Effect of tris-extender supplemented with various concentrations of strawberry(Fragaria spp.) on bull semen preservability
El-Sheshtawy RI, El-Nattat WS
Animal Reproduction and Artificial Insemination Department, Veterinary Division, National Research Center, Dokki, Giza
1. Introduction
Spermatozoa are produced by spermatogenesis process. Nowadays,semen freezing is important for maintaining the supergenetic constitutions of males, and for the use of frozen semen in artificial insemination as well as in vitro fertilization (IVF)[1]. Artificial insemination with frozen semen is of great importance in breeding and selection programs implicated in increasing productivity of farm animals.
The spermatozoal potency of preserved semen is affected by many factors including storage temperature, cooling regime, chemical ingredients of the diluent, cryoprotectant concentration, oxygen free radicals level, seminal plasma constituents and sanitary measures[2].Cryopreservation of spermatozoa induces reduced semen quality,thus so many efforts are being done to improve sperm viability postfreezing. Variations in the extender and protocol used for freezing process differ according to species. These variations are due to their specific species and seminal plasma composition specialities[3].Experimental trials still try to obtain the best extender for each species using different cryoprotectants. Cryoprotectants are included in the semen extender to minimize the oxidative cryo-injury resulting from sperm cooling, freezing and thawing of sperm cells and consequently enhancing semen quality and fertilizing capacity[4-6].A new generation of semen extenders based on the presence of natural products is the target of our study to minimize the risk of contamination and improve the potential of cryopreservation.
Fruits containing natural antioxidants are more acceptable than synthetic antioxidants. Strawberry fruit is rich in natural antioxidants including phytochemicals mainly anthocyanins, flavonoids,phenolic compounds and ellagic acid, which have strong antioxidant activity[7]. Strawberry juice protects living cells from oxidative agents due to its strong antioxidant capacity[8]. The strawberry fruits contain adequate amounts of potassium, vitamin C and E, folic acid, carotenoids as well as phenolic compounds, so it has strong antioxidant capacity[9]. Natural extracts and infusions from fruits are added to semen extenders for preserving sperm cells in cattle due to its strong protective property[10].
2. Materials and methods
2.1. Fruit juice preparation
Fresh mature strawberry (Fragaria spp.) fruits (SB) were purchased from local market. They were well cleaned and cut to be squeezed in a blender machine with filter mesh. Stock solution of the SB juice(10%) in tris-citric acid-egg yolk-fructose was prepared. Then, the 10% juice was added to tris-strawberry (TSB) at concentrations of 1%-6%.
2.2. Semen processing
A basic control extender tris-citric acid-egg yolk-fructose (TCYF)was prepared according to Foote[11]. Semen samples were diluted in TCYF (control, 0% SB) and in the former concentrations of TSB(1%-6%) to ensure 60 million motile spermatozoa per milliliter.Extended semen was exposed to freezing program.
2.3. Semen quality assessment
Semen evaluation was carried out after thawing of bull spermatozoa. Also, sperm motility of raw semen was assessed for 2 h post-cooling and post-chilling daily up to 10 d. Frozen straws were thawed at 37 ℃ for 30 s. The measured parameters were motility%,alive%, abnormality% and membrane integrity using hypo-osmotic swelling test (HOST%).
2.3.1. Sperm motility
Sperm motility was performed microscopically with closed circuit television system[12].
2.3.2. Live and abnormal spermatozoa (%)
This was assessed using eosin-Nigrosin stained smear[13].
2.3.3. Sperm membrane integrity (%)
The integrity of sperm membrane was performed using the HOST[14].
2.3.4. Conception rate
Conception rate no. of buffalo cows (n=145) were inseminated with TSB extended buffalo bull semen. Another no. of cows was inseminated with bull semen diluted with TCYF (control group).Pregnancy was confirmed by rectal palpation 2 months later after insemination. The inseminated cows were used via the cooperation in Beni-Suef Governorate. Conception rate was calculated according to the equation:
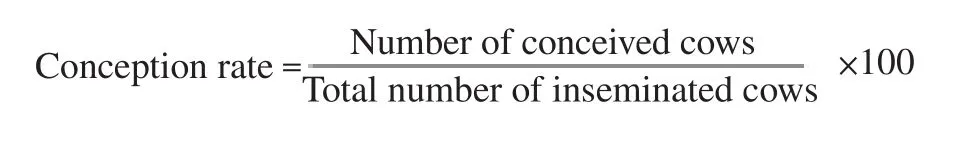
2.4. Statistical analysis
Data were analyzed by means of the SPSS (2005)[15] computerized program v. 14.0 to calculate the analysis of variance (ANOVA)[16] for the different parameters between control and additives replications. Significant differences between means were calculated using Duncan multiple range test at P<0.05.
