螺靛红呋喃衍生物的合成及活性研究
谢建武, 邵云霞, 张 雪, 吴佳顺
(浙江师范大学 化学与生命科学学院,浙江 金华 321004)
靛红衍生物,特别是螺靛红衍生物,是一类具有特殊结构的杂环化合物,具有很好的生物及药物活性[1-2].靛红中的3-羰基是一个很好的亲电试剂,可以与其他亲核试剂发生反应从而构造螺杂环化合物,若将3-羰基还原为3-羟基,那么可以作为亲核试剂,与亲电试剂发生串联反应,构造成其他杂环化合物.因此,靛红作为一个非常有用的合成中间体,被广泛应用在医药、农药、染料和精细化工等方面[3-5].螺环化合物是一类特殊的骨架,是一类两平面互相垂直且具有特殊刚性结构的化合物,广泛存在于众多的天然产物中.近年来,化学家们基于靛红已经合成了一系列具有生理活性的螺靛红衍生物[6-7].
长期以来,本课题组一直专注于杂环化合物的合成,并取得了一系列的研究成果.以靛红为原料,通过设计合成新底物,构造新型的螺靛红杂环化合物并取得了一定的进展,高选择性地合成了一系列螺杂环化合物[8-12].基于已经取得的一些成果,如果从靛红出发,通过与丙二腈发生克脑文盖尔缩合反应(Knoevenagel condensation),接着还原得到3-丙二腈基靛红1a.底物1a在一定条件下与醛,如苯甲醛2a,发生羟醛缩合/环化/质子迁移串联反应,就可以获得新型的螺靛红呋喃衍生物3aa,具体见图1.
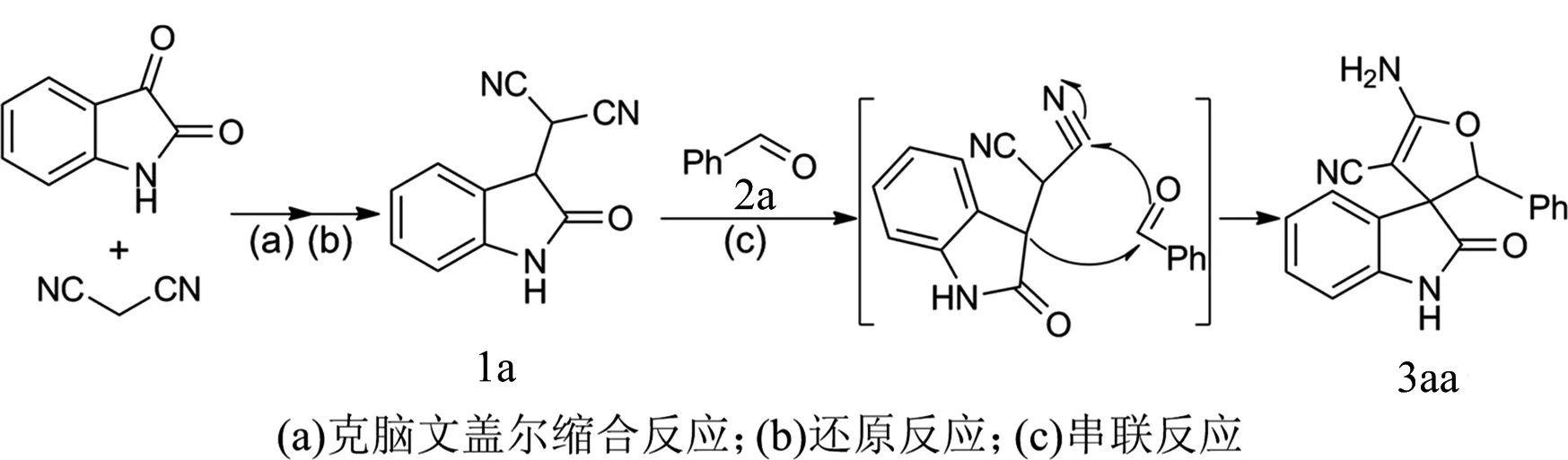
图1 研究策略
1 实验部分
1.1 试剂与仪器
WRS-1B 数字熔点仪(温度计未经校正);Bruker Avance 400型或600型核磁共振波谱仪(DMSO或CDCl3为溶剂,TMS为基准物质);有机反应用薄层硅胶板(TLC)跟踪监测.德国Bruker高分辨质谱仪(BioTOFⅢQ).
1.2 羟醛缩合/环化/质子迁移串联反应的一般步骤
靛红腈基底物1[13](0.20 mmol)、醛2(0.24 mmol)溶于1 mL二氯甲烷,接着加入三乙胺(0.04 mmol),室温搅拌6 h.经快速柱层析(V(乙酸乙酯)/V(石油醚)=1/5)分离得到对应的产物3或者4.
2 结 果
2.1 条件优化
为了获得该串联反应的优化条件,以靛红腈基底物1a及苯甲醛2a为反应模板,对各种溶剂(氯仿、乙醇、甲苯、四氢呋喃和二氯甲烷)及碱(三乙胺、碳酸钾和氢氧化锂等)进行了筛选,发现该反应在三乙胺的催化下,以二氯甲烷为溶剂,常温下反应6 h得到71%的产率及2∶1的非对映选择性,相对于其他溶剂和碱,该条件为最好(见图2).因此,羟醛缩合/环化/质子迁移串联反应优化条件为:靛红腈基底物1及醛2在三乙胺的催化下,以二氯甲烷为溶剂,常温下反应6 h.根据此优化条件,对底物的适用性进行了研究,实验结果如表1所示.
