Effect of exogenous progesterone on cumulus characteristics of buffalo oocytes by allowing passage of more number of sperm through cumulus but not essentially fertilization
Madhusmita Panda, Asmita, Sandeep Kumar, Purusottam Mishra, Mahesh Chandra Sahu, Sachinandan De,Tirtha Kumar Datta, Rakesh Kumar
1Nodal Officer, Chief District Veterinary Office, Kadambeda, Bhadrak, 756100, Odisha, India
2Institute of Microbial Technology, Council of Scientific and Industrial Research, Chandigarh, India
3BSL-4, National Institute of Virology, Sus Road, Pashan, Pune - 411021, India
4Goat Breeding Farm, AT/PO-Salapada, District-Keonjhar, Pin code-758020, State-Odisha, India
5Directorate of Medical Research, IMS and SUM Hospital, Siksha'O' Anusandhan University, K8, Kalinga Nagar, Bhubaneswar-751003, Odisha,India
6Animal Biotechnology Centre, National Dairy Research Institute, Karnal 132001, Haryana, India
1. Introduction
In spite of having a large domain of knowledge surrounding oocyte biology, in vitro embryo production still remains a vexed issue with less than 10% of the total oocytes reaching up to blastocyst stage.This can be attributed to both oocyte causes as well as insufficient culture conditions[1]. Immature oocytes are regularly selected on the basis of more than 3-4 layers of compact cumulus and cytoplasm homogeneity[2]. Brilliant cresyl blue (BCB) stain is one of the best known non-invasive methods which permit the categorization of good and poor quality oocytes among a heterogeneous pool[3,4].BCB-oocytes which are still in their growing phase have reduced abundance of important transcripts known to impart developmental competence when compared to BCB+ones[5]. A diverse plethora of factors which are produced by cumulus cells that participate in bidirectional communication thus affecting oocyte quality have been identified[6]. In a recent study, differential transcripts in cumulus cells of BCB+and BCB-oocytes were observed[7]. These factors synthesized by cumulus cells contribute vitally to the subsequent oocyte development.
Progesterone has been found to be secreted by cumulus cells during in-vitro maturation (IVM)[8], which then induces the progesterone receptors in cumulus cells leading to meiotic resumption in porcine and bovine oocytes[9,10]. Its importance during IVM can be judged from the fact that blocking these progesterone receptor (PGR) in porcine oocytes resulted in inhibition of cumulus expansion[11].Several other evidences exist where inhibiting progesterone synthesis and secretion by cumulus cells significantly reduced the cumulus expansion and thus oocyte maturation in cattle[12]. Besides,progesterone also regulates expression of TNFα-converting enzyme(TACE)/A disintegrin and metalloprotease-17 (ADAM17) which in turn controls expression of epidermal growth factor (EGF)-signalling factors like amphiregulin (AREG), epiregulin. These factors have been implicated in the process of cumulus expansion and oocyte maturation[13]. Further its role not only as a chemo-attractant for spermatozoa[14,15], but also as a one which tends to modulate sperm functional features like hyperactivation, acrosome reaction, zona penetration rates and motility has been reviewed extensively[16].
Recent evidences hint towards requirement of progesterone during follicular development in animals. In a study, it has been shown that lactating cows with low progesterone (P4) were having high amount of luteinizing hormone which in turn compromised early stages of embryonic development[17-19]. Further, the amount of progesterone secreted has been observed to be positively correlated with number of cumulus cells surrounding the oocyte. Poor morphology oocytes with less number of cumulus cells are unable to produce enough level of progesterone required to proceed through development[20].In humans, a momentary expression of progesterone receptor has also been shown in cumulus cells during in vitro fertilization(IVF) program which probably adds to further developmental competence[21]. To our knowledge, the present study is probably the first of its kind where the study has estimated the progesterone concentration in BCB+and BCB-cumulus oocyte complexs (COCs)during IVM and subsequent incorporation of the different doses of P4 in IVM medium of BCB-ve COCs to assess if the progesterone has any effect in improving the maturation, fertilizing ability and subsequently the developmental competence of BCB-oocytes.