3. Results
In chilled semen, concentrations of strawberry from 1% to 5% in tris-extender exhibited higher sperm motility% (P<0.000 1) at 6 days post-chilling (60.00 ± 5.77, 53.33 ± 3.33, 58.33 ± 1.67, 68.33 ± 4.41 and 53.33± 3.33, respectively) (Table 1).
Sperm motility% of frozen semen in concentrations 3%, 4%, 5%and 6% were significantly different (40.00 ± 0.00, 40.00 ± 0.00,43.33 ± 3.33 and 43.33 ± 3.33%, respectively) with control(31.67 ± 1.67) (P<0.000 1). Sperm alive% of concentration 1% was significantly different in comparison with the control (85.33 ± 0.33 and 80.33 ± 0.33, respectively) (P<0.000 1). HOST% was maintained as the control. Sperm abnormality% of concentration 3% gave the lowest value (20.33 ±0.33) (Table 2).
The conception rate upon using frozen semen in insemination showed higher conception rate in concentrations of 5% and 6% TSB in cattle (Table 3).
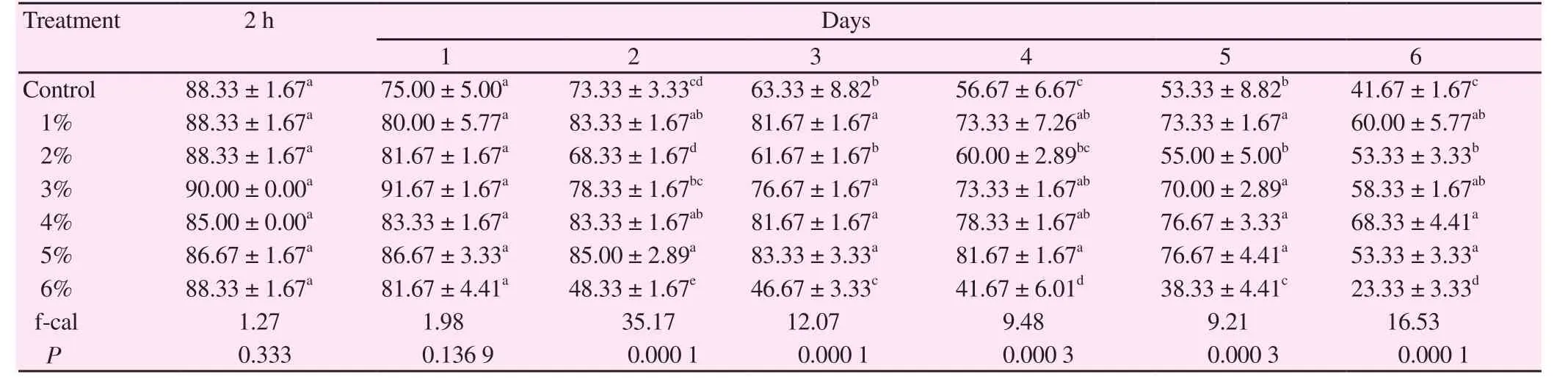
Table 1 Sperm motility% of chilled cattle semen using different concentrations of TSB extender.

Table 2 Sperm parameters of post-thawed cattle semen using different concentrations of TSB extender.
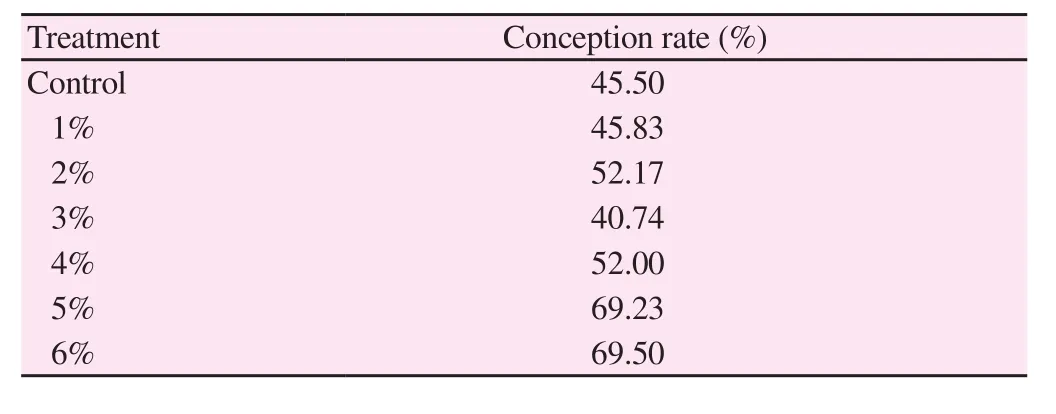
Table 3 Effect of addition of TSB to the TCYF on a field conception rate test in cattle.