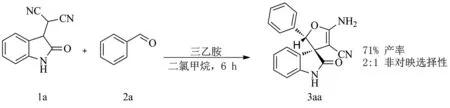
图2 羟醛缩合/环化/质子迁移串联反应的优化条件
2.2 底物扩展
获得优化该串联反应的优化条件后,为了获取更多的螺靛红呋喃衍生物,随后对有各种取代基靛红衍生的腈基底物及芳香甲醛进行了适用性研究,实验结果如图3和表1所示.首先,靛红腈基底物1a分别与不同取代基的芳香甲醛(2a—2i)进行反应,以研究电子效应对该串联反应的影响.芳香甲醛中芳环上的取代基的电子效应对产物产率有非常大的影响.芳环上有拉电子基团,如氟(2b)、三氟甲基(2d)和氯(2h)等基团时,该串联反应顺利进行,螺靛红呋喃衍生物可以获得70%以上的收率.特别是,当芳环上有三氟甲基强拉电子基团时,螺靛红呋喃衍生物(3ad,3ad′)收率最高可达到91%.另外,所有的产物非对应选择性不是特别好,普遍为2∶1.不过,部分非对映异构体可以很好地分离,如3ad和3ad′,3ae和3ae′,3ah和3ah′.此外,还对靛红腈基底物进行了研究.靛红腈基底物上的氮带有其他取代基时,反应活性较差,只有中等收率,非对映选择性也仅有2∶1(3ba,3ca).
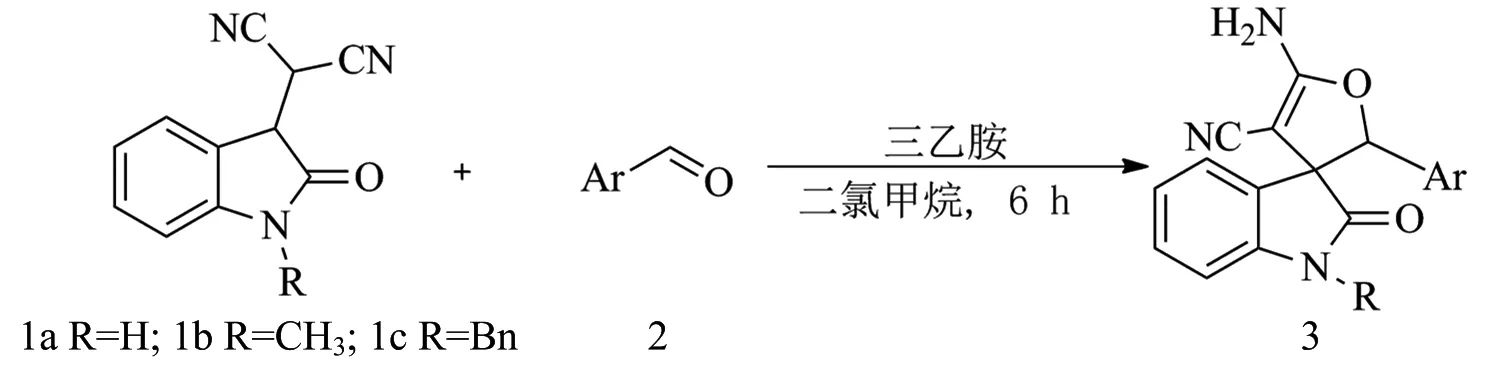
图3 靛红腈基底物1与芳香甲醛2发生串联反应的研究
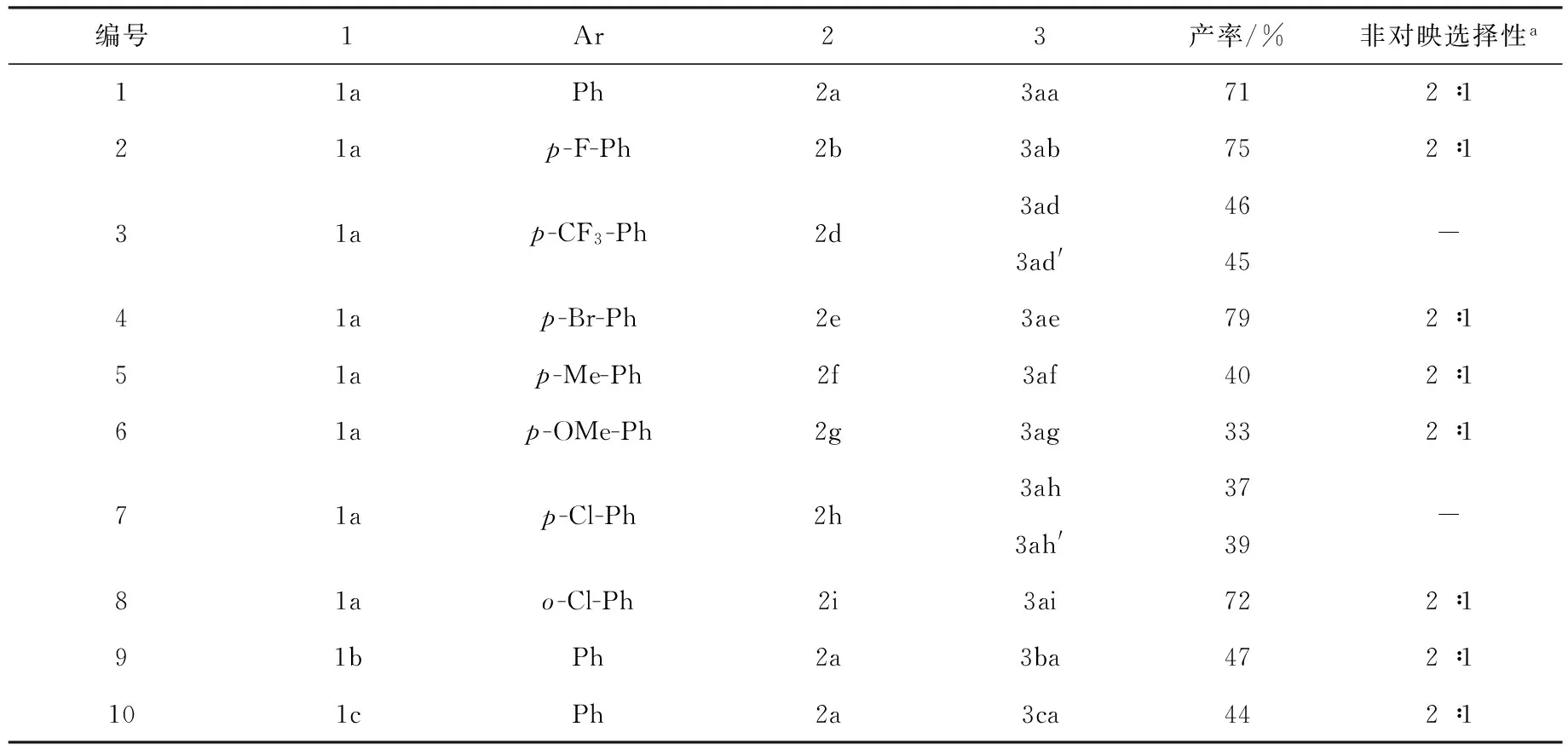
编号1Ar23产率/%非对映选择性a11aPh2a3aa712∶121ap-F-Ph2b3ab752∶131ap-CF3-Ph2d3ad463ad'45-41ap-Br-Ph2e3ae792∶151ap-Me-Ph2f3af402∶161ap-OMe-Ph2g3ag332∶171ap-Cl-Ph2h3ah373ah'39-81ao-Cl-Ph2i3ai722∶191bPh2a3ba472∶1101cPh2a3ca442∶1
注:a非对映选择性测定通过核磁共振氢谱测定.