2. Materials and methods
Progesterone secreted by different quality of buffalo oocytes was estimated by enzyme linked immunosorbent assay (ELISA)and the concentration differences were translated into P4 doses to be incorporated in the maturation medium of BCB-ve COCs followed by expression analysis of genes involved in the cumulus expansion, extracellular matrix disintegration and progesterone receptor signaling. In addition, the study also evaluated the effect of exogenous P4 on sperm-cumulus interaction. The details of the work explained as follows.
2.1. Oocyte recovery and BCB staining
Buffalo ovaries were obtained from a local abattoir (Ghazipur slaughter house, Delhi, India) and transported to laboratory in normal saline (0.9% NaCl) containing 50 µg/mL streptopenicillin at 37 ℃. Then after, ovaries were washed in normal saline at 37 ℃ in the laboratory. COCs were recovered by aspirating antral follicles (3-8 mM in diameter) using aspiration unit (Cook) with pressure at 59 mM Hg in a 50 mL tube (Nunc). Only oocytes with 3-4 layers compact cumulus layers and homogeneous cytoplasm were selected for BCB staining. Oocytes were washed in modified Dulbecco’s phosphate buffered saline (mDPBS) containing 0.4%bovine serum albumin and exposed to 26 µM BCB (B-5388,Sigma) diluted in mDPBS for 90 min at 38.5 ℃ in a 5% CO2humidified atmosphere[5]. Following this, oocytes were washed twice in mDPBS and classified into two groups, according to their cytoplasm coloration under stereo zoom microscope. Oocytes with or without blue cytoplasmic coloration were designated as BCB+and BCB-, respectively. The oocytes were washed three times in the maturation medium [tissue culture medium-199 supplemented with 10% fetal bovine serum (v/v), 0.05 IU/mL follicle stimulating hormone), 0.05 IU/mL luteinizing hormone, 1.00 µg/mL 17β-estradiol, 24.20 mg/L sodium pyruvate, 0.10 mM cysteamine,and 10.00 ng/mL EGF]. After screening, groups of 10 COCs were cultured in 100 µL droplets of maturation medium overlaid with mineral oil and incubated for 24 h at 38.5 ℃ in a humidified 5%CO2atmosphere.
2.2. Semen sample preparation
For capillary cumulus model experiment, fresh semen was collected from bull of proven fertility from Animal Breeding Research Centre,National Dairy Research Institute, Karnal following all measures of ethical committee. After visible analysis of mass motility, sperm were prepared by swim-up method followed by counting using haemocytometer (Neubauer Improved, Marienfeld, Germany) and adjusted to get a concentration of 10 million sperm/mL in IVF media(Bracket and Oliphant media supplemented with 1.0% fatty acid free bovine serum albumin, 10 µg/mL heparin, 0.014% sodium pyruvate& 0.190% caffeine sodium benzoate).
2.3. Collection of cumulus oophorus
After 24 h of maturation, cumulus oophorus were dissected mechanically from the three COCs from each group i.e. BCB+ve,BCB-ve, BCB-ve supplemented with 10 ng/mL of P4 using a glass pipette. Cumulus en masse was pooled and washed in IVF medium.
2.4. Establishment of in-vitro capillary cumulus model
A capillary model was established as previously described[22], with few modifications to study the interaction of sperm with cumulus cells and to show the effect of progesterone treated cumulus on sperm. A sterile glass capillary (Microcaps, Drummund, USA) was attached to 1 mL insulin syringe. Sperm capacitation media was aspirated to a length of 10 cm from the end of pipette followed by aspiration of cumulus oophorus up to length of 3 cm. The open end of capillary was dipped into 200 µL of droplet containing viable motile sperm fixed to 10 million numbers and overlaid with mineral oil. Whole set up was kept in CO2incubator for 1 h. After incubation the capillary was cut at 3 cm and the upper medium column was expelled in 15 mL falcon tube and penetrated spermatozoa which had crossed the cumulus barrier was counted by haemocytometer(Neubauer improved, Marienfeld, Germany). The experiment was repeated 4 times with semen collected from the same bull.