4. Discussion
Recently, scientists are interested in exploring the beneficial and the synergistic effects of the natural extracts and their multiple constituents compared to the single purified active compounds[17].Semen cryopreservation can induce oxidative damage to spermatozoa leading to a reduction in semen quality[18], but it is important to preserve the valuable genetic constitution of our local breeds of cattle bulls.
Cryodamage induced by freezing and thawing can be minimized by adding lipoproteins, or using the convenient cryoprotectant[19].Semen freezing is associated with accumulation of reactive oxygen species and an alteration in the antioxidant capacity as manifested by a decrease in intracellular glutathione content that induce damage in integrity and function of spermatozoal membrane[20-22].
Seminal plasma has limited antioxidant capacity, thus the use of an extender with strong antioxidant effect is recommended to maintain the viability and subsequent fertilizing capacity of frozen spermatozoa[4]. The motility of sperm is the most important criterion used for semen assessment, both for chilled and frozen semen[23].Concannon and Battista[24] stated that 40%-50% of sperm motility is needed for artificial insemination success. However, Linde-Forsberg and Forsberg[25] postulated that 20%-30% sperm motility is necessary for pregnancy.
In recent years, extensive researches have been conducted to investigate the effect of natural and synthetic antioxidants (herbal origins) on the viability of animal sperm during cooling and cryopresevation. Present investigation on chilled semen explored significant maintenance of sperm motility in some concentrations of strawberry up to the second, third and sixth day of chilling. This indicates that it could be used in field insemination up to these days of chilling. Frozen semen explored improvement in sperm motility post-thawing at some concentrations. The conception rate upon using frozen semen in insemination showed higher conception rate in concentrations 5% and 6%. The higher conception rates at these concentrations coincide with the higher sperm motility at these concentrations as sperm motility is the main criterion in semen evaluation[26].
Improved results in semen preservability by using strawberry juice as a cryoprotectant in semen tris-extender are mainly due to strong antioxidant properties. Strong antioxidant capacity is attributed to its high contents of vitamins, phenolic and flavonoid compounds as these components are strong antioxidants[27,28]. Canuto et al[29]recorded that strawberry was rich in anthocyanins that had strong antioxidant property as radical scavenger and alleviating oxidative stress and cellular damage. Main polyphenolic compounds in strawberry responsible for antioxidation are anthocyanins[30]. The mechanisms to prevent oxidation are associated with the defense system, including antioxidant enzymes and antioxidants, which play an important role in preventing oxidative injury through elimination of the excess of oxygen free radicals that cause sperm damage[31].
Conflict of interest statement
The authors declare that they have no conflict of interest.
[1] Medeiros CM, Forell F, Oliveira AT, Rodrigues JL. Current status of sperm cryopreservation: Why isn’t better? Theriogenology 2002; 57(1):327-344.
[2] Barbas JP, Mascarenhas RD. Cryopreservation of domestic animal sperm cells. Cell Tissue Bank 2009; 10(1): 49-62.
[3] Salmon S, Maxwell WMC. Storage of ram semen. Anim Reprod Sci 2000;62(1-3): 77-111.
[4] Gadea J, Gumbao D, Cánovas S, García-Vázquez FA, Grullón LA,Gardón JC. Supplementation of the dilution medium after thawing with reduced glutathione improves function and the in vitro fertilizing ability of frozen-thawed bull spermatozoa. Andrology 2008; 31(1): 40-49.
[5] Uysal O, Bucak MN. Effects of oxidized glutathione, bovine serum albumin, cysteine and lycopene on the quality of frozen–thawed ram semen. Acta Vet Brno 2007; 76(3): 383-390.
[6] Bucak MN, Atessahin A, Yuce A. Effect of anti-oxidants and oxidative stress parameters on ram semen after the freeze-thawing process. Small Rum Res 2008; 75(2-3): 128-134.
[7] Asghari M, Hasanlooe AR. Interaction effects of salicylic acid and methyl jasmonate on totalantioxidant content, catalase and peroxidase enzymes activity in “Sabrosa” strawberry fruit during storage. Sci Hortic 2015; 197:490-495.
[8] Asghari M, Aghdam MS. Impact of salicylic acid on postharvest physiology of horticulture crop. Trends Food Sci Technol 2010; 21(10):502-509.
[9] Basu A, Nguyen A, Betts NM, Lyons TJ. Strawberry as a functional food:An evidence-based review. Crit Rev Food Sci Nutr 2014; 54(6): 790-806.
[10] Sansone G, Nastri MJF, Fabbrocini A. Storage of buffalo (Bubalus bubalis) semen. Anim Reprod Sci 2000; 62(1-3): 55-76.
[11] Foote RH. Fertility of bull semen at high extension rates in tris buffered extenders. J Dairy Sci 1970; 53: 1475-1477.
[12] Graham EF, Schmehl MKL, Maki-Laurila M. Some physical and chemical methods of evaluating semen. Columbia: National Association of Animal Breeders; 1970, p. 44-48.