为了进一步研究该串联反应的适用性,对肉桂醛及其取代肉桂醛2′进行了研究和拓展,如图4和表2所示.在优化的条件下,该羟醛缩合/环化/质子迁移串联反应都能够顺利进行,获得了一系列螺靛红呋喃衍生物.肉桂醛衍生的芳基上取代基的电子效应对该反应收率影响特别明显.肉桂醛衍生物芳环上有拉电子基团时,产物螺靛红呋喃衍生物可以获得很好的收率,普遍在65%以上;若是肉桂醛衍生物芳环上有供电子基团时,则产率有所降低.特别值得注意的是,肉桂醛衍生物芳环上有拉电子基团,所得到2种螺靛红衍生物的非对映异构体可以在普通柱子上分离.此外,含有杂环的不饱和醛与靛红腈基底物发生反应,同样可以获得较好的收率,而且非对映选择性最好,可以达到3∶1(4ae).
图4 靛红腈基底物1与α,β-不饱和醛2发生串联反应的研究
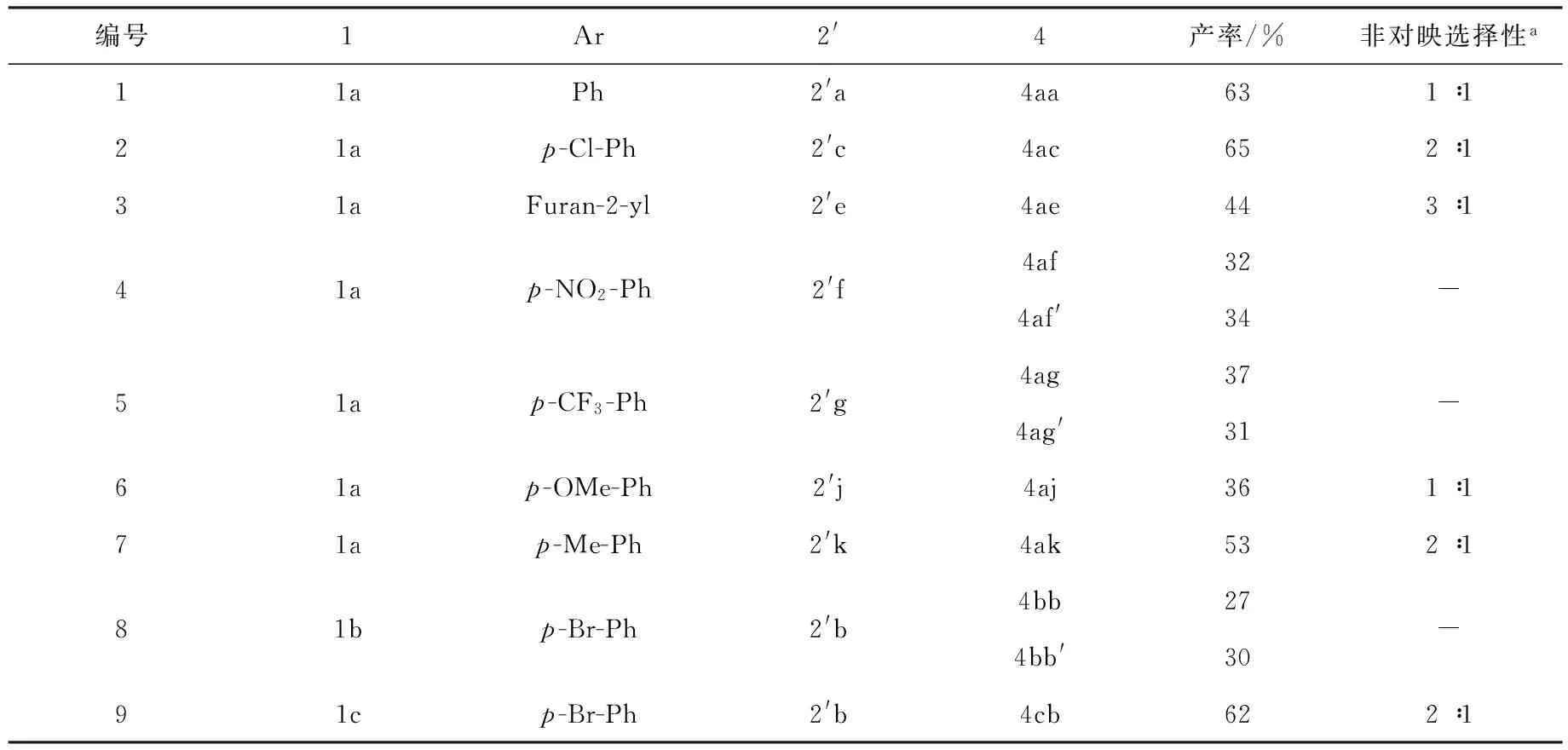
编号1Ar2'4产率/%非对映选择性a11aPh2'a4aa631∶121ap-Cl-Ph2'c4ac652∶131aFuran-2-yl2'e4ae443∶141ap-NO2-Ph2'f4af324af'34-51ap-CF3-Ph2'g4ag374ag'31-61ap-OMe-Ph2'j4aj361∶171ap-Me-Ph2'k4ak532∶181bp-Br-Ph2'b4bb274bb'30-91cp-Br-Ph2'b4cb622∶1
注:a非对映选择性测定通过核磁共振氢谱测定.