2.5. Progesterone estimation via ELISA
A group of 10 COCs (categorized into BCB+and BCB-by brilliant cresyl blue staining) were cultured in 100 µL IVM media for each group. After 8 h, 16 h and 24 h of culture, 70 µL of IVM culture medium was collected from both groups to analyze the level of progesterone secreted by 10 COCs. The number of cumulus cells of respective groups was counted by neubauer cell counting chamber and checked for viability by trypan blue staining. The number of cumulus cells was fixed to 6 000 viable cells and accordingly the volume of media was collected from respective samples and diluted for estimation of progesterone secreted during time course of IVM.Analyses of progesterone concentrations were performed with the validated solid-phase immunoassay method (Progesterone ELISA kit, Enzo Life Sciences Inc, NY, USA), with the sensitivity of 0.007 ng/mL.The intra-assay coefficient of variation was 7.8% and the inter-assay coefficient of variation was 12.1%.
2.6. Progesterone supplementation and in-vitro embryo production
To observe the effect of exogenous progesterone on developmental competence of BCB-oocytes, screened oocytes were matured (10 COC/100 µL of IVM medium) for 24 h in presence of 0 ng/mL (control),5 ng/mL and 10 ng/mL of exogenous progesterone. Following this,matured COCs were co-incubated with frozen-thawed buffalo spermatozoa prepared as described earlier[23], for 12 h at 38.5 ℃in 5% CO2humidified air in in-vitro fertilization media drop of 100 µL. Presumptive zygotes were then cultured in a group of 12 COCs/100 µL drop in modified Charles Rosenkrans 2 medium with amino acids media (supplemented with bovine serum albumin and 2% fetal bovine serum). Cleavage and blastocyst rates were assessed across groups and expressed as percentages.
2.7. Ribonucleic acid (RNA) isolation and real time polymerase chain reaction(PCR) analysis
For RNA analysis, cumulus cells were removed separately from BCB-control, BCB-supplemented with 5 ng/mL P4 and BCB-supplemented with 10 ng/mL P4 (n=4 pools of 10 COCs for each group) at 0, 8, 16 and 24 h of in vitro maturation. Total RNA was isolated using the RNAqueous®-Micro total RNA Isolation Kit(Ambion™, USA) according to the manufacturer’s instructions. The integrity and quantity of total RNA was detected using NanoDrop®ND-1000 Spectrophotometer (NanoDrop Technologies, Thermo Fisher Scientific, Wilmington, DE, USA). Fixed amount (100 ng) of total RNA from each sample was utilized for reverse transcription using Revert Aid™ First Strand cDNA Synthesis Kit (Thermo Scientific, Massachusetts, USA), according to manufacturer’s instructions. After termination of cDNA synthesis, each reversetranscriptase reaction was diluted with nuclease-free water to a final volume of 60 µL. Quantification of all gene transcripts was done by real-time quantitative PCR using SYBR green PCR master mix on LightCycler® 96 Real-Time PCR system (Roche Diagnostics,Mannheim, Germany). All PCR primers (Table 1) used in this study were designed by using Lasergene’s PrimerSelect software(DNASTAR, Madison, WI, USA). Primer sequences of the genes analyzed were shown in Table 1. Transcript abundance for gene of interest was normalized relative to abundance of endogenous control ribosomal protein S18. Relative fold changes were calculated usingthe formula of

Table 1 Primer sequences used for expression analysis of genes under study.
2.8. Statistical analysis
Experiments were performed four times independently and statistical evaluations were performed with GraphPad Prism(GraphPad software, San Diego, CA). Analysis of variance(ANOVA) was used to determine the significance of experimental variables followed by Bonferroni Post-tests to compare the significance of effect of treatment between groups at a 95%confidence interval. Calculation and analysis of ELISA was carried out by using four parameter logistic curve fitting program in Graph pad prism software. For experiments studying the effect of exogenous progesterone supplementation on early embryonic development stages, data was arcsine transformed prior to analysis and presented as mean ± standard error. The differences between means were then analyzed by one-way ANOVA followed by Fisher’s least significant difference test. Differences were considered to be significant at P < 0.05.
3. Results
3.1. Different quality buffalo COCs secrete diverse levels of progesterone during in vitro maturation
ELISA based assay was standardized for estimating progesterone concentration in maturation medium of different quality of buffalo COC’s at 8, 16 and 24 h of IVM (S1). Buffalo COC’s were observed to follow a time dependent increase in the amount of progesterone secreted with maxima at 24 h (Figure 1). However, at any given point of time, amount secreted by good quality COC (BCB+) was higher than poor quality ones (BCB-). The difference in progesterone level between these two groups of oocytes was found to be significantly different at 24 h of IVM (P<0.05) with BCB+COCs secreting more than 3 times higher amount of progesterone than BCB-COCs.

