011,193(4):259-266.
[19]Chan D,Citro A,Cordy JM,et al.Rac1 protein rescues neurite retraction caused by G2019S leucine-rich repeat kinase 2(LRRK2).J Biol Chem,2011,286(18):16140-16149.
[20]MacLeod DA,Rhinn H,Kuwahara T,et al.RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson’s disease risk.Neuron,2013,77(3):425-439.
[21]Tang BL.Rabs,Membrane Dynamics,and Parkinson’s Disease.J Cell Physiol,2017,232(7):1626-1633.PMID:27925204.
[22]Hakeda-Suzuki S,Ng J,Tzu J,et al.Rac function and regulation during Drosophila development.Nature,2002,416(6879):438-442.
[23]Loucks FA,Le SS,Zimmermann AK,et al.Rho family GTPase inhibition reveals opposing effects of mitogen-activated protein kinase kinase/extracellular signal-regulated kinase and Janus kinase/signal transducer and activator of transcription signaling cascades on neuronal survival.J Neurochem,2006,97(4):957-967.
[24]Nikolic M.The role of Rho GTPases and associated kinases in regulating neurite outgrowth.Int J Biochem Cell Biol,2002,34(7):731-745.
[25]Etienne-Manneville S,Hall A.Rho GTPases in cell biology.Nature,2002,420(6916):629-635.
[26]Jeon S,Park JK,Bae CD,et al.NGF-induced moesin phosphorylation is mediated by the PI3K,Rac1 and Akt and required for neurite formation in PC12 cells.Neurochem Int,2010,56(6-7):810-818.
[27]Kiraly DD,Eipper-Mains JE,Mains RE,et al.Synaptic plasticity,a symphony in GEF.ACS Chem Neurosci,2010,1(5):348-365.
[28]Yan Y,Tian J,Mo X,et al.Genetic variants in the RAB7L1 and SLC41A1 genes of the PARK16 locus in Chinese Parkinson’s disease patients.Int J Neurosci,2011,121(11):632-636.
[29]Massmann S,Schürmann A,Joost HG.Cloning of two splicing variants of the novel Ras-related GTPase Rab29 which is predominantly expressed in kidney.Biochim Biophys Acta,1997,1352(1):48-55.
[30]Wang S,Ma Z,Xu X,et al.A role of Rab29 in the integrity of the trans-Golgi network and retrograde trafficking of mannose-6-phosphate receptor.PLoS One,2014,9(5):e96242.
[31]Steger M,Tonelli F,Ito G,et al.Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases.Elife,2016,29(5):pii:e12813.
[32]Plowey ED,Cherra SJ 3rd,Liu YJ,et al.Role of autophagy in G2019S-LRRK2-associated neurite shortening in differentiated SH-SY5Y cells.J Neurochem,2008,105(3):1048-1056.
[33]Colgan L,Liu H,Huang SY,et al.Dileucine motif is sufficient for internalization and synaptic vesicle targeting of vesicular acetylcholine transporter.Traffic,2007,8(5):512-522.
[34]Onnis A,Finetti F,Patrussi L,et al.The small GTPase Rab29 is a common regulator of immune synapse assembly and ciliogenesis.Cell Death Differ,2015,22(10):1687-1699.
[35]Smith WW,Pei Z,Jiang H,et al.Kinase activity of mutant LRRK2 mediates neuronal toxicity.Nat Neurosci,2006,9(10):1231-1233.
[36]Esufali S,Charames GS,Pethe VV,et al.Activation of tumorspecific splice variant Rac1b by dishevelled promotes canonical Wnt signaling and decreased adhesion of colorectal cancer cells.Cancer Res,2007,67(6):2469-2479.
[37]Chae YC,Kim JH,Kim KL,et al.Phospholipase D activity regulates integrin-mediated cell spreading and migration by inducing GTP-Rac translocation to the plasma membrane.Mol Biol Cell,2008,19(7):3111-3123.
[38]Chamberlain MD,Berry TR,Pastor MC,et al.The p85alpha subunit of phosphatidylinositol 3′-kinase binds to and stimulates the GTPase activity of Rab proteins.J Biol Chem,2004,279(47):48607-48614.
[39]Fu X,Zang K,Zhou Z,et al.Retrograde neurotrophic signaling requires a protein interacting with receptor tyrosine kinases via C2H2 zinc fingers.Mol Biol Cell,2010,21(1):36-49.
[40]Zhang YW,Chen Y,Liu Y,et al.APP regulates NGF receptor trafficking and NGF-mediated neuronal differentiation and survival.PLoS One,2013,8(11):e80571.
[41]Winckler B,Yap CC.Endocytosis and endosomes at the crossroads of regulating trafficking of axon outgrowth-modifying receptors.Traf fic,2011,12(9):1099-1108.
 THE JOURNAL OF BIOMEDICAL RESEARCH2018年2期
THE JOURNAL OF BIOMEDICAL RESEARCH2018年2期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Legal protection of the rights of clinical trial subjects in China
- Re-evaluating the role of epithelial-mesenchymal-transition in cancer progression
- Multifunctional quantum dots and liposome complexes in drug delivery
- Evaluation of cardiolipin nanodisks as lipid replacement therapy for Barth syndrome
- Pathological significance and regulatory mechanism of lymphotoxin β receptor overexpression in T cells of patients with systemic lupus erythematosus
- A novel entry point for pedicle screw placement in the thoracic spine