通过对产物3ae的单晶培养,确定了产物的相对构型,见图5.
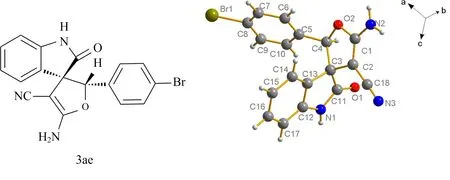
图5 单晶结构图
2.3 活性研究
为了研究该类化合物的抗菌活性,随后采用速率生长法测试化合物对真菌的抑制活性.化合物3—4抑制黄瓜枯萎病菌(Fusariumoxysporum),苦瓜枯萎病菌(Fusariumsemitectum),番茄早疫病菌(Alternariasolani),杨树溃疡病菌(Dothiorellagregaria),小麦赤霉病菌(Fusariumgraminearum)和水稻稻瘟病菌(Magnaportheoryzae) 6种植物致病真菌菌丝生长活性如表3所示.结果表明,化合物4ac能很好地抑制供试菌番茄早疫病菌菌丝生长,而化合物4ag也能强烈抑制供试菌小麦赤霉病菌菌丝生长,其抑制率分别为65.0%,85.9%,与阳性对照药放线菌酮(75.7%,100.0%)抑制效果相当.同时,根据表3中数据,发现在同一浓度下,不同化合物对同种植物致病真菌的抑制效果和同一化合物对不同植物致病真菌的抑制作用均不相同.但是,从整体上看,该类化合物对番茄早疫病菌和小麦赤霉病菌的抑制作用相对较好,而对其他4种植物致病真菌的抑制效果则较差,且部分化合物(如3af,3ba,4af和4ag)对个别植物致病真菌表现出微弱的抑制活性.以上结果再次表明,在螺环结构中引入卤素或者三氟甲基后,其抑菌效果会得到相应的提高.
表3 部分化合物在50 mg/L下对真菌的抑制率 %
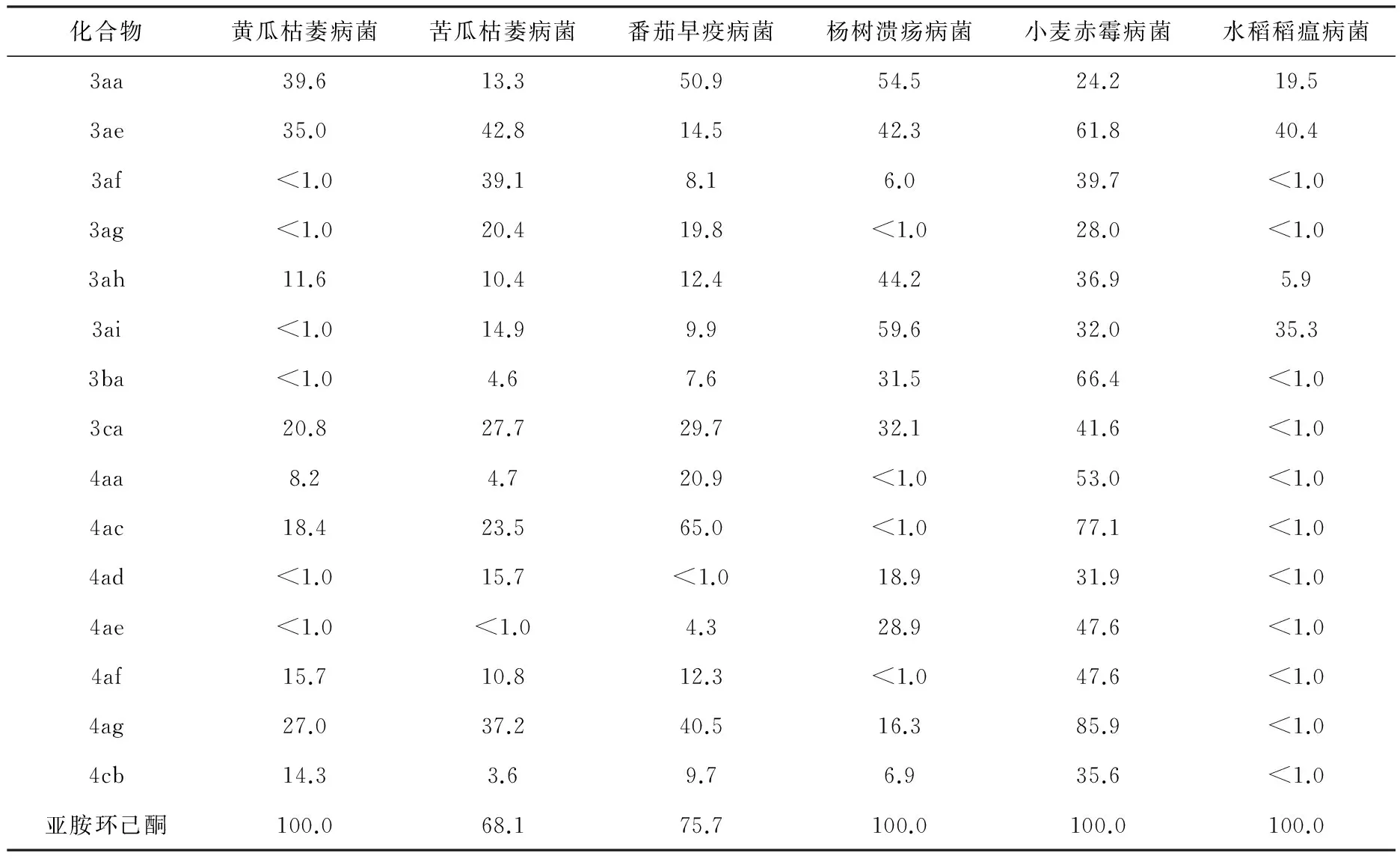
化合物黄瓜枯萎病菌苦瓜枯萎病菌番茄早疫病菌杨树溃疡病菌小麦赤霉病菌水稻稻瘟病菌3aa39.613.350.954.524.219.53ae35.042.814.542.361.840.43af<1.039.18.16.039.7<1.03ag<1.020.419.8<1.028.0<1.03ah11.610.412.444.236.95.93ai<1.014.99.959.632.035.33ba<1.04.67.631.566.4<1.03ca20.827.729.732.141.6<1.04aa8.24.720.9<1.053.0<1.04ac18.423.565.0<1.077.1<1.04ad<1.015.7<1.018.931.9<1.04ae<1.0<1.04.328.947.6<1.04af15.710.812.3<1.047.6<1.04ag27.037.240.516.385.9<1.04cb14.33.69.76.935.6<1.0亚胺环己酮100.068.175.7100.0100.0100.0
2.4 产物的表征数据:
1)3aa 淡黄色固体;熔点101~103 ℃;1H NMR(600 MHz;DMSO-d6)δ:10.59(s;1H),7.71(s,2H),7.21(t,J=7.3 Hz,2H),7.17(t,J=7.3 Hz,1H),7.03(d,J=7.3 Hz,2H),6.94~6.91(m,1H),6.69(d,J=7.7 Hz,1H),6.58(d,J=7.4 Hz,1H),5.82(s,1H).13C NMR (150 MHz,DMSO)δ:178.5,169.0,141.0,135.3,129.2,128.6,128.3,128.1,125.5,125.2,122.2,121.4,117.7,109.4,88.3,62.1;高分辨质谱m/z:计算值C18H13N3NaO2+[M+Na+] 326.090 0,测量值326.091 6.