Figure 1. Progesterone concentration (for fixed 6 000 cumulus cells) in BCB+ and BCB- group during course of in vitro maturation.
3.2. Transcript abundance variations with exogenous supplementation of progesterone expression of receptor related genes
Exogenous progesterone was supplemented in the IVM medium of BCB-COCs with doses decided on the basis of difference observed in the ELISA assay. Figure 2 showed expression analysis of progesterone receptor genes-PGR and PGRMC1 in cumulus cells from control and treatment groups. PGR was found to be significantly up regulated by more than 10 fold progesterone supplemented group. This increase was evident only at 8 and 16 h of IVM after which it dropped to basal levels by 24 h. PGRMC1 expression was not influenced by exogenous progesterone. On the contrary, it was found to be inhibited during initial hours of maturation in treatment group.

Figure 2. Expression pattern of PGR(A) and PGRMC1(B) genes in cumulus cells from BCB-ve, BCB-ve 5 ng/mL P4 & BCB- 10 ng/mL P4 groups at different hours of maturation.
3.3. Expression of maturation related genes
Additionally, expression of few maturation related genes viz. HAS2,TNFAIP6, AREG and TACE/ADAM17 was also assessed in cumulus cells from control and progesterone supplemented groups. No significant effect was observed on TNFAIP6 expression in treatment groups with respect to control. No significant effect was observed on TNFAIP6 expression in treatment groups at 8 h and 16 h of maturation with respect to control whereas with 10 ng of P4 in the treatment group the expression was significant indicating better extracellular matrix stability. HAS2 was up regulated at 8 h and 16 h of maturation but subsequently dropped to basal levels by the end of maturation. Besides, TACE/ADAM17 expression which was known to be regulated by P4-PGR pathway was significantly elevated at 8 h in10 ng/mL group and at 16 and 24 h in 5 ng/mL group (Figure 3A-D). In case of AREG also, significant increase in expression was observed during initial hours of maturation in treatment groups which any how could not get carried over to later stages.

Figure 3. Expression pattern of AREG (A), TNFAIP6 (B), HAS2 (C) and TACE/ADAM17 (D) gene in cumulus cells from BCB- , BCB- 5 ng/mL P4 &BCB- 10 ng/mL P4 groups at different hours of maturation.
3.4. Exogenous progesterone supplementation could not improve fertilizing ability of BCB- COCs
In addition to documenting the effect of exogenous progesterone on maturation, its effect on fertilizing ability of oocytes was also monitored. Figure 4 represents cleavage and blastocyst rates assessed for different treatment groups as well as control. Cleavage, rate of progression to successive stages as well as blastocyst rates could not improve in different treatment groups when compared to control(Table 2).

Figure 4. Cleavage (A) & blastocyst (B) percentage in BCB+, BCB- control,BCB- 5 ng/mL P4 & BCB- 10 ng/mL P4 groups.
3.5. Progesterone supplementation did improve number of sperm passing through cumulus barrier
As described in the materials and methods section, spermatozoa obtained by swim up of fresh buffalo bull semen was incubated with cumulus cell en mass isolated from 2-3 COCs. The mean percentage of spermatozoa that was able to cross cumulus cell barrier in different groups under this experiment was documented (Table 2). Only one dose was tested in this experiment as both the doses were more or less equivalent in terms of affecting gene expression or fertilizing ability of treated oocytes with respect to control. Percentage of penetrated spermatozoa across the progesterone treated (10 ng/mL)cumulus mass [(0.520±0.147a)%] were significantly higher than the BCB-control group [(0.226±0.025b)%] and were almost at par with BCB+group [(0.650±0.085a)%].
4. Discussion
Buffaloes in India suffer from numerous reproductive problems viz late maturity, silent heat, irregular oestrous cycle, anoestrus, low conception rate, long postpartum interval, repeat breeding, etc[25,26].This happens mainly due to climatic, managemental, nutritional and disease factors[26]. Attempts to improve productivity in this species stem their basis on improving its reproductive capacity which in turn demands a comprehensive understanding of oocyte biology[27]. Pertinent to this issue, the present study was conducted with emphasis on modulating the present in vitro culture conditions of buffalo oocytes in an attempt to improve their fertilizing ability.Progesterone has been reviewed as a vital candidate involved in oocyte maturation[9]. Significantly different amounts of progesterone secreted in the medium by good and poor quality COCs as observed in the present study further reinforces this fact. Throughout the period of assessment, progesterone secretion was higher by good quality oocytes as compared to poor quality ones but this difference was significant at 24 h of IVM. This could be due to higher steroidogenic activity of good quality COCs enabling them to produce higher amount of progesterone which probably in turn activates maturation processes in an autocrine fashion[20].