[13] Sidhu KS, Guraya SS. Buffalo bull semen morphology, biochemistry,physiology and methodology. Ludhiana: USA Publishers and Distributors;1985, p. 152-154.
[14] Jeyendran RS, Vander Ven HH, Perez Pelaez M, Crabo BG, Zaneveld LJD. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil 1984; 70(1): 219-228.
[15]SPSS 14.0 for windows evaluation version. Chicago: SPSS Inc.; 2005.
[16] Snedecor GW, Cochran WG. Statistical methods. Ames, IA: Iowa State University Press; 1967.
[17] Seeram NP, Adams LS, Hardy ML, Heber D. Total cranberry extract versus its phytochemical constituents: Antiproliferative and synergistic effects against human tumor cell lines. J Agric Food Chem 2004; 52(9):2512-2517.
[18] Watson PF. The causes of reduced fertility with cryopreserved semen.Anim Reprod Sci 2000; 60-61: 481-492.
[19] Agarwal A, Prahakaran SA, Said TM. Prevention of oxidative stress injury to sperm. J Androl 2005; 26(6): 653-660.
[20] Ball BA, Medina V, Gravance CG, Baumber I. Effect of antioxidant on preservation of motility, viability and acrosomal integrity of equine spermatozoa during storage at 5 ℃. Theriogenology 2001; 56(4): 577-569.
[21] Bilodeau JF, Blanchette S, Gagnon IC, Sirard MA. Thiols prevent H2O2- mediated loss of sperm motility in cryopreserved bull semen.Theriogenology 2001; 56(2): 275-286.
[22] Gadea J, Selles E, Marco MA, Coy P, Matas C, Romar R, et al. Decrease in glutathione content in boar sperm after cryopreservation. Effect of the addition of reduced glutathione to the freezing and thawing extenders.Theriogenology 2004; 62(3-4): 690-701.
[23] Rota A. Studies on preservation, capacitation and fertility of dog spermatozoa. Thesis. Swedish University of Agricultural Sciences; 1998.
[24] Concannon PW, Battista M. Canine semen freezing and artificial insemination. In: Kirk RW, editor. Current veterinary therapy.Philadelphia: W.B. Saunders Company; 1989.
[25] Linde-Forsberg C, Forsberg M. Fertility in dogs in relation to semen quality and the time and site of insemination with fresh or frozen semen.J Reprod Fertil Suppl 1989; 39: 299-310.
[26] El-Sheshtawy RI, El-Nattat WS, Sabra HA, Ali AH. Effect of honey solution on semen preservability of local breeds of cattle bulls. World Appl Sci J 2014; 32(10): 2076-2078.
[27] Yang D, Xie H, Jiang Y, Wei X. Phenolics from strawberry cv. Falandi and their antioxidant and a-glucosidase inhibitory activities. Food Chem 2016; 194: 857-863.
[28] Ortega RH, Krisa S, García-Parrilla MC, Richard T. Effects of gluconic and alcoholic fermentation on anthocyanin composition and antioxidant activity of beverages made from strawberry. LWT - Food Sci Technol 2016; 69: 382-389.
[29] Canuto GA, Oliveira DR, da Conceição LS, Farah JP, Tavares MF.Development and validation of a liquid chromatography method for anthocyanins in strawberry (Fragaria spp.) and complementary studies on stability, kinetics and antioxidant power. Food Chem 2016; 192: 566-574.
[30] Alvarez-Fernandez MA, Hornedo-Ortega R, Cerezo AB, Troncoso AM,Garcia-Parrilla MC. Effects of the strawberry (Fragaria ananassa)purée elaboration process on non-anthocyanin phenolic composition and antioxidant activity. Food Chem 2014; 164: 104-112.
[31] Xu XY, Xie HH, Wei XY. Jasmonoid glucosides, sesquiterpenes and coumarins from the fruit of Clausena lansium. LWT-Food Sci Technol 2014; 59: 65-69.
 Asian Pacific Journal of Reproduction2018年2期
Asian Pacific Journal of Reproduction2018年2期
- Asian Pacific Journal of Reproduction的其它文章
- Sperm counts in Asian men: Reviewing the trend of past 50 years
- Effect of exogenous progesterone on cumulus characteristics of buffalo oocytes by allowing passage of more number of sperm through cumulus but not essentially fertilization
- Improvement in cryosurvival of buffalo bull (Bubalus bubalis) sperm by altering freezing rate within critical temperature range
- Effect of voltage-gated sodium channels blockers on motility and viability of human sperm in vitro
- Homeostatic relevance of vitamin D in maintaining male fertility in human: Downregulation of oxidative stress and up-regulation of anti-oxidative defense and steroidal hormones
- Germline cells derived from mesenchymal stem cells, with the focus on Wharton's jelly