2)3ab 淡黄色固体;熔点 113~115 ℃;1H NMR(600 MHz,DMSO-d6)δ:10.63(s,1H),7.76(s,2H),7.11~7.01(m,6H),6.71(d,J=7.5 Hz,1H),6.58(d,J=7.4 Hz,1H),5.84(s,1H).13C NMR(150 MHz,DMSO)δ:178.5(d,JC-F=246.4 Hz),169.0,162.6,160.9,141.2,131.7,128.8,128.1,127.9,127.6,125.8,121.6,117.8,115.2,115.0,109.6,87.7,62.1;高分辨质谱m/z:计算值C18H12FN3NaO2+[M+Na+] 344.080 6,测量值344.081 6.
3)3ad 淡黄色固体;熔点 124~126 ℃;1H NMR(600 MHz,DMSO-d6)δ:10.17(s,1H),7.84(s,2H),7.68(d,J=8.3 Hz,2H),7.63(d,J=7.3 Hz,1H),7.33~7.26(m,1H),7.21~7.07(m,3H),6.77(d,J=7.7 Hz,1H),6.03(s,1H).13C NMR(150 MHz,DMSO)δ:176.7,169.4,142.4,139.0,129.5,129.0(q,JC-F=31.6 Hz),128.8,128.7,128.3,126.2(q,JC-F=4.6 Hz),125.1,124.9,124.7,123.2(q,JC-F=266.3 Hz),122.4,117.8,109.7,88.4,61.9;高分辨质谱m/z:计算值C19H12F3N3NaO2+[M+Na+] 394.077 4,测量值394.079 8.
4)3ad′ 淡黄色固体;熔点 124~126 ℃;1H NMR(600 MHz,DMSO-d6)δ:10.69(s,1H),7.82(s,2H),7.61(d,J=8.1 Hz,2H),7.26(d,J=8.1 Hz,2H),7.09~6.98(m,1H),6.73(d,J=7.7 Hz,1H),6.67(t,J=7.5 Hz,1H),6.55(d,J=7.5 Hz,1H),5.96(s,1H).13C NMR(150 MHz,DMSO-d6)δ:178.2,168.8,141.1,140.1,128.9(q,JC-F=31.8 Hz),128.7,128.5,127.9,126.7,126.1,125.6(q,JC-F=6.1 Hz),125.1,124.9,123.1(q,JC-F=269.3 Hz),121.5,117.5,109.7,87.3,61.9;高分辨质谱m/z:计算值C19H12F3N3NaO2+[M+Na+] 394.077 4,测量值394.079 8.
5)3ae 淡黄色固体;熔点 130~132 ℃;1H NMR(600 MHz,DMSO-d6)δ:10.11(s,1H),7.77(s,2H),7.57(d,J=7.4 Hz,1H),7.47(d,J=8.5 Hz,2H),7.31~7.22(m,1H),7.11(t,J=7.9 Hz,1H),6.85(d,J=8.5 Hz,2H),6.74(d,J=7.7 Hz,1H),5.86(s,1H).13C NMR(150 MHz,DMSO-d6)δ:176.7,169.4,142.4,138.1,133.5,131.0,129.4,128.3,127.6,124.6,122.3,121.8,117.8,109.6,88.7,61.9;高分辨质谱m/z:计算值C18H12BrN3NaO2+[M+Na+] 404.000 5,测量值404.003 1.
6)3af 淡黄色固体;熔点 112~114 ℃;1H NMR(600 MHz,DMSO-d6)δ:10.59(s,1H),7.69(s,2H),7.07~6.99(m,4H),6.93(d,J=8.0 Hz,2H),6.70(d,J=7.7 Hz,2H),5.77(s,1H),2.18(s,3H).13C NMR(150 MHz,DMSO-d6)δ:178.1,168.6,142.1,140.6,137.1,131.8,128.8,128.3,128.1,125.2,124.9,121.1,117.4,109.1,88.2,61.8,20.3;高分辨质谱m/z:计算值C19H15N3NaO2+[M+Na+] 340.105 6,测量值340.107 3.
7)3ag 淡黄色固体;熔点 119~121 ℃;1H NMR(600 MHz,DMSO-d6)δ:10.56(s,1H),7.66(s,2H),7.26~7.15(m,1H),7.03~7.00(m,1H),6.97(d,J=8.6 Hz,2H),6.76(d,J= 8.8 Hz,2H),6.72~6.68(m,2H),5.74(d,J=5.0 Hz,1H),3.64(s,3H).13C NMR(150 MHz,DMSO-d6)δ:178.6,169.0,159.0,141.1,129.2,128.4,127.2,126.8,125.9,124.4,121.5,117.8,113.4,109.5,88.5,62.2,54.8;高分辨质谱m/z:计算值C19H15N3NaO3+[M+Na+] 356.100 6,测量值356.101 7.