These differences in the amount of progesterone secreted were translated into doses to be incorporated in the IVM medium of poor quality buffalo COCs. To assess the P4 supplementation on oocyte maturation, mRNA expression studies of few genes involved incumulus expansion and extracellular matrix disintegration were done in the study. BCB-oocytes were supplemented with different concentrations of progesterone during IVM. Progesterone has been observed to mediate its action via both nuclear (genomic)and membrane (non genomic) receptors[28,29]. But in the study, exogenous progesterone enhanced expression of nuclear progesterone receptor signifying the fact that perhaps in buffalo oocytes, progesterone mediated most of its action using this receptor. This information is further propounded by inhibition of the membrane receptor during initial stages of IVM. Another important element in this experiment was the dynamics of PGR expression which was highest during mid maturation phases and dropped to basal levels at 24 h suggesting that progesterone is probably active during early stages during which it activates downstream targets like TACE/ADAMTS17-proteases which are important for optimum maturation[13]. On the contrary, requirement of PGRMC1 has been propounded by some authors in order to mediate the action of P4 in granulosa cells and in prevention of apoptosis by activating the antiapoptotic genes[30].
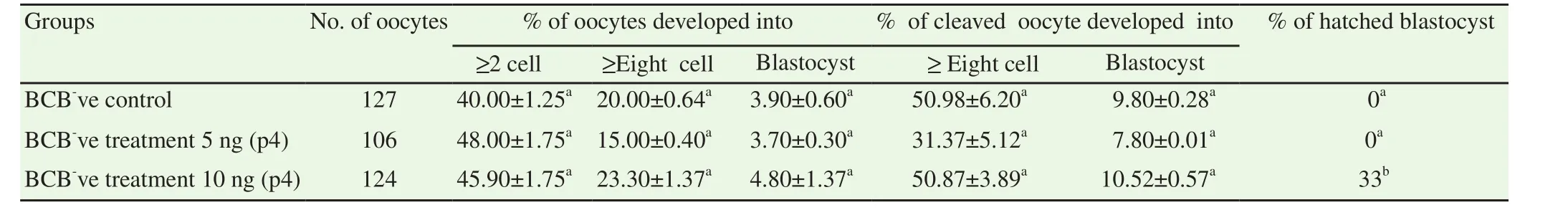
Table 2 Oocytes cleavage, 8-cell, Blastocyst & hatched blastocyst rate in BCB- (control) and BCB- with added P4 (5 ng/mL & 10 ng/mL) treatment groups.
Further, exogenous progesterone was observed not to affect cumulus expansion genes viz. HAS 2 and TACE/ADAMTS17. HAS 2 has been reported to be expressed during early stages of maturation and thereafter dropping to basal levels by the time of ovulation[31].Present work is in conformity with these studies, but no significant difference between control and progesterone supplemented groups suggests that progesterone is probably not involved in cumulus expansion process. The EGF-like growth factor family member i.e. AREG is expressed in cumulus cells of buffalo COCs during the course of maturation. In the present study, mRNA expression of AREG was highest at initial stages (8 h) of IVM where it could have supported the process of cumulus expansion. The interactome between TACE/ ADAM17 is essential for ectodomain shedding of AREG and therefore on exogenous progesterone addition, through P4-PGR-TACE/ADAM17 pathway, it might have affected AREG expression at 8 h of IVM but the effect could not get carried over to later stages due to the lower transcriptional activation signalling input from the PGR to regulate the downstream extracellular regulated protein kinases pathways in cumulus cells. Similar results were shown, where they observed a high expression of AREG during early stages of in vitro maturation of porcine oocytes and the expression decreased to basal levels at the end of maturation process[32].However, it was observed that increase in AREG expression at initial stage of maturation was not concomitantly followed by the increase in expression of HAS2 and TNFAIP6 mRNA in the current study.In the study, it was found that on supplementation of 5 and 10 ng/mL progesterone, the effect of upregulation of PGR-TACE-AREG pathway could not get carried over to later stages which might have increased the transcript abundance of genes regulating cumulus expansion. This could be due to the fact that this regulation might be precisely dependent on P4 dosage and regulated in a stage specific manner[13].