8)3ah 淡黄色固体;熔点 127~129 ℃;1H NMR(600 MHz,DMSO-d6)δ:10.12(s,1H),7.78(s,2H),7.59(d,J=7.4 Hz,1H),7.45~7.32(m,2H),7.29~7.21(m,1H),7.18~7.07(m,1H),6.93(d,J=8.5 Hz,2H),6.75(d,J=7.7 Hz,1H),5.90(s,1H).13C NMR(150 MHz, DMSO-d6)δ:176.7,169.4,142.4,133.1,133.1,129.4,128.3,128.1,127.4,124.6,122.3,117.8,109.6,99.5,88.7,61.9;高分辨质谱m/z:计算值C18H12ClN3NaO2+[M+Na+] 360.051 0,测量值360.052 9.
9)3ah′ 淡黄色固体;熔点 127~129 ℃;1H NMR(600 MHz,DMSO-d6)δ:10.64(s,1H),7.75(s,2H),7.29(d,J=8.4 Hz,2H),7.05(dd,J=13.5,8.1 Hz,3H),6.78~6.68(m,2H),6.60 (d,J=7.3 Hz,1H),5.84(s,1H).13C NMR(150 MHz,DMSO)δ:178.8,169.3,141.5,134.8,133.1,129.2,128.6,128.4,127.6,126.1,125.8,122.0,118.0,110.0,87.9,62.4;高分辨质谱m/z:计算值C18H12ClN3NaO2+[M+Na+] 360.051 0,测量值360.052 9.
10)3ai 淡黄色固体;熔点 167~169 ℃;1H NMR(600 MHz,DMSO-d6)δ:10.57(s,1H),7.73(s,2H),7.54~7.47(m,2H),7.28(t,J=6.9 Hz,2H),7.20(t,J=7.4 Hz,1H),7.08~7.04(m,1H),6.75(d,J=7.7 Hz,1H),6.65~6.54(m,1H),6.02(s,1H).13C NMR(150 MHz,DMSO-d6)δ:179.3,168.5,142.1,133.9,132.4,131.1,129.9,129.5,129.0,127.4,126.5,125.3,124.5,121.2,117.3,109.5,84.4,60.9;高分辨质谱m/z:计算值C18H12ClN3NaO2+[M+Na+] 360.051 0,测量值360.052 1.
11)3ba 淡黄色固体;熔点 128~130 ℃;1H NMR(600 MHz,DMSO-d6)δ:7.76(s,2H),7.24~7.16(m,5H),6.99(d,J=7.1 Hz,2H),6.86(d,J=6.8 Hz,2H),5.83(s,1H),3.18(s,3H).13C NMR(150 MHz,DMSO-d6)δ:176.9,169.2,142.6,135.2,129.5,128.8,128.2,127.4,125.4,125.2,124.2,122.1,117.7,108.5,88.3,61.7,26.6;高分辨质谱m/z:计算值C19H15N3NaO2+[M+Na+] 340.105 6,测量值340.107 5.
12)3ca 淡黄色固体;熔点 124~126 ℃;1H NMR(600 MHz,CDCl3)δ:7.36~7.28(m,5H),7.13(d,J=6.5 Hz,3H),7.02~6.97(m,3H),6.77(t,J=4.0 Hz,2H),6.59(d,J=7.8 Hz,1H),6.10(s,1H),4.99(dd,J=84.8,15.7 Hz,2H).13C NMR(150 MHz,CDCl3)δ:177.3,169.0,141.6,135.3,134.4,129.0,128.9,128.5,128.2,127.8,127.3,126.7,126.3,126.1,125.3,122.8,116.9,109.2,90.4,62.6,44.3;高分辨质谱m/z:计算值C25H19N3NaO2+[M+Na+] 416.136 9,测量值416.139 0.
13)4aa 淡黄色固体;熔点 117~119 ℃;1H NMR(400 MHz,CDCl3)δ:8.73(s,1H),7.22~7.18(m,7H),7.11(s,2H),6.86~6.78(m,2H),6.58(d,J=15.8 Hz,1H),5.89(dd,J=15.8,7.8 Hz,1H),5.53(d,J=7.8 Hz,1H).13C NMR(150 MHz,CDCl3)δ:175.2,168.9,143.0,134.6,133.2,130.9,128.9,127.7,127.2,123.3,122.9,121.9,120.7,116.3,107.9,99.2,90.8,60.7;计算值C20H16N3O2+[M+H+] 330.123 7,测量值330.122 4.
14)4ac 淡黄色固体;熔点 124~126 ℃;1H NMR(600 MHz,DMSO-d6)δ:10.56(s,1H),7.57(s,2H),7.38(s,1H),7.36~7.24(m,4H),7.19(t,J=7.7 Hz,1H),6.99(t,J=7.6 Hz,1H),6.81(d,J=7.7 Hz,1H),6.62(d,J=15.8 Hz,1H),6.20(dd,J=15.9,8.1 Hz,1H),5.28(d,J=8.0 Hz,1H).13C NMR(150 MHz,CDCl3)δ:176.3,169.5,141.0,134.9,133.4,131.5,129.9,127.2,127.2,125.4,124.4,123.8,123.3,123.0,117.7,110.6,91.1,62.0;计算值C20H15ClN3O2+[M+H+] 364.084 7,测量值364.085 4.
15)4ae 淡黄色固体;熔点 133~135 ℃;1H NMR(600 MHz,DMSO-d6)δ:10.60(s,1H),7.59(s,2H),7.24~7.19(m,2H),7.03(d,J=0.6 Hz,1H),6.83(d,J=7.6 Hz,1H),6.55(d,J=15.7 Hz,1H),6.49~6.39(m,3H),5.92~5.79(m,1H),5.31~5.25(m,1H).13C NMR(150 MHz,DMSO-d6)δ:177.9,169.3,168.6,150.5,143.7,142.3,141.3,129.0,128.6,125.7,122.9,122.0,120.3,111.8,110.8,110.0,87.7,61.1;高分辨质谱m/z:计算值C25H19N3NaO3+[M+Na+] 432.131 9,测量值432.133 2.
16)4af 淡黄色固体;熔点 143~145 ℃;1H NMR(600 MHz,DMSO-d6)δ:10.43(s,1H),8.17(s,2H),7.72~7.61(m,4H),7.48(d,J=7.4 Hz,1H),7.25(t,J=7.7 Hz,1H),7.09(t,J=7.6 Hz,1H),6.80(d,J=7.6 Hz,1H),6.66(d,J=16.0 Hz,1H),6.54(dd,J=15.9,7.9 Hz,1H),5.40(d,J=7.9 Hz,1H).13C NMR(150 MHz,DMSO-d6)δ:177.2,169.2,147.0,142.2,141.7,132.7,129.3,128.9,127.9,127.0,124.6,124.1,122.4,117.9,109.7,88.5,61.2;高分辨质谱m/z:计算值C20H14N4NaO4+[M+Na+] 397.090 7,测量值397.090 9.
17)4af′ 淡黄色固体;熔点 143~145 ℃;1H NMR(600 MHz,DMSO)δ:10.62(s,1H),8.13(s,2H),7.65(s,2H),7.57(d,J=8.8 Hz,2H),7.29(d,J=7.3 Hz,1H),7.22~7.16(m,1H), 6.98(t,J=7.5 Hz,1H),6.82(d,J=7.7 Hz,1H),6.76(d,J=16.0 Hz,1H),6.46(dd,J=15.9,6.8 Hz,1H),5.36(d,J=6.8 Hz,1H).13C NMR(150 MHz,DMSO)δ:178.2,168.6,146.9,141.9,141.4,131.3,129.1,128.7,128.3,127.7,126.2,124.0,121.9,117.7,110.0,87.2,61.1;高分辨质谱m/z:计算值C20H14N4NaO4+[M+Na+] 397.090 7,测量值397.091 2.
18)4ag 淡黄色固体;熔点 137~139 ℃;1H NMR(600 MHz,DMSO-d6)δ:10.40(s,1H),7.67(d,J=8.3 Hz,2H),7.63(s,2H),7.57(d,J=8.2 Hz,2H),7.48(d,J=7.4 Hz,1H),7.26~7.22(m,1H),7.09(t,J=7.6 Hz,1H),6.80(d,J=7.7 Hz,1H),6.61(d,J=16.0 Hz,1H),6.45(dd,J=16.0,8.1 Hz,1H),5.37(d,J=8.1 Hz,1H).13C NMR(150 MHz,DMSO-d6)δ:176.8,167.2,140.0,137.9,130.6,127.6,126.9,125.9,125.2,124.7,124.1,123.6,121.8,120.4,116.3,108.5,86.0,59.7;高分辨质谱m/z:计算值C21H14F3N3NaO2+[M+Na+] 398.111 1,测量值398.111 3.
19)4ag′ 淡黄色固体;熔点 137~139 ℃;1H NMR(600 MHz,DMSO-d6)δ:10.60(s,1H),7.63(s,2H),7.62(d,J=2.9 Hz,2H),7.51(d,J=8.2 Hz,2H),7.29(d,J=7.4 Hz,1H),7.23~7.13(m,1H),6.99(t,J=7.3 Hz,1H),6.82(d,J=7.7 Hz,1H),6.71(d,J=15.9 Hz,1H),6.35(dd,J=15.9,7.8 Hz,1H),5.34(d,J=7.7 Hz,1H).13C NMR(150 MHz,DMSO-d6)δ:178.6,169.0,141.8,139.7,132.4,129.4,128.8,127.7,127.1,126.5,126.0,125.4,123.6,122.3,118.1,110.3,87.8,61.6;高分辨质谱m/z:计算值C21H14F3N3NaO2+[M+Na+]398.111 1,测量值398.111 0.
20)4aj 淡黄色固体;熔点 141~143 ℃;1H NMR(600 MHz,CDCl3)δ:8.94(s,1H),7.35(d,J=7.3 Hz,1H),7.18~7.11(m,4H),7.05~7.00(m,3H),6.71(d,J=8.2 Hz,2H),6.67(d,J=15.6 Hz,1H),6.49(dd,J=15.6,8.0 Hz,1H),5.16(d,J=8.2 Hz,1H),3.72(s,3H).13C NMR(150 MHz,CDCl3)δ:175.6,169.7,149.8,145.0,141.1,140.4,133.9,131.5,128.3,124.6,123.9,120.8,120.1,114.0,113.6,108.1,90.8,62.1,41.4;计算值C21H18N3O3+[M+H+]360.134 3,测量值360.132 8.
21)4ak 淡黄色固体;熔点 129~131 ℃;1H NMR(600 MHz,CDCl3)δ:8.85(s,1H),7.36(d,J=7.5 Hz,2H),7.23(d,J=7.4 Hz,1H),7.13~7.11(m,2H),7.02~6.99(m,5H),6.53(d,J=15.7 Hz,1H),5.84(dd,J=15.8,8.0 Hz,1H),5.18(d,J=7.6 Hz,1H),2.27(s,3H).13C NMR(150 MHz,CDCl3)δ:179.3,169.1,141.1,140.0,138.6,136.9,135.5,132.5,129.3,127.9,127.1,126.9,124.3,120.6,119.3,110.8,89.9,62.1,21.3;高分辨质谱m/z:计算值C21H18N3O2+[M+H+]344.139 4,测量值344.139 7.
22)4bb 淡黄色固体;熔点 122~124 ℃;1H NMR(600 MHz,CDCl3)δ:7.42(d,J=7.3 Hz,1H),7.34~7.28(m,3H),7.17(t,J=7.5 Hz,1H),7.08~6.99(m,2H),6.77(d,J=7.7 Hz,1H),6.39~6.30(m,1H),6.27~6.18(m,1H),5.87(d,J=11.6 Hz,2H),5.19(d,J=8.5 Hz,1H),3.13(s,3H).13C NMR(150 MHz,CDCl3)δ:176.1,169.7,143.8,135.4,134.0,131.7,129.7,128.5,128.0,124.1,123.7,122.7,121.5,117.1,108.7,100.0,91.6,61.5,26.6;高分辨质谱m/z:计算值C21H16BrN3NaO2+[M+Na+] 444.031 8,测量值422.032 3.