Despite exogenous P4 could not improve maturation significantly,the study proceeded with in vitro fertilization and subsequent culture of embryos produced from control and progesterone supplemented oocytes. With little surprise, no significant difference was observed in the rate of total oocytes or cleaved oocytes developing into blastocysts between the control and treated groups (P<0.05).Although, exogenous progesterone was able to increase overall cleavage rate in the treatment group up to certain extent when compared with control but this increase was not significant. In a sharp contrast, a study reported that poor quality oocytes (≤2 layer of cumulus cell) secretes less progesterone in culture medium and the addition of progesterone to this group of oocytes significantly increased their fertilization rate[20]. Distinct evidences are available reporting effect of exogenous progesterone on developmental ability of oocytes. Improved embryo development rate with addition of P4 were observed in a study conducted previously[33]. Conversely,negative effects of progesterone on developmental ability of oocytes rates have also been documented which were significantly reversed by addition of anti-progestin molecules[34,35].
Besides, sperm cumulus interaction under treated (P4 supplemented)and control conditions was also studied. Here, it is hypothesized that progesterone might be changing the cumulus characteristics of buffalo COCs which may in turn improve their fertilizing ability[36].Chemo-attractant properties of spermatozoa has been reported which are crucial in helping spermatozoa in the process of fertilization[14].To prove this, a capillary cumulus sperm interaction experiment was done in the study. It is observed that exogenous progesterone supplemented indeed made more number of spermatozoa to cross through the cumulus barrier. This could have been due to either increased penetration of progesterone treated cumulus cells by spermatozoa or due to chemo-attractant property of progesterone at higher concentrations[16]. Hong et al in 2004 had used a capillary cumulus model in which they had shown that more number of acrosome reacted spermatozoa could cross cumulus barrier thereby signifying the role of cumulus cells in selection of best sperm in large numbers for fertilization[22,23]. From the current study results,it seems that exogenous P4 supplementation in BCB-oocytes might have modulated the properties of cumulus cells, thus making them more receptive for the passage of sperm[14].
In conclusion, this study revealed that progesterone might have an important role in oocyte maturation by improving cumulus features such as making them more penetrative to spermatozoa, but perhaps not fertilization at least with the doses used under this study.
Conflict of interest statement
The authors declare that they have no conflict of interest.
This project was supported by National Fund project (Grant No.NF2049/3040) of Indian Council of Agricultural Research, New Delhi, India.
[1] Rizos D, Clemente M, Bermejo-Alvarez P, de-La-Fuente J, Lonergan P,Gutiérrez-Adán A. Consequences of in vitro culture conditions on embryo development and quality. Reprod Domest Anim 2008; 4: 44-50.
[2] Madison V, Avery B, Greve T. Selection of immature bovine oocytes for developmental potential in vitro. Anim Reprod Sci 1992; 27(1): 1-11.
[3] Alm H, Torner H, Lohrke B, Viergutz T, Ghoneim IM, Kanitz W. Bovine blastocyst development rate in vitro is influenced by selection of oocytes by brillant cresyl blue staining before IVM as indicator for glucose-6-phosphate dehydrogenase activity. Theriogenology 2005; 63: 2194-2205.
[4] Catalá MG, Izquierdo D, Uzbekova S, Morató R, Roura M, Romaguera R, et al. Brilliant Cresyl Blue stain selects largest oocytes with highest mitochondrial activity, maturation-promoting factor activity and embryo developmental competence in prepubertal sheep. Reproduction 2011; 142:517-527.
[5] Torner H, Ghanem N, Ambros C, Holker M, Tomek W, Phatsara C, et al.Molecular and subcellular characterisation of oocytes screened for their developmental competence based on glucose-6-phos-phate dehydrogenase activity. Reproduction 2008; 135: 197-212.
[6] Atef A, Francois P, Christian V, Marc-Andre S. The potential role of gap junction communication between cumulus cells and bovine oocytes during in vitro maturation. Mol Reprod Dev 2005; 71: 358-367.