23)4bb′ 淡黄色固体;熔点 122~124 ℃;1H NMR(600 MHz,CDCl3)δ:7.35(d,J=8.4 Hz,2H),7.31~7.28(m,1H),7.26(d,J=0.7 Hz,1H),7.12~7.09(m,1H),7.00~6.93(m,2H),6.82(d,J=7.8 Hz,1H),6.49(d,J=15.8 Hz,1H),5.91~5.81(m,1H),5.49~5.46(m,1H),5.46(d,J=11.0 Hz,2H),3.23(s,3H).13C NMR(150 MHz,CDCl3)δ:176.5,168.6,142.8,134.2,134.1,131.8,129.7,128.3,127.3,125.8,123.2,122.6,122.5,116.7,108.9,100.0,89.5,61.5,27.0;高分辨质谱m/z:计算值C21H16BrN3NaO2+[M+Na+]444.031 8,测量值422.032 3.
24)4cb 淡黄色固体;熔点 134~136 ℃;1H NMR(600 MHz,CDCl3)δ:7.34~7.31(m,2H), 7.31~7.27(m,2H),7.18~7.11(m,7H),7.07(t,J=7.8 Hz,2H),6.91(t,J=7.5 Hz,2H),6.62(d,J=7.8 Hz,1H),5.92~5.84(m,1H),5.55~5.49(m,1H),4.93(dd,J=105.5,16.0 Hz,2H).13C NMR(150 MHz,CDCl3)δ:176.8,168.9,141.7,135.5,134.9,134.6,134.0,131.8,129.5,128.8,128.6,128.3,127.6,126.7,125.7,124.2,123.7,123.3,122.7,109.9,89.9,61.8,44.1;高分辨质谱m/z:计算值C27H20BrN3NaO2+[M+Na+] 520.063 1,测量值520.065 4.
3 结 论
本文首次以各种不同取代基的靛红腈基底物为原料,在常用碱三乙胺的催化下,以二氯甲烷为溶剂,常温下分别与芳香甲醛、肉桂醛衍生物发生羟醛缩合/环化/质子迁移串联反应,以中等至良好的产率高效合成了螺靛红环呋喃衍生物.同时,还对该类化合物对真菌的抑制活性也进行了初步研究,发现部分化合物如化合物4ac能很好地抑制供试菌番茄早疫病菌菌丝生长,而化合物4ag也能强烈抑制供试菌小麦赤霉病菌菌丝生长,它们的抑制率分别为65.0%和85.9%.此外,这些化合物具有2个季碳中心并且同时具有多个可以官能团化的氨基、腈基及双键,为进一步合成其他活性物质提供了很好的骨架单元.
[1]Ramu P,Augustine A P T,Scholastica M V.Synthesis,characterization and biological activity of novel spiroheterocycles from isatin derivatives[J].Der Pharm Chem,2014,6(4):30-36.
[2]Neelakandan V L,Yuvara J A,Paramasivam T,et al.Unusual mechanistic pathway for the synthesis of novel spiro-oxindoles via 3-phenyl-5-isoxazolone ring cleavage by secondary amino acids[J].Tetrahedron Lett,2011,52(27):3437-3442.
[3]Kassab S E,Hegazy G H,Eid N M,et al.Synthesis of 1H-indole-2,3-dione-3-thiosemicarbazone ribonucleosides as antibacterial agents[J].Nucleosides,Nucleotides Nucleic Acids,2010,29(1):72-80.
[4]Mei L,Wei Y,Xu Q,et al.Diastereo- and enantioselective construction of oxindole-fused spirotetrahydrofuran scaffolds through palladium-catalyzed asymmetric [3+2] cycloaddition of vinyl cyclopropanes and isatins[J].Organomet Chem,2013,32(12):3544-3556.
[5]Singh U K,Pandeya S N,Singh A,et al.Synthesis and antimicrobial activity of schiff’s andN-mannich bases of isatin and its derivatives with 4-amino-N-carbamimidoyl benzene sulfonamide[J].Int J Pharm Sci Drug Res,2010,2(2):151-154.
[6]Dandia A,Singh R,Khaturia S,et al.Efficient microwave enhanced regioselective synthesis of a series of benzimidazolyl/triazolyl spiro [indole-thiazolidinones] as potent antifungal agents and crystal structure of spiro[3H-indole-3,2′-thiazolidine]-3′(1,2,4-triazol-3-yl)-2,4′(1H)-dione[J].Bioorg Med Chem,2006,14(7):2409-2417.
[7]Girija S S,Zelalem Y D.Isatins as privileged molecules in design and synthesis of spiro-fused cyclic frameworks[J].Chem Rev,2012,112(11):6104-6155.
[8]谢建武,余超波,徐美兰.手性伯胺催化麦克尔/烷基化串联反应:螺靛红环丙烷衍生物的高效合成[J].浙江师范大学学报:自然科学版,2017,40(1):51-57.
[9]Zhu X Q,Wu J S,Xie J W.Stereoselective construction of bi-spirooxindole frameworks via a michael addition/cyclization and an unexpected redox/oxidative coupling/cyclization[J].Tetrahedron,2016,72(50):8327-8334.
[10]Wu J S,Zhang X,Xie J W,et al.Synthesis and antifungal activities of novel polyheterocyclic spirooxindole derivatives[J].Org Biomol Chem,2015,13(17):4967-4975.
[11]Fu Z K,Pan J Y,Xie J W,et al.Organocatalytic domino michael/cyclization reaction:Efficient synthesis of multi-functionalized tetracyclic spirooxindoles with multiple stereocenters[J].RSC Advances,2014,4(93):51548-51557.
[12]Huang X,Zhang Y R,Xie J W,et al.Construction of functionalized spiro 1,4-benzoxazine oxindole derivatives via domino mannich-alkylation of α-halocarbonyl compounds with imines[J].Tetrahedron Lett,2013,54(44):5857-5860.
[13]Garden S J,Guimaraes C R W,Corréa M B,et al.Synthetic and theoretical studies on the reduction of electron withdrawing croup conjugated olefins using the hantzsch 1,4-dihydropyridine ester[J].J Org Chem,2003,68(23):8815-8822.