[7] Ashry M, Lee K, Mondal M, Datta T, Folger JK, Rajput SK, et al.Expression of TGF-βsuperfamily components and other markers of oocyte quality in oocytes selected by brilliant cresyl blue staining:Relevance to early embryonic development. Mol Reprod Dev 2015; 82(3):251-264.
[8] Chian RC, Ao A, Clarke HJ, Tulandi T, Tan S. Production of steroids from human cumulus cells treated with different concentrations of gonadotropins during culture in vitro. Fertil Steril 1999; 71: 61-66.
[9] Shimada M, Terada T. FSH and LH induce progesterone production and progesterone receptor synthesis in cumulus cells: A requirement for meiotic resumption in porcine oocytes. Mol Hum Reprod 2002; 8(7): 612-618.
[10] Eroglu A. Experimental studies on in vitro maturation of porcine oocytes.II. Effects of estradiol-17 beta and progesterone. Berl Munch Tierarztl Wochenschr 1993; 106: 157-159.
[11] Shimada M, Nihsibori M, Yamashita Y, Ito J, Mori T, Richards JS.Down- regulated expression of A disintegrin and metalloproteinase with thrombospondin-like repeats-1 by progesterone receptor is associated with impaired expression of porcine cumulus-oocyte complexes.Endocrinology 2004; 145: 4603-4610.
[12] Wang HF, Isobe N, Kumamoto K, Yamashiro H, Yamashita Y, Terada T. Studies of the role of steroid hormone in the regulation of oocyte maturation in cattle. Reprod Biol Endocrinol 2006; 4: 4.
[13] Yamashita Y, Kawashima I, Gunji Y, Hishinuma M, Shimada M.Progesterone is essential for maintenance of Tace ∕Adam17 mRNA expression, but not EGF-like factor, in cumulus cells, which enhances the EGF receptor signaling pathway during in vitro maturation of porcine COCs. J Reprod Dev 2010; 56: 315-323.
[14] Oren-Benaroya R, Orvieto R, Gakamsky A, Pinchasov M, Eisenbach M. The sperm chemoattractant secreted from human cumulus cells is progesterone. Hum Reprod 2008; 23(10): 2339-2345.
[15] Teves ME, Barbano F, Guidobaldi HA, Sanchez R, Miska W, Giojalas LC. Progesterone at the pico-molar range is a chemoattractant for mammalian spermatozoa. Fertil Steril 2006; 86: 745-749.
[16] Campbell JM, Savage AL, Madamidola O, Tamhane K, Soriano R,Adya AK, et al. Progesterone significantly enhances the mobility of boar spermatozoa. Bio Discovery 2013; 9: 5.
[17] Cerri RL, Chebel RC, Rivera F, Narciso CD, Oliveira RA, Amstalden M, et al. Concentration of progesterone during the development of the ovulatory follicle: II. Ovarian and uterine responses. J Dairy Sci 2011;94: 3352-3365.
[18] Cerri RL, Chebel RC, Rivera F, Narciso CD, Oliveira RA, Thatcher WW, et al. Concentration of progesterone during the development of the ovulatory follicle: I. Ovarian and embryonic responses. J Dairy Sci 2011;94: 3342-3351.
[19] Rivera FA, Mendonca LG, Lopes G Jr, Santos JEP, Perez RV, Amstalden M, et al. Reduced progesterone concentration during growth of the first follicular wave affects embryo quality but has no effect on embryo survival post transfer in lactating dairy cows. Reproduction 2011; 141:333-342.
[20] Kusaka CS, Utsunomiya T, Kumasako Y, Otsu E, Mori T, Shimada M.The relationship between level of progesterone secreted from cumulus cells and oocyte developmental competence in in-vitro matured human cumulus oocytes complexes. J Mamm Ova Res 2012; 29: 41-47.
[21] Shimada M, Nishibori M, Isobe N, Kawano N and Terada T. Luteinizing hormone receptor formation in cumulus cells surrounding porcine oocytes and its role during meiotic maturation of porcine oocytes. Biol Reprod 2003; 68: 1149-1159.
[22] Hong, SJ, Chiu PC, Lee KF, Tse JMY, Ho PC, Yeung WSB.Establishment of a capillary-cumulus model to study the selection of sperm for fertilization by the cumulus oophorus. Hum Reprod 2004;19(7): 1562-1569.
[23] Chauhan MS, Singla SK, Palta P, Manik RS, Madan ML. In vitro maturation and fertilization, and subsequent development of buffalo(Bubalus bubalis) embryos: Effects of oocyte quality and type of serum.Reprod Fertil Dev 1998; 10: 173-177.
[24] Madan ML, Das SK, Palta P. Application of reproductive technology to buffaloes. Anim Reprod Sci 1996; 42: 299-306.
[25] Madan ML, Prakash BS, Jailkhani S, Singla SK, Palta P, Manik RS.Buffalo endocrinology with special reference to embryo transfer. Karnal,India: Embryo Biotechnology Centre, National Dairy Research Institute;1993, p. 32.
[26] Perera BM. Reproduction in domestic buffalo. Reprod Domest Anim 2008;2: 200-206.
[27] Beker Van Woudenberg A, Grollers-Mulderij M, Snel C, Jeurissen N,Stierum R, Wolterbeek A. The bovine oocyte in vitro maturation model:A potential tool for reproductive toxicology screening. Reprod Toxicol 2012; 34(2): 251-260.
[28] Rekawiecki R, Kowalik MK, Kotwica J. Nuclear progesterone receptor isoforms and their functions in the female reproductive tract. Pol J Vet Sci 2011; 14(1): 149-158.
[29] Dressing GE, Goldberg JE, Charles NJ, Schwertfeger KL, Lange CA.Membrane progesterone receptor expression in mammalian tissues:A review of regulation and physiological implications. Steroids 2011;76(1-2): 11-17.
[30] Peluso JJ, Liua X, Gawkowskaa A, Loddea V, Wuc CA. Progesterone inhibits apoptosis in part by PGRMC1-regulated gene expression. Mol Cell Endocrinol 2010; 320:153-161.
[31] Adriaenssens T, Segers I, Wathlet S, Smitz J. The cumulus cell gene expression profile of oocytes with different nuclear maturity and potential for blastocyst formation. J Assist Reprod Genet 2011; 28: 31-40.
[32] Kawashima I, Okazaki T, Noma N, Nishibori M, Yamashita Y, Shimada M. Sequential exposure of porcine cumulus cells to FSH and/or LH is critical for appropriate expression of steroidogenic and ovulation-related genes that impact oocyte maturation in vivo and in vitro. Reproduction 2008; 136: 9-12.
[33] Ferguson CE, Davidson TR, Mello MRB, Lima AS, Kesler DJ, Wheeler MB, et al. Evidence for a direct effect of P4 on IVF-derived bovine 8-cell embryos. Reprod Fertil Dev 2005; 17: 219.
[34] Silva CC, Knight PG. Effects of androgens, progesterone and their antagonists on the developmental competence of in vitro matured bovine oocytes. J Reprod Fertil 2000; 119: 261-269.
[35] Clemente M, de-La-Fuente J, Fair T, Al-Naib A, Gutierrez-Adan A,Roche JF, et al. Progesterone and conceptus elongation in cattle: A direct effect on the embryo or an indirect effect via the endometrium?Reproduction 2009; 138: 507-517.
[36] Salhab M, Tosca L, Cabau C, Papillier P, Perreau C, Dupont JK, et al. Kinetics of gene expression and signalling in bovine cumulus cells throughout IVM in different mediums in relation to oocyte developmental competence, cumulus apoptosis and progesterone secretion. Theriogenology 2011; 75: 90-104.
 Asian Pacific Journal of Reproduction2018年2期
Asian Pacific Journal of Reproduction2018年2期
- Asian Pacific Journal of Reproduction的其它文章
- Effect of tris-extender supplemented with various concentrations of strawberry(Fragaria spp.) on bull semen preservability
- Sperm counts in Asian men: Reviewing the trend of past 50 years
- Improvement in cryosurvival of buffalo bull (Bubalus bubalis) sperm by altering freezing rate within critical temperature range
- Effect of voltage-gated sodium channels blockers on motility and viability of human sperm in vitro
- Homeostatic relevance of vitamin D in maintaining male fertility in human: Downregulation of oxidative stress and up-regulation of anti-oxidative defense and steroidal hormones
- Germline cells derived from mesenchymal stem cells, with the focus on Wharton's jelly
